The Effects of Blue Light and Supplemental Far-Red on an In Vitro Multiple Harvest System for the Production of Cannabis sativa
Abstract
1. Introduction
2. Results and Discussion
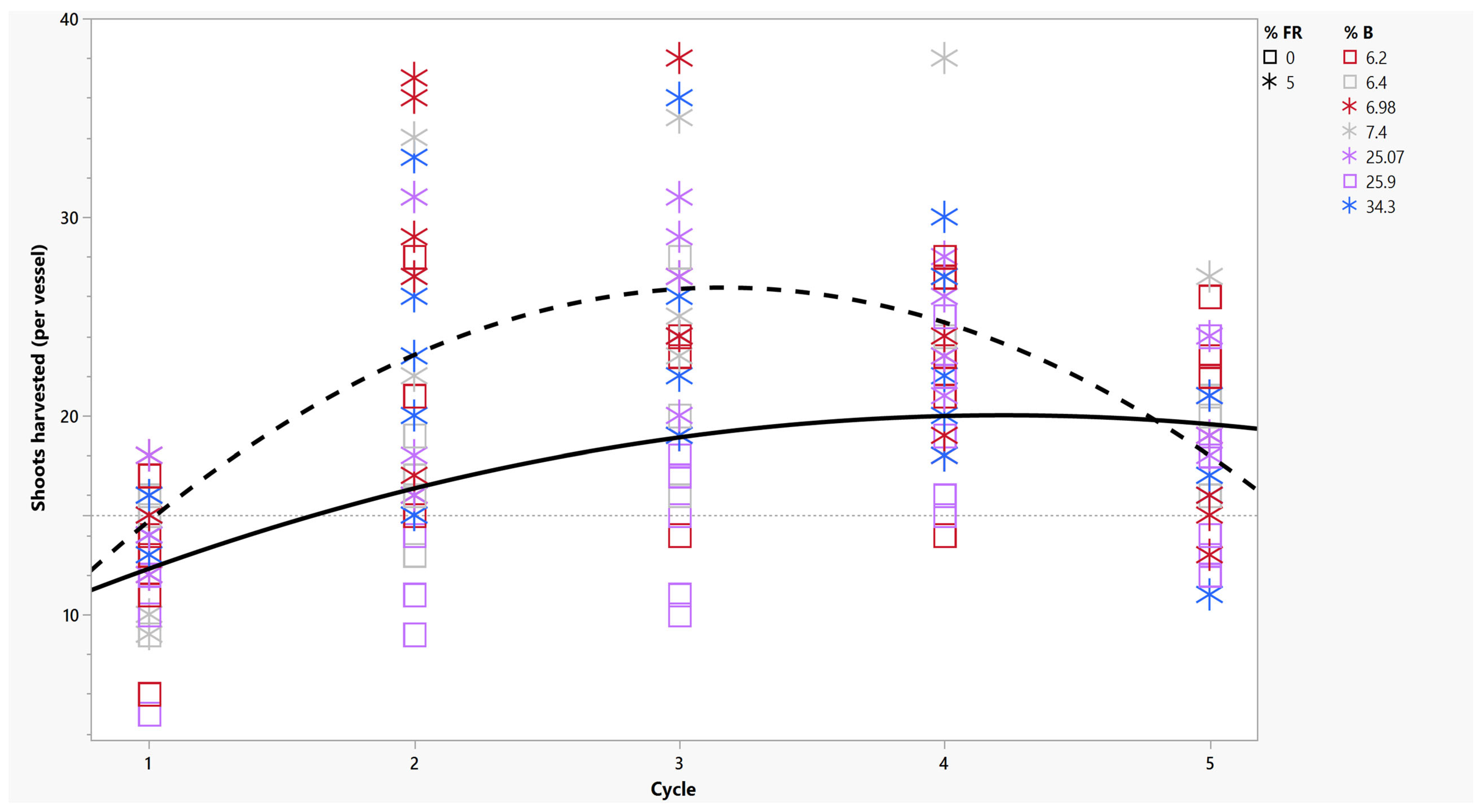
2.1. Number of Shoots Harvested
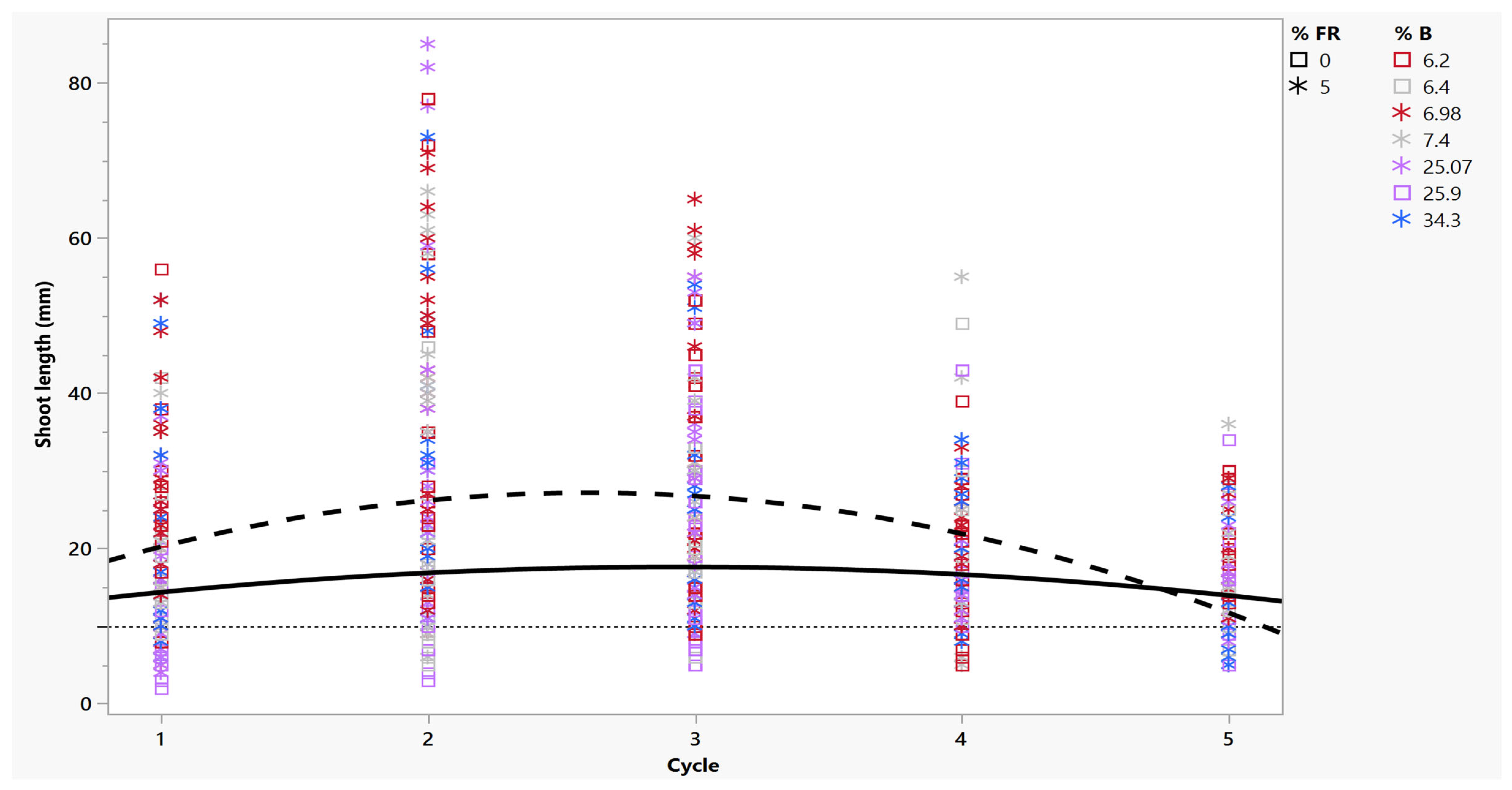
2.2. Length of Harvested Shoots
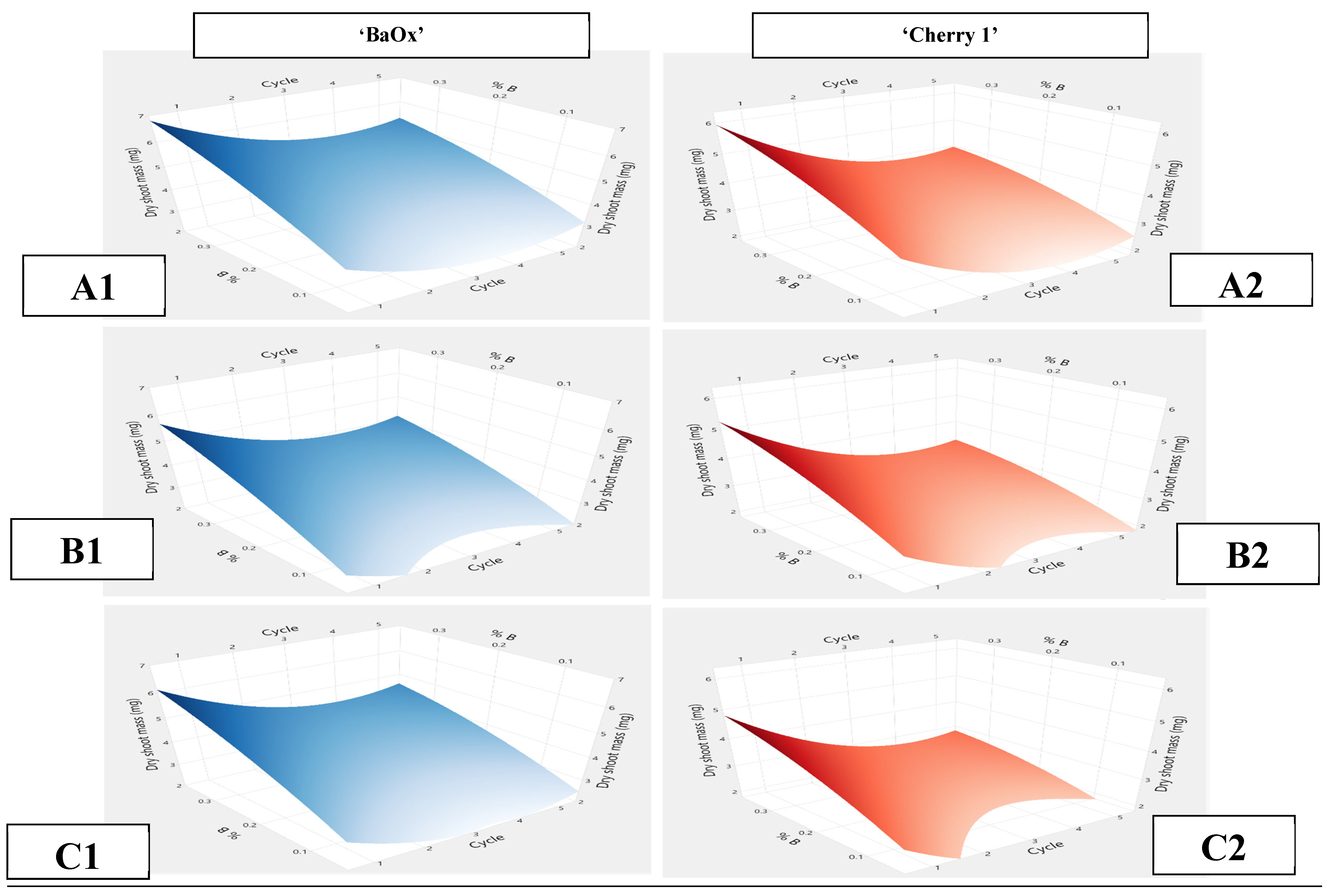
2.3. Dry Shoot Mass
2.4. Ex Vitro Rooting
3. Methods and Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batista, D.S.; Felipe, S.H.S.; Silva, T.D.; de Castro, K.M.; Mamedes-Rodrigues, T.C.; Miranda, N.A.; Ríos-Ríos, A.M.; Faria, D.V.; Fortini, E.A.; Chagas, K.; et al. Light quality in plant tissue culture: Does it matter? Vitr. Cell Dev. Biol. Plant 2018, 54, 195–215. [Google Scholar] [CrossRef]
- Cavallaro, V.; Pellegrino, A.; Muleo, R.; Forgione, I. Light and Plant Growth Regulators on In Vitro Proliferation. Plants 2022, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Boros, F.; Székely, G.; Balázs, L.; Csambalik, L.; Sipos, L. Effects of LED lighting environments on lettuce (Lactuca sativa L.) in PFAL systems. Sci. Hortic. 2023, 321, 112351. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental lighting quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Biol. 2009, 67, 59–64. [Google Scholar]
- Westmoreland, F.M.; Kusuma, P.; Bugbee, B. Cannabis lighting: Decreasing blue photon fraction increases yield but efficacy is more important for cost effective production of cannabinoids. PLoS ONE 2021, 16, e0248988. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; van Iersel, M.W. Far-red light effects on lettuce growth and morphology in indoor production are cultivar specific. Plants 2022, 11, 2714. [Google Scholar] [CrossRef] [PubMed]
- Morello, V.; Brousseau, V.D.; Wu, N.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. Light Quality Impacts Vertical Growth Rate, Phytochemical Yield and Cannabinoid Production Efficiency in Cannabis sativa. Plants 2022, 11, 2982. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-C.; Jeon, S.-Y.; Choi, Y.-J.; Byun, B.-H.; Kim, D.-H.; Yu, G.-R.; Kim, H.; Lim, D.-W. Establishment of an In Vitro Micropropagation System for Cannabis sativa ‘Cheungsam’. Horticulturae 2024, 10, 1060. [Google Scholar] [CrossRef]
- Murphy, R.; Adelberg, J. Physical factors increased quantity and quality of micropropagated shoots of Cannabis sativa L. in a repeated harvest system with ex vitro rooting. Vitr. Cell. Dev. Biol. Plants 2021, 57, 923–931. [Google Scholar] [CrossRef]
- McKay, M.; Adelberg, J.; Faust, J.; Taylor, M. Modified media for repeated in vitro cutting cycles of Cannabis sativa L. without the use of cytokinin. Plants 2024, submitted in press.. [Google Scholar]
- Debergh, P.C.; Topoonyanont, N.; Van Huylenbroeck, J.; Moreira da Silva, H.; Oyaert, E. Preparation of microplants for ex vitro establishment. Acta Hort. 2000, 530, 269–275. [Google Scholar] [CrossRef]
- Sae-Tang, W.; Heuvelink, E.P.; Kohlen, W.; Argyri, E.; Celine, N.; Leo, M. Effects of far-red and blue light on rooting in medicinal cannabis cuttings and related changes in endogenous auxin and carbohydrates. Sci. Hortic. 2024, 325, 112614. [Google Scholar] [CrossRef]
- Kong, Y.; Nemali, K. Blue and Far-Red Light Affect Area and Number of Individual Leaves to Influence Vegetative Growth and Pigment Synthesis in Lettuce. Front. Plant Sci. 2021, 12, 667407. [Google Scholar] [CrossRef] [PubMed]



| Light Treatment | R:B | B (%) | Measured Total PPFD. (µmol m−2 s−1) |
|---|---|---|---|
| White (W) | 9.1 | 6.4 | 211 |
| High red (RHIGH) | 15.2 | 6.2 | 209 |
| Medium red (RMID) | 2.9 | 25.9 | 238 |
| White w/5% far-red (W + FR) | 7.8 | 7.4 | 192 |
| High red w/5% far-red (RHIGH + FR) | 13.3 | 6.9 | 214 |
| Medium red w/5% far-red (RMID + FR) | 3.0 | 25.1 | 243 |
| Low red w/5% far-red (RLOW + FR) | 1.9 | 34.3 | 198 |
| Source | Shoots Harvested | Shoot Length | Dry Mass per Shoot | Ex Vitro Rooting |
|---|---|---|---|---|
| Cycle (C) | <0.0001 | 0.0597 | <0.0001 | 0.2724 |
| C C | <0.0001 | <0.0001 | 0.0040 | 0.0022 |
| Genotype (G) | 0.7085 | 0.7892 | 0.0008 | 0.1578 |
| Far-red (FR) | <0.0001 | <0.0001 | 0.0679 | 0.6218 |
| % Blue (B) | 0.0025 | <0.0001 | <0.0001 | 0.3943 |
| Standard (S) | 0.4636 | 0.0051 | <0.0001 | 0.0787 |
| C G | 0.0505 | 0.3263 | 0.4218 | 0.3172 |
| C FR | 0.1238 | 0.0001 | 0.1750 | 0.5680 |
| C B | 0.9931 | 0.0744 | 0.4135 | 0.8669 |
| G FR | 0.9145 | 0.2100 | 0.5090 | 0.0102 |
| G B | 0.2010 | 0.1135 | 0.1830 | 0.5226 |
| FR B | 0.1207 | 0.8704 | 0.0937 | 0.6359 |
| B B | 0.9986 | 0.5571 | 0.6067 | 0.3307 |
| Whole model | 0.4550 | 0.1718 | 0.4631 | 0.0400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKay, M.; Faust, J.E.; Taylor, M.; Adelberg, J. The Effects of Blue Light and Supplemental Far-Red on an In Vitro Multiple Harvest System for the Production of Cannabis sativa. Plants 2025, 14, 966. https://doi.org/10.3390/plants14060966
McKay M, Faust JE, Taylor M, Adelberg J. The Effects of Blue Light and Supplemental Far-Red on an In Vitro Multiple Harvest System for the Production of Cannabis sativa. Plants. 2025; 14(6):966. https://doi.org/10.3390/plants14060966
Chicago/Turabian StyleMcKay, Molly, James E. Faust, Matthew Taylor, and Jeffrey Adelberg. 2025. "The Effects of Blue Light and Supplemental Far-Red on an In Vitro Multiple Harvest System for the Production of Cannabis sativa" Plants 14, no. 6: 966. https://doi.org/10.3390/plants14060966
APA StyleMcKay, M., Faust, J. E., Taylor, M., & Adelberg, J. (2025). The Effects of Blue Light and Supplemental Far-Red on an In Vitro Multiple Harvest System for the Production of Cannabis sativa. Plants, 14(6), 966. https://doi.org/10.3390/plants14060966







