Optimal Irradiation Strategy to Induce Male Sterility in Cotton Mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae)
Abstract
1. Introduction
2. Results
2.1. Optimal Day Age for P. solenopsis Sterility
2.2. Preliminary Selection of Optimal Radiation Dose for P. solenopsis Sterility
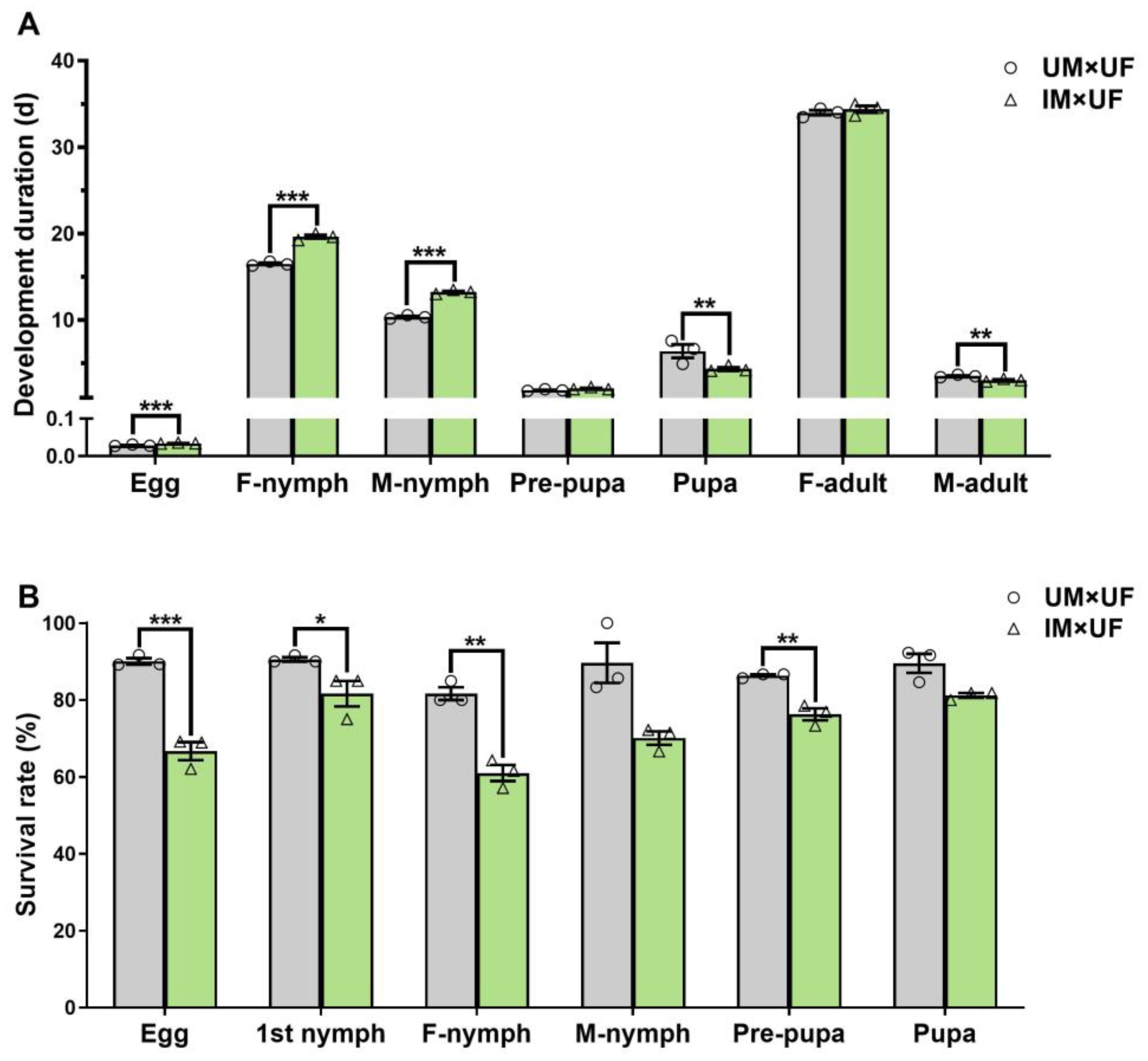
2.3. Effect of 60 Gy Gamma Radiation on Mating Competitiveness of Irradiated F0 Males
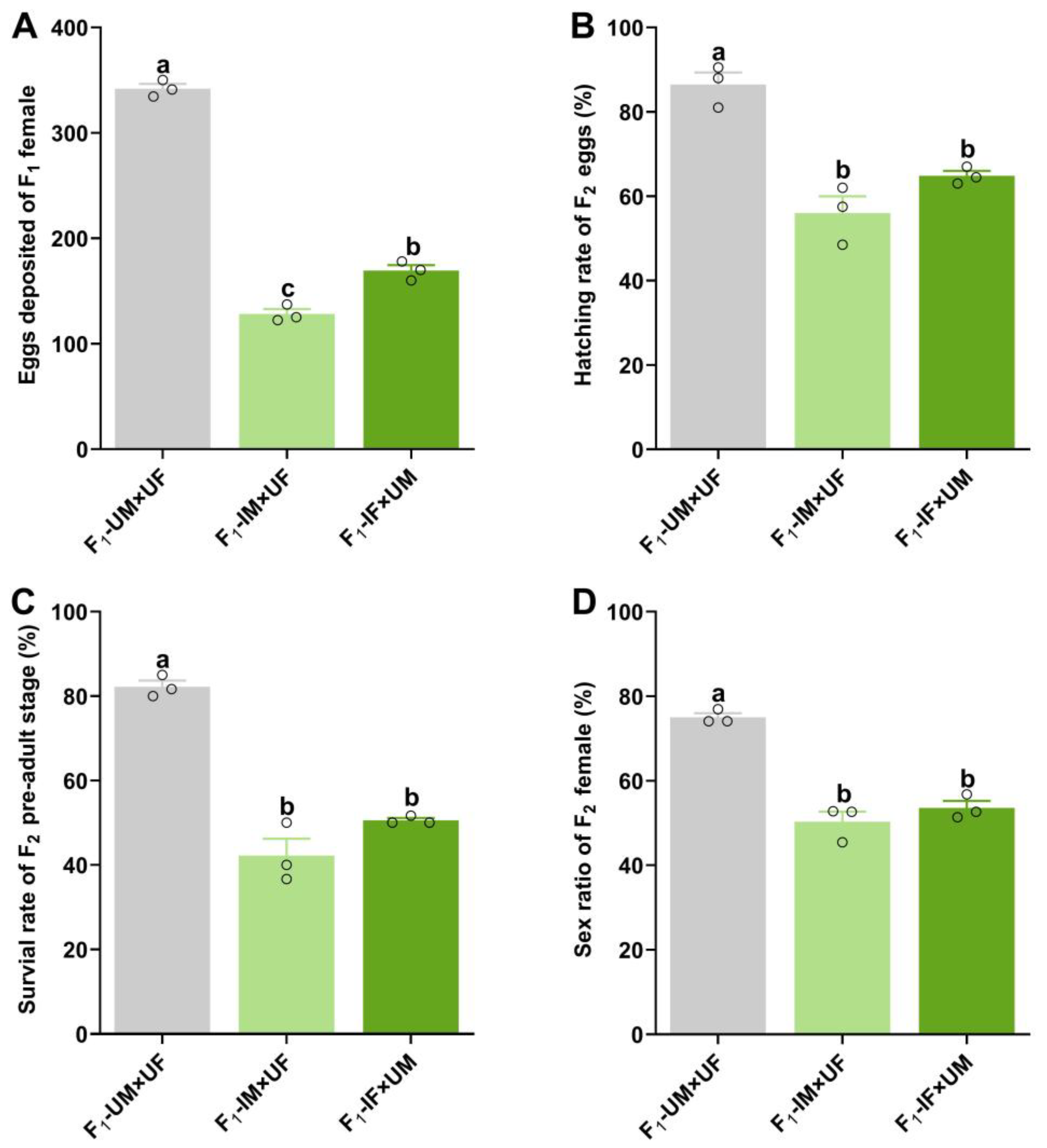
2.4. Effect of 60 Gy Gamma Radiation on Descendants (F1 and F2) of P. solenopsis Irradiated F0 Males
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Selection of Optimal Day Age for P. solenopsis Sterility
4.3. Preliminary Selection of Optimal Radiation Dose for P. solenopsis Sterility
4.4. Effect of 60 Gy Gamma Radiation on Mating Competitiveness of F0 Males
- Fifteen unirradiated males (UMs) and fifteen unirradiated females (UFs) (1:0:1);
- Fifteen irradiated males (IMs) and fifteen UFs (0:1:1);
- Ten UMs, ten IMs, and ten UFs (1:1:1).
4.5. Effect of 60 Gy Gamma Radiation on Descendants of Irradiated F0 Males
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tinsley, J.B. An ant’s-nest coccid from New Mexico. Can. Entomol. 1898, 30, 47–48. [Google Scholar] [CrossRef]
- Fand, B.B.; Suroshe, S.S. The invasive mealybug Phenacoccus solenopsis Tinsley, a threat to tropical and subtropical agricultural and horticultural production systems-A review. Crop Prot. 2015, 69, 34–43. [Google Scholar] [CrossRef]
- Abdul-Rassoul, M.S.; Al-Malo, I.M.; Hermiz, F.B. First record and host plants of Solenopsis Mealybug, Phenacoccus solenopsis Tinsley, 1898 (Hemiptera: Pseudococcidae) from Iraq. J. Biodivers. Environ. Sci. 2015, 7, 216–222. [Google Scholar]
- Wu, S.A.; Zhang, R.Z. A new invasive pest, Phenacoccus solenopsis, threatening seriously to cotton production. Chin. Bull. Entomol. 2009, 46, 159–162. (In Chinese) [Google Scholar]
- Çaliskan, A.F.; Kaydan, M.B.; Mustu, M. Demographic parameters and biological features of Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcodae) on four ornamental plants. Phytoparasitica 2016, 44, 75–82. [Google Scholar] [CrossRef]
- Ma, J.; Hu, X.N.; Liu, H.J.; Liang, F.; Zhao, J.P.; Feng, L.X.; Chen, N.Z. Phenacoccus solenopsis was found on Hibiscus rosa-sinensis in Guangzhou. Plant Quar. 2009, 23, 35–36. (In Chinese) [Google Scholar]
- Huang, T.; Jiang, X.L.; Zhou, T.Y. Be Alert to the Threat of Phenacoccus solenopsis to Cotton Production. China Cust. 2022, 56. (In Chinese) [Google Scholar]
- Ahmad, M.; Akhtar, S. Development of resistance to insecticides in the invasive mealybug Phenacoccus solenopsis (Hemiptera: Pseudococcidae) in Pakistan. Crop Prot. 2016, 88, 96–102. [Google Scholar] [CrossRef]
- Lachance, L.E.; Richard, R.D.; Proshold, F.I. Radiation response in the pink bollworm: A comparative study of sperm bundle production, sperm transfer, and oviposition response elicited by native and laboratory-reared males. Environ. Entomol. 1975, 4, 321–324. [Google Scholar] [CrossRef]
- Klassen, W.; Curtis, C.F.; Hendrichs, J. History of the sterile insect technique. In Sterile Insect Technique Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 3–38. [Google Scholar]
- Bakri, A.; Mehta, K.; Lance, D.R. Sterilizing insects with ionizing radiation. In Sterile Insect Technique Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 355–398. [Google Scholar]
- Neven, L.G.; Wakie, T. Effects of irradiation on codling moth (Lepidoptera: Tortricidae) first through third instars in sweet cherries. J. Entomol. Sci. 2020, 55, 570–577. [Google Scholar] [CrossRef]
- Zhou, S.; Li, X.; Zhang, J.; Liu, C.; Huang, J.; Zhang, Z.; Ren, X.; Chen, L.; Hafeez, M.; Han, P.; et al. Screening the optimal dose of gamma radiation for Tuta absoluta sterility: Paving the way for sterile insect technique programs. Entomol. Gen. 2024, 44, 415–422. [Google Scholar] [CrossRef]
- Chu, S.; Liu, B.; Li, H.; Lu, K.; Lu, Y. Effect of X-ray irradiation on the biological parameters of Xestia c-nigrum. Front. Physiol. 2024, 15, 1362991. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.T.; He, W.; Jiang, S.; Ge, S.S.; Chu, B.; Liang, G.M.; Yang, X.M.; Wu, K.M. The evaluation on control potential using X-ray to irradiate adult Spodoptera frugiperda (Lepidoptera: Noctuidae). Pest Manag. Sci. 2025, 81, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Koyama, J.; Kakinohana, H.; Miyatake, T. Eradication of the melon fly, Bactrocera cucurbitae, in Japan: Importance of behavior, ecology, genetics, and evolution. Annu. Rev. Entomol. 2004, 49, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Plá, I.; de Oteyza, J.G.; Tur, C.; Martínez, M.Á.; Laurín, M.C.; Alonso, E.; Martínez, M.; Martín, Á.; Sanchis, R.; Navarro, M.C.; et al. Sterile insect technique programme against Mediterranean fruit fly in the Valencian Community (Spain). Insects 2021, 12, 415. [Google Scholar] [CrossRef]
- Ramírez-Santos, E.; Rendon, P.; Gouvi, G.; Zacharopoulou, A.; Bourtzis, K.; Cáceres, C.; Bloem, K. A novel genetic sexing strain of Anasrepha ludens for cost-effective sterile insect technique applications: Improved genetic stability and rearing efficiency. Insects 2021, 12, 499. [Google Scholar] [CrossRef]
- Panduranga, G.S.; Sharma, K.; Singh, B.; Sharma, R.K. Effect of gamma irradiation on quality parameters sterility and mating competitiveness of melon fly, Bactrocera cucurbitae (Coquillett). Int. J. Trop. Insect Sc. 2021, 42, 875–883. [Google Scholar] [CrossRef]
- Ma, C.; Liu, H.; Liu, B.; Zhao, J.P.; Zhao, Q.Y.; Song, Z.J.; Han, X.; Zhan, G.P. Gamma and X-ray irradiation as a phytosanitary treatment against various stages of Planococcus lilacinus (Hemiptera: Pseudococcidae). J. Asia-Pac. Entomol. 2022, 25, 102009. [Google Scholar] [CrossRef]
- Song, Z.J.; Zhao, Q.Y.; Ma, C.; Chen, R.R.; Ma, T.B.; Li, Z.H.; Zhan, G.P. Quarantine disinfestation of papaya mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae) using gamma and X-rays irradiation. Insects 2023, 14, 682. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Ma, F.H.; Deng, W.; Li, Z.H.; Song, Z.J.; Ma, C.; Ren, Y.L.; Du, X.; Zhan, G.P. Phytosanitary irradiation treatment of the aerial root mealybug, Pseudococcus baliteus (Hemiptera: Pseudococcidae). J. Econ. Entomol. 2023, 116, 1567–1574. [Google Scholar] [CrossRef]
- Huang, F.; Li, W.; Li, X.; Bei, Y.; Lin, W.; Lu, Y.; Wang, B. Irradiation as a quarantine treatment for the solenopsis mealybug, Phenacoccus solenopsis. Radiat. Phys. Chem. 2014, 96, 101–106. [Google Scholar] [CrossRef]
- Seth, R.K.; Zarin, M.; Khan, Z.; Seth, R. Ionizing radiation as a phytosanitary treatment against Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Fla. Entomol. 2016, 99, 76–87. [Google Scholar]
- Gavrilov-Zimin, I.A. Chromosomal and reproductive features of some Oriental and Australasian scale insect (Homoptera, Coccinae). Comp. Cytogen. 2020, 14, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Indarwatmi, M.; Dadang, D.; Ridwani, S.; Ratna, E.S. The bionomics of the cocoa mealybug, Exallomochlus hispidus (Morrison) (Hemiptera: Pseudocoddiae), on mangosteen fruit and three alternative hosts. Insects 2017, 8, 75. [Google Scholar] [CrossRef]
- Vennila, S.; Deshmukh, A.J.; Pinjarkar, D.; Agarwal, M.; Ramamurthy, V.V.; Joshi, S.; Kranthi, K.R.; Bambawale, O.M. Biology of the mealybug, Phenacoccus solenopsis on cotton in the laboratory. J. Insect Sci. 2010, 10, 115. [Google Scholar] [CrossRef]
- Hodgson, C.J.; Abbas, G.; Arif, M.J.; Saeed, S.; Karar, H. Phenacoccus solenopsis Tinsley (Sternorrhyncha: Coccoidea: Pseudococcidae), an invasive mealybug damaging cotton in Pakistan and India, with a discussion on seasonal morphological variation. Zootaxa 2008, 1913, 1–35. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, J.M.; Zhang, P.J.; Lu, Y.B. Reproduction of the solenopsis mealybug, Phenacoccus solenopsis: Males play an important role. J. Insect Sci. 2013, 13, 137. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Huang, F.; Lu, Y.B. Bionomics of mealybug Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) on cotton. Acta Entomol. Sin. 2011, 54, 246–252. (In Chinese) [Google Scholar]
- Zhao, J.; Li, S.; Xu, L.; Li, C.; Li, Q.; Dewer, Y.; Wu, K. Effects of X-ray irradiation on biological parameters and induced sterility of Ephestia elutella: Establishing the optimum irradiation dose and stage. Front. Physiol. 2022, 13, 895882. [Google Scholar] [CrossRef]
- Jiang, S.; He, L.M.; He, W.; Zhao, H.Y.; Yang, X.M.; Yang, X.Q.; Wu, K.M. Effects of X-ray irradiation on the fitness of the established invasive pest fall armyworm Spodoptera frugiperda. Pest Manag. Sci. 2022, 78, 2806–2815. [Google Scholar] [CrossRef]
- Ferrater, J.B.; Gómez-Marco, F.; Yoshimoto, A.K.; Greene, T.D.; Simmons, G.S.; Daugherty, M.P.; Rugman-Jones, P.F. Development of a sterile insect technique as a control strategy for the Asian citrus psyllid: Establishing the effect of sterilizing X-rays on fecundity, fertility, and survival. J. Econ. Entomol. 2024, 117, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.F.; Maier, M.J.; Gilles, J.; Jacquet, M.; Lemperiere, G.; Quilici, S.; Vreysen, M.J.B.; Schooneman, F.; Chadee, D.D.; Boyer, S. Effects of irradiation, presence of females, and sugar supply on the longevity of sterile males Aedes albopictus (Skuse) under semi-field conditions on Reunion Island. Acta Trop. 2013, 125, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Villalun, M.; Geib, S.M.; Goodman, C.L.; Ringbauer, J.; Stanley, D. Pupal X-ray irradiation influences protein expression in adults of the oriental fruit fly, Bactrocera dorsalis. J. Insect Physiol. 2015, 76, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Bakri, A.; Heather, N.; Hendrichs, J.; Ferris, I. Fifty years of radiation biology in entomology: Lessons learned from IDIDAS. Ann. Entomol. Soc. Am. 2005, 98, 1–12. [Google Scholar] [CrossRef]
- Rull, J.; Arredondo, J.; Diaz-Fleischer, F. Improved mating performance of male Anastrepha ludens (Diptera: Tephritidae) irradiated at low doses for release in sterile insect technique programmes. Int. J. Trop. Insect Sci. 2014, 34, S28–S34. [Google Scholar] [CrossRef]
- Muthu, L.B.C.; Panduranga, G.S.; Rajesh, A.; Reddy, K.; Ramanamurthy, B. Gamma radiation for sterile insect quality in melon fly, Zeugodacus cucurbitae (Coquillet). Int. J. Trop. Insect Sci. 2023, 43, 949–960. [Google Scholar]
- Parker, A.G.; Vreysen, M.J.B.; Bouyer, J.; Calkins, C.O. Sterile insect quality control/assurance. In Sterile Insect Technique Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 400–426. [Google Scholar]
- Rendón, P.; Mcinnis, D.; Lance, D.; Stewart, J. Medfly (Diptera: Tephritidae) genetic sexings: Large-scale field comparison of males-only and bisexual sterile fly releases in Guatemala. Ecol. Behav. 2004, 97, 1547–1553. [Google Scholar]
- Mastrangelo, T.; Kovaleski, A.; Botteon, V.; Scopel, W.; Costa, M. Optimization of the sterilizing doses and overflooding ratios for the South American fruit fly. PLoS ONE 2018, 13, e201026. [Google Scholar] [CrossRef]
- Wee, S.L.; Suckling, D.M.; Burnip, G.M.; Hackett, J.; Barrington, A.; Pedley, R. Effects of substerilizing doses of gamma radiation on adult longevity and level of inherited sterility in Teia anartoides (Lepidoptera: Lymantriidae). J. Econ. Entomol. 2005, 98, 732–738. [Google Scholar] [CrossRef]
- Osouli, S.; Ahmadi, M.; Kalantarian, N. Radiation biology and inherited sterility in Helicoverpa armigera Hübner (Lepidoptera: Nuctuidae). Int. J. Trop. Insect Sci. 2021, 41, 2421–2429. [Google Scholar] [CrossRef]
- Marec, F.; Bloem, S.; Carpenter, J.E. Inherited sterility in insects. In Sterile Insect Technique Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 164–186. [Google Scholar]
- North, D.T.; Holt, G.G. Population suppression by transmission of inherited sterility to progeny of irradiated cabbage loopers, Trichoplusia ni. Can. Entomol. 1969, 101, 513–520. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Gross, H.R. Suppression of feral Helicoverpa zea (Lepidoptera: Noctuidae) populations following the infusion of inherited sterility from released substerile males. Environ. Entomol. 1993, 22, 1084–1091. [Google Scholar] [CrossRef]
- Boersma, N. The suppression of the false codling moth in South Africa using an AW-IPM approach with a sit component. In Area-Wide Integrated Pest Management, 1st ed.; Hendrichs, J., Pereira, R., Vreysen, M.J.B., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 93–109. [Google Scholar]
- Zhao, C.; Huang, F.; Wang, Z.; Lu, Y. Individual development of Phenacoccus solenopsis males (Homoptera: Pseudococcidae). Acta Agric. Zhejiangensis 2016, 28, 1544–1549. (In Chinese) [Google Scholar]
- Fried, M. Determination of sterile insect competitiveness. J. Econ. Entomol. 1971, 64, 869–872. [Google Scholar] [CrossRef]
- Hooper, G.H.S.; Horton, I.F. Competitiveness of sterilized male insects: A method of calculating the variance of the value derived from competitive mating tests. J. Econ. Entomol. 1981, 74, 119–121. [Google Scholar] [CrossRef]


| Target Variable | Source | Type III SS | df | MS | F | p |
|---|---|---|---|---|---|---|
| Adjusted mortality | Radiation dose | 27,755.311 | 4 | 6938.828 | 1782.452 | <0.001 |
| Age | 3105.778 | 4 | 776.444 | 199.454 | <0.001 | |
| Radiation dose × Age | 2182.929 | 16 | 136.433 | 35.047 | <0.001 | |
| Emergence rate | Radiation dose | 36,313.534 | 5 | 7262.707 | 1131.385 | <0.001 |
| Age | 3235.275 | 4 | 808.819 | 125.998 | <0.001 | |
| Radiation dose × Age | 2139.412 | 20 | 106.971 | 16.664 | <0.001 | |
| Deformity rate | Radiation dose | 24,583.731 | 5 | 4916.746 | 509.003 | <0.001 |
| Age | 3703.982 | 4 | 925.996 | 95.863 | <0.001 | |
| Radiation dose × Age | 4275.637 | 18 | 237.535 | 24.591 | <0.001 |
| Pupae Day Age | Number | Slope ± SE | Estimated Doses (95% CI) (Gy) | χ2 (df) | p Values | |
|---|---|---|---|---|---|---|
| ED50 | ED99 | |||||
| 1-day-old | 559 | 2.30 ± 0.39 | 75.23 (64.99–85.09) | 206.54 (160.22–335.86) | 2.64 (12) | 0.998 |
| 2-day-old | 644 | 2.67 ± 0.58 | 100.96 (85.96–112.52) | 241.65 (189.77–427.10) | 9.74 (12) | 0.639 |
| 3-day-old | 662 | 2.33 ± 0.46 | 121.94 (101.95–137.37) | 331.43 (256.82–577.82) | 5.16 (12) | 0.852 |
| 4-day-old | 694 | 1.67 ± 0.32 | 137.98 (114.62–159.22) | 554.53 (385.05–1204.22) | 3.81 (12) | 0.987 |
| 5-day-old | 672 | 1.58 ± 0.35 | 172.84 (145.51–209.03) | 752.34 (466.27–2473.90) | 1.33 (12) | 1.000 |
| Cross Ratio (UM × IM × UF) | Fecundity (Per Female) | %Observed Egg Hatch | Competitiveness Value (C) | Variance of C |
|---|---|---|---|---|
| 1:0:1 | 228.84 ± 3.35 a | 73.75 ± 1.30 a | ||
| 0:1:1 | 200.68 ± 4.21 b | 44.94 ± 1.74 c | ||
| 1:1:1 | 211.53 ± 1.94 b | 52.57 ± 1.04 b | 2.21 | 6.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Lei, Y.; Liu, C.; Ullah, F.; Huang, J.; Zhou, Z.; Lu, Y. Optimal Irradiation Strategy to Induce Male Sterility in Cotton Mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Plants 2025, 14, 912. https://doi.org/10.3390/plants14060912
Dong W, Lei Y, Liu C, Ullah F, Huang J, Zhou Z, Lu Y. Optimal Irradiation Strategy to Induce Male Sterility in Cotton Mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Plants. 2025; 14(6):912. https://doi.org/10.3390/plants14060912
Chicago/Turabian StyleDong, Wanying, Yang Lei, Chaogang Liu, Farman Ullah, Jun Huang, Zhongshi Zhou, and Yaobin Lu. 2025. "Optimal Irradiation Strategy to Induce Male Sterility in Cotton Mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae)" Plants 14, no. 6: 912. https://doi.org/10.3390/plants14060912
APA StyleDong, W., Lei, Y., Liu, C., Ullah, F., Huang, J., Zhou, Z., & Lu, Y. (2025). Optimal Irradiation Strategy to Induce Male Sterility in Cotton Mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Plants, 14(6), 912. https://doi.org/10.3390/plants14060912









