Seed Characteristics and Terpene Variability of Mediterranean Fir Species (Abies nebrodensis, A. pinsapo, and A. alba)
Abstract
1. Introduction
2. Results and Discussion
2.1. Seed Characteristics
2.2. Terpene Profile
3. Materials and Methods
3.1. Plant Material
3.2. Seed Moisture Content and Morphological Features
3.3. Terpene Isolation
3.4. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, R.J. World markets for coniferous forest products: Recent trends and future prospects. Acta Hortic. 2003, 615, 349–353. [Google Scholar] [CrossRef]
- Farjon, A. A Handbook of the World’s Conifers; BRILL: Leiden, The Netherlands, 2010; Volume 1. [Google Scholar]
- Liu, T.S. A Monograph of the Genus Abies; Department of Forestry, College of Agriculture, National Taiwan University: Taipei, China, 1971. [Google Scholar]
- Ran, J.H.; Shen, T.T.; Wu, H.; Gong, X.; Wang, X.Q. Phylogeny and evolutionary history of Pinaceae updated by transcriptomic analysis. Mol. Phylogenet. Evol. 2018, 129, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Goswami, P.; Chanotiya, C.S. Chemical analysis of volatile oils from West Himalayan Pindrow Fir Abies pindrow. Nat. Prod. Commun. 2014, 9, 1181–1184. [Google Scholar] [CrossRef]
- Sękiewicz, K.; Sękiewicz, M.; Jasińska, A.K.; Boratyńska, K.; Iszkuło, G.; Romo, A.; Boratyński, A. Morphological diversity and structure of West Mediterranean Abies species. Plant Biosyst. 2013, 147, 125–134. [Google Scholar] [CrossRef]
- Liepelt, S.; Mayland-Quellhorst, E.; Lahme, M.; Ziegenhagen, B. Contrasting geographical patterns of ancient and modern genetic lineages in Mediterranean Abies species. Plant Syst. Evol. 2010, 284, 141–151. [Google Scholar] [CrossRef]
- Terrab, A.; Talavera, S.; Arista, M.; Paun, O.; Stuessy, T.F.; Tremetsberger, K. Genetic diversity at chloroplast microsatellites (cpSSRs) and geographic structure in endangered West Mediterranean firs (Abies spp., Pinaceae). Taxon 2007, 56, 409–416. [Google Scholar] [CrossRef]
- Caudullo, G.; Tinner, W. Abies_spp. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; p. e015be7+. [Google Scholar]
- Wajs-Bonikowska, A.; Sienkiewicz, M.; Stobiecka, A.; Maciąg, A.; Szoka, Ł.; Karna, E. Chemical Composition and Biological Activity of Abies alba and A. Koreana Seed and Cone Essential Oils and Characterization of Their Seed Hydrolates. Chem. Biodivers. 2015, 12, 407–418. [Google Scholar] [CrossRef]
- Pokorska, O.; Dewulf, J.; Amelynck, C.; Schoon, N.; Šimpraga, M.; Steppe, K.; Van Langenhove, H. Isoprene and terpenoid emissions from Abies alba: Identification and emission rates under ambient conditions. Atmos. Environ. 2012, 59, 501–508. [Google Scholar] [CrossRef]
- Wajs, A.; Urbańska, J.; Zaleśkiewicz, E.; Bonikowski, R. Composition of essential oil from seeds and cones of Abies alba. Nat. Prod. Commun. 2010, 5, 1291–1294. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Flexas, J.; Galmés, J.; Niinemets, Ü.; Gil-Pelegrín, E. Light acclimation of photosynthesis in two closely related firs (Abies pinsapo Boiss. and Abies alba Mill.): The role of leaf anatomy and mesophyll conductance to CO2. Tree Physiol. 2016, 36, 300–310. [Google Scholar] [CrossRef]
- Sanchez-Morales, J.; Pardo-Igúzquiza, E.; Rodríguez-Tovar, F.J.; Dowd, P.A. A new method for reconstructing past-climate trends using tree-ring data and kernel smoothing. Dendrochronologia 2019, 55, 1–15. [Google Scholar] [CrossRef]
- De Vita, P.; Serrano, M.S.; Luchi, N.; Capretti, P.; Trapero, A.; Sánchez, M.E. Susceptibility of Abies pinsapo and its tree cohort species to Heterobasidion abietinum. For. Pathol. 2010, 40, 129–132. [Google Scholar] [CrossRef]
- Linares, J.C.; Carreira, J.A. Temperate-like stand dynamics in relict Mediterranean-fir (Abies pinsapo, Boiss.) forests from southern Spain. Ann. For. Sci. 2009, 66, 610. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Interacting effects of changes in climate and forest cover on mortality and growth of the southernmost European fir forests. Glob. Ecol. Biogeogr. 2009, 18, 485–497. [Google Scholar] [CrossRef]
- Sánchez-Robles, J.M.; Balao, F.; García-Castaño, J.L.; Terrab, A.; Navarro-Sampedro, L.; Talavera, S. Nuclear microsatellite primers for the endangered relict fir, abies pinsapo (Pinaceae) and cross-amplification in related Mediterranean species. Int. J. Mol. Sci. 2012, 13, 14243–14250. [Google Scholar] [CrossRef] [PubMed]
- Frascella, A.; Della Rocca, G.; Barberini, S.; Emiliani, G.; Secci, S.; Lambardi, M.; Benelli, C.; Tarraf, W.; Izgu, T.; Schicchi, R.; et al. Innovative In Situ and Ex Situ Conservation Strategies of the Madonie Fir Abies nebrodensis. Sustainability 2022, 14, 12643. [Google Scholar] [CrossRef]
- Pasta, S.; Sala, G.; La Mantia, T.; Bondì, C.; Tinner, W. The past distribution of Abies nebrodensis (Lojac.) Mattei: Results of a multidisciplinary study. Veg. Hist. Archaeobot. 2020, 29, 357–371. [Google Scholar] [CrossRef]
- Tarraf, W.; Izgu, T.; Jouini, N. Strategies for the conservation by biotechnological approaches of Abies nebrodensis, a relict conifer of Sicily. Acta Hortic. 2023, 1359, 215–221. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Gijzen, M.; Croteau, R. Mechanisms of conifers. Plant Physiol. 1991, 96, 44–49. [Google Scholar] [CrossRef]
- Lundborg, L.; Sampedro, L.; Borg-Karlson, A.K.; Zas, R. Effects of methyl jasmonate on the concentration of volatile terpenes in tissues of Maritime pine and Monterey pine and its relation to pine weevil feeding. Trees 2019, 33, 53–62. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.S. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef] [PubMed]
- Šimpraga, M.; Ghimire, R.P.; Van Der Straeten, D.; Blande, J.D.; Kasurinen, A.; Sorvari, J.; Holopainen, T.; Adriaenssens, S.; Holopainen, J.K.; Kivimäenpää, M. Unravelling the functions of biogenic volatiles in boreal and temperate forest ecosystems. Eur. J. For. Res. 2019, 138, 763–787. [Google Scholar] [CrossRef]
- Zulak, K.G.; Bohlmann, J. Terpenoid biosynthesis and specialized vascular cells of conifer defense. J. Integr. Plant Biol. 2010, 52, 86–97. [Google Scholar] [CrossRef]
- Kopaczyk, J.M.; Warguła, J.; Jelonek, T. The variability of terpenes in conifers under developmental and environmental stimuli. Environ. Exp. Bot. 2020, 180, 104197. [Google Scholar] [CrossRef]
- Zorić, M.; Kostić, S.; Kladar, N.; Božin, B.; Vasić, V.; Kebert, M.; Orlović, S. Phytochemical screening of volatile organic compounds in three common coniferous tree species in terms of forest ecosystem services. Forests 2021, 12, 928. [Google Scholar] [CrossRef]
- Raber, A.G.; Peachey-Stoner, R.J.; Cessna, S.G.; Siderhurst, M.S. Headspace GC-MS analysis of differences in intra- and interspecific Terpene profiles of Picea pungens Engelm. and P. abies (L.) Karst. Phytochemistry 2021, 181, 112541. [Google Scholar] [CrossRef]
- Hanover, J.W. Applications of terpene analysis in forest genetics. New For. 1992, 6, 159–178. [Google Scholar] [CrossRef]
- Plomion, C.; Yani, A.; Marpeau, A. Genetic determinism of δ3-carene in maritime pine using RAPD markers. Genome 1996, 39, 1123–1127. [Google Scholar] [CrossRef]
- Casano, S.; Grassi, G.; Martini, V.; Michelozzi, M. Variations in Terpene Profiles of Different Strains of Cannabis sativa L. CRA-CIN Consiglio per la Ricerca e la Sperimentazione in Agricoltura Centro di Ricerca per le Colture Industriali Rovigo Italy. Acta Hortic. 2011, 925, 115–122. [Google Scholar] [CrossRef]
- Nikolić, B.; Ristić, M.; Tešević, V.; Marin, P.D.; Bojović, S. Terpene chemodiversity of relict conifers picea omorika, pinus heldreichii, and pinus peuce, endemic to balkan. Chem. Biodivers. 2011, 8, 2247–2260. [Google Scholar] [CrossRef] [PubMed]
- Mitić, Z.S.; Jovanović, S.; Zlatković, B.K.; Nikolić, B.M.; Stojanović, G.S.; Marin, P.D. Needle terpenes as chemotaxonomic markers in Pinus: Subsections Pinus and Pinaster. Chem. Biodivers. 2017, 14, e1600453. [Google Scholar] [CrossRef] [PubMed]
- Mukrimin, M.; Kovalchuk, A.; Ghimire, R.P.; Kivimäenpää, M.; Sun, H.; Holopainen, J.K.; Asiegbu, F.O. Evaluation of potential genetic and chemical markers for Scots pine tolerance against Heterobasidion annosum infection. Planta 2019, 250, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, J.S.; Zlatković, B.K.; Jovanović, S.; Stojanović, G.S.; Marin, P.D.; Mitić, Z.S. Needle volatiles as chemophenetic markers in differentiation of natural populations of Abies alba, A. x borisii-regis, and A. cephalonica. Phytochemistry 2021, 183, 112612. [Google Scholar] [CrossRef]
- Rajčević, N.; Nikolić, B.; Marin, P.D. Different responses to environmental factors in terpene composition of pinus heldreichii and P. peuce: Ecological and chemotaxonomic considerations. Arch. Biol. Sci. 2019, 71, 629–637. [Google Scholar] [CrossRef]
- Sadeghi, H.; Tahery, Y.; Moradi, S. Intra- and inter-specific variation of turpentine composition in Eldar pine (Pinus eldarica Medw.) and black pine (Pinus nigra Arnold). Biochem. Syst. Ecol. 2013, 48, 189–193. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Jovanović, S.; Zlatković, B.K.; Milanovici, S.J.; Nikolić, B.M.; Petrović, G.M.; Stojanović, G.S.; Marin, P.D. Variation of needle volatiles in native populations of Pinus mugo–evidence from multivariate statistical analysis. Plant Biosyst. 2021, 155, 700–710. [Google Scholar] [CrossRef]
- Axelsson, K.; Zendegi-Shiraz, A.; Swedjemark, G.; Borg-Karlson, A.K.; Zhao, T. Chemical defence responses of Norway spruce to two fungal pathogens. For. Pathol. 2020, 50, e12640. [Google Scholar] [CrossRef]
- Kshatriya, K.; Whitehill, J.G.A.; Madilao, L.; Henderson, H.; Kermode, A.; Kolotelo, D.; Bohlmann, J. Histology of resin vesicles and oleoresin terpene composition of conifer seeds. Can. J. For. Res. 2018, 48, 1073–1084. [Google Scholar] [CrossRef]
- Calvo-Yuste, J.; Ruiz-Rodríguez, Á.L.; Hermosilla, B.; Agut, A.; Martínez-Ortega, M.M.; Tejero, P. Classification Importance of Seed Morphology and Insights on Large-Scale Climate-Driven Strophiole Size Changes in the Iberian Endemic Chasmophytic Genus Petrocoptis (Caryophyllaceae). Plants 2024, 13, 3208. [Google Scholar] [CrossRef]
- Singh, O.; Bordoloi, S.; Mahanta, N. Variability in cone, seed and seedling characteristics of Pinus kesiya Royle ex. Gordon. J. For. Res. 2015, 26, 331–337. [Google Scholar] [CrossRef]
- Hodžić, M.M.; Hajrudinović-Bogunić, A.; Bogunić, F.; Marku, V.; Ballian, D. Geographic variation of Pinus heldreichii Christ from the Western Balkans based on cone and seed morphology. Dendrobiology 2020, 84, 81–93. [Google Scholar] [CrossRef]
- Mustafa, E.; Tigabu, M.; Aldahadha, A.; Li, M. Variations in cone and seed phenotypic traits among and within populations of Aleppo pine in Jordan. New For. 2024, 55, 289–304. [Google Scholar] [CrossRef]
- Ghimire, B.; Yeom, D.; Jeong, M.J. Seed Atlas of Korea I. Conifers. J. Asia-Pac. Biodivers. 2019, 12, 459–466. [Google Scholar] [CrossRef]
- Shen, X.; Cho, M.J. Factors affecting seed germination and establishment of an efficient germination method in sugar pine (Pinus lambertiana Dougl.). HortScience 2021, 56, 299–304. [Google Scholar] [CrossRef]
- Skrzyszewska, K.; Chłanda, J. A study on the variation of morphological characteristics of silver fir (Abies alba Mill.) seeds and their internal structure determined by X-ray radiography in the beskid sâdecki and beskid niski mountain ranges of the Carpathians (southern Poland). J. For. Sci. 2009, 55, 403–414. [Google Scholar] [CrossRef]
- Harper, J.L.; Lovell, P.H.; Moore, K.G. The Shapes and Sizes of Seeds. Annu. Rev. Ecol. Syst. 1970, 1, 327–356. [Google Scholar] [CrossRef]
- Trujillo-Ríos, M.; Gazol, A.; Seco, J.I.; Linares, J.C. Phenotypic variation in cone scales and seeds as drivers of seedling germination dynamics of co-occurring cedar and fir species. Forests 2025, 16, 252. [Google Scholar] [CrossRef]
- Benelli, C.; Tarraf, W.; İzgü, T.; Anichini, M.; Faraloni, C.; Salvatici, M.C.; Antonietta, M.; Danti, R.; Lambardi, M. Long-term conservation for the safeguard of Abies nebrodensis: An endemic and endangered species of Sicily. Plants 2024, 13, 1682. [Google Scholar] [CrossRef]
- Arista, M.; Talavera, S. Density effect on the fruit-set, seed crop viability and seedling vigour of Abies pinsapo. Ann. Bot. 1996, 77, 187–192. [Google Scholar] [CrossRef]
- Hunt, R.S.; von Rudloff, E. Chemosystematic studies in the Genus Abies. IV. Introgression in Abies lasiocarpa and Abies bifolia. Taxon 1979, 28, 297–305. [Google Scholar] [CrossRef]
- Yang, X.W.; Li, S.M.; Shen, Y.H.; Zhang, W.D. Phytochemical and biological studies of Abies species. Chem. Biodivers. 2008, 5, 56–81. [Google Scholar] [CrossRef]
- Schicchi, R.; Geraci, A.; Rosselli, S.; Maggio, A.; Bruno, M. Chemodiversity of the essential oil from leaves of Abies nebrodensis (Lojac.) Mattei. Chem. Biodivers. 2017, 14, e1600254. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.M.; Martinoli, A.; Pitton, M.; Di Fabio, D.; Caruso, E.; Banfi, S.; Tosi, G.; Wauters, L.A.; Martinoli, A. Food choice of Eurasian red squirrels and concentrations of anti-predatory secondary compounds. Mamm. Biol. 2012, 77, 332–338. [Google Scholar] [CrossRef]
- Barrero, A.F.; Sanchez, J.F.; Alvarez-Manzaneda R., E.J.; Dorado, M.M. Sesquiterpenoids related to juvabione in Abies pinsapo. Phytochemistry 1989, 28, 2617–2619. [Google Scholar] [CrossRef]
- Barrero, A.F.; Sanchez, J.F.; Alvarez-Manzaneda, E.J.; Dorado, M.M.; Haidour, A. Terpenoids and sterols from the wood of Abies pinsapo. Phytochemistry 1993, 32, 1261–1265. [Google Scholar] [CrossRef]
- Nikolić, B.M.; Ballian, D.; Mitić, Z.S. Autochthonous conifers of family Pinaceae in Europe: Broad review of morpho-anatomical and phytochemical properties of needles and genetic investigations. Forests 2024, 15, 989. [Google Scholar] [CrossRef]
- Yang, S.A.; Jeon, S.K.; Lee, E.J.; Im, N.K.; Jhee, K.H.; Lee, S.P.; Lee, I.S. Radical scavenging activity of the essential oil of silver fir (Abies alba). J. Clin. Biochem. Nutr. 2009, 44, 253–259. [Google Scholar] [CrossRef]
- Bagci, E.; Babaç, M.T. A morphometric and chemosystematic study on the Abies Miller (Fir) species in Turkey. Acta Bot. Gall. 2003, 150, 355–367. [Google Scholar] [CrossRef]
- Warren, R.L.; Keeling, C.I.; Yuen, M.M.S.; Raymond, A.; Taylor, G.A.; Vandervalk, B.P.; Mohamadi, H.; Paulino, D.; Chiu, R.; Jackman, S.D.; et al. Improved white spruce (Picea glauca) genome assemblies and annotation of large gene families of conifer terpenoid and phenolic defense metabolism. Plant J. 2015, 83, 189–212. [Google Scholar] [CrossRef]
- Celedon, J.M.; Yuen, M.M.S.; Chiang, A.; Henderson, H.; Reid, K.E.; Bohlmann, J. Cell-type- and tissue-specific transcriptomes of the white spruce (Picea glauca) bark unmask fine-scale spatial patterns of constitutive and induced conifer defense. Plant J. 2017, 92, 710–726. [Google Scholar] [CrossRef]
- Fady, B.; Arbez, M.; Marpeau, A. Geographic variability of terpene composition in Abies cephalonica Loudon and Abies species around the Aegean: Hypotheses for their possible phylogeny from the Miocene. Trees 1992, 6, 162–171. [Google Scholar] [CrossRef]
- Fojtová, J.; Lojková, L.; Kubáň, V. Supercritical fluid extraction as a tool for isolation of monoterpenes from coniferous needles and walnut-tree leaves. Cent. Eur. J. Chem. 2010, 8, 409–418. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Stojanović-Radić, Z.Z.; Jovanović, S.; Cvetković, V.J.; Nikolić, J.S.; Ickovski, J.D.; Mitrović, T.L.; Nikolić, B.M.; Zlatković, B.K.; Stojanović, G.S. Essential Oils of three Balkan Abies Species: Chemical profiles, antimicrobial activity and toxicity toward Artemia salina and Drosophila melanogaster. Chem. Biodivers. 2022, 19, e202200235. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Maffei, M. Biodiversity and chemotaxonomic significance of specialized metabolites. In Plant Specialized Metabolism; CRC Press: Boca Raton, FL, USA, 2016; pp. 35–76. [Google Scholar]
- Niederbacher, B.; Winkler, J.B.; Schnitzler, J.P. Volatile organic compounds as non-invasive markers for plant phenotyping. J. Exp. Bot. 2015, 66, 5403–5416. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Volker, W. Sesqui-, di-, and triterpenoids as chemosystematic markers in extant conifers—A review. Bot. Rev. 2001, 67, 141–238. [Google Scholar] [CrossRef]
- Naydenov, K.D.; Tremblay, F.M.; Alexandrov, A.; Fenton, N.J. Structure of Pinus sylvestris L. populations in Bulgaria revealed by chloroplast microsatellites and terpenes analysis: Provenance tests. Biochem. Syst. Ecol. 2005, 33, 1226–1245. [Google Scholar] [CrossRef]
- von Rudloff, E. Volatile leaf oil analysis in chemosystematic studies of North American conifers. Biochem. Syst. Ecol. 1975, 2, 131–167. [Google Scholar] [CrossRef]
- Duquesnoy, E.; Castola, V.; Casanova, J. Composition and chemical variability of the twig oil of Abies alba Miller from Corsica Emilie. Flavour Fragr. J. 2008, 22, 293–299. [Google Scholar] [CrossRef]
- Mitsopoulos, D.J.; Panetsos, C.P. Origin of variation in Fir forests of Greece. Silvae Genet. 1987, 36, 1–7. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Salmerón-Gómez, R.; García-García, C.; García-Pérez, J. A Guide to Using the R package “multiColl” for detecting multicollinearity. Comput. Econ. 2021, 57, 529–536. [Google Scholar] [CrossRef]
- Salmeron, A.R.; Garcia, C.; Garcia, J.; Salmeron, M.R. Collinearity Detection in a Multiple Linear Regression Model. Available online: http://colldetreat.r-forge.r-project.org/ (accessed on 24 February 2025).
- Hastie, T.; Tibshirani, R.; Friedman, J.F. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer Science & Business Media: New York, NY, USA, 2009. [Google Scholar]
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. In Data Analysis; Wickham, H., Ed.; Springer: New York, NY, USA, 2016; pp. 189–201. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Brunson, J.C. Ordr: A Tdyverse Extension for Ordinations and Biplots. Available online: https://cran.r-project.org/web/packages/ordr/ordr.pdf (accessed on 24 February 2025).
- Karolis Koncevicius Package ‘matrixTests’. Available online: https://cran.r-project.org/web/packages/matrixTests/matrixTests.pdf (accessed on 24 February 2025).
- Pohler, T. Calculate Pairwise Multiple Comparisons of Mean Rank Sums. Available online: https://cran.r-project.org/web/packages/PMCMRplus/PMCMRplus.pdf (accessed on 24 February 2025).
- Labouriau, R. postHoc: Tools for Post-Hoc Analysis. R Package Version 0.1.3. Available online: https://tildeweb.au.dk/au33031/astatlab/software/posthoc/ (accessed on 24 February 2025).
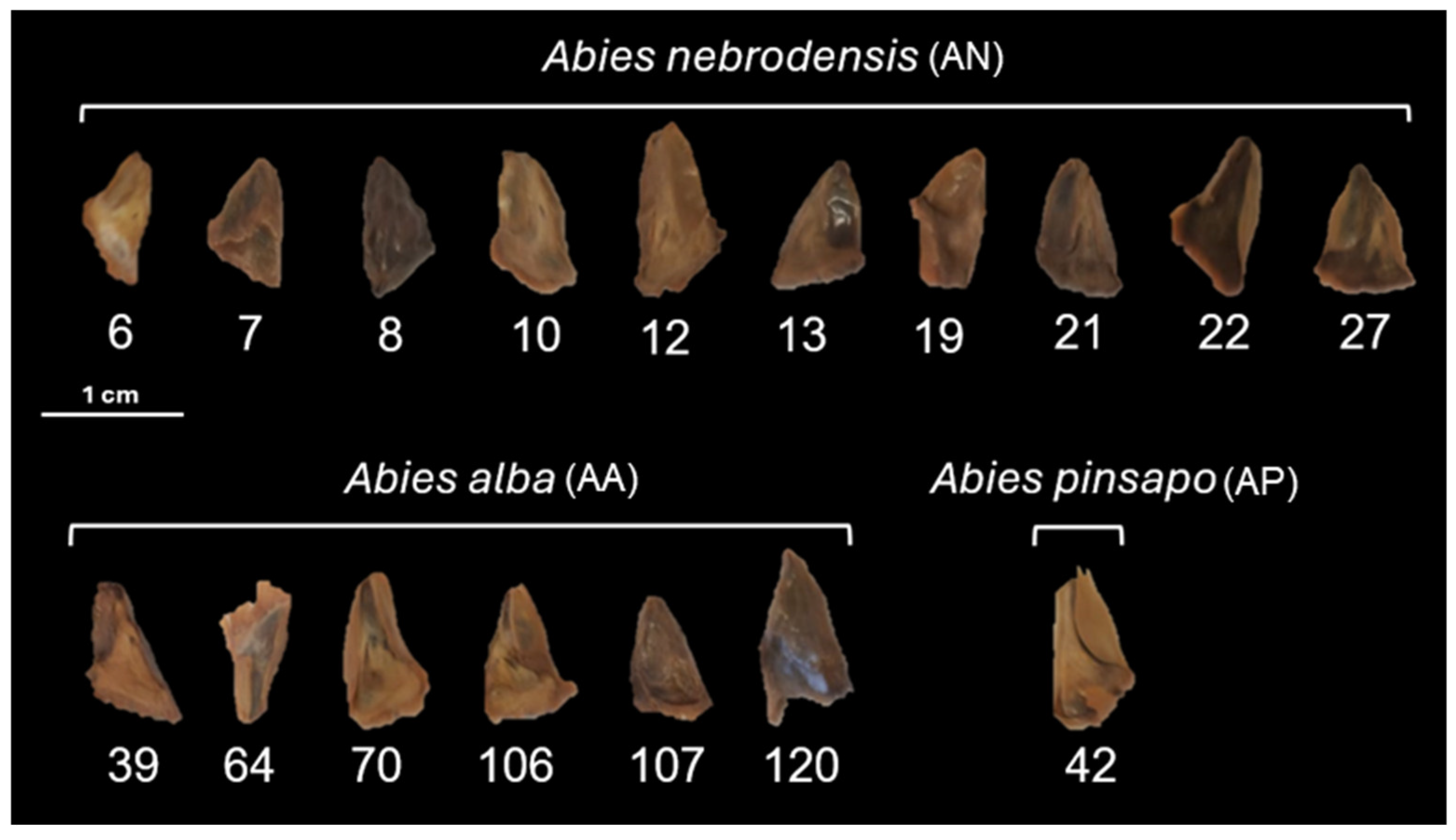

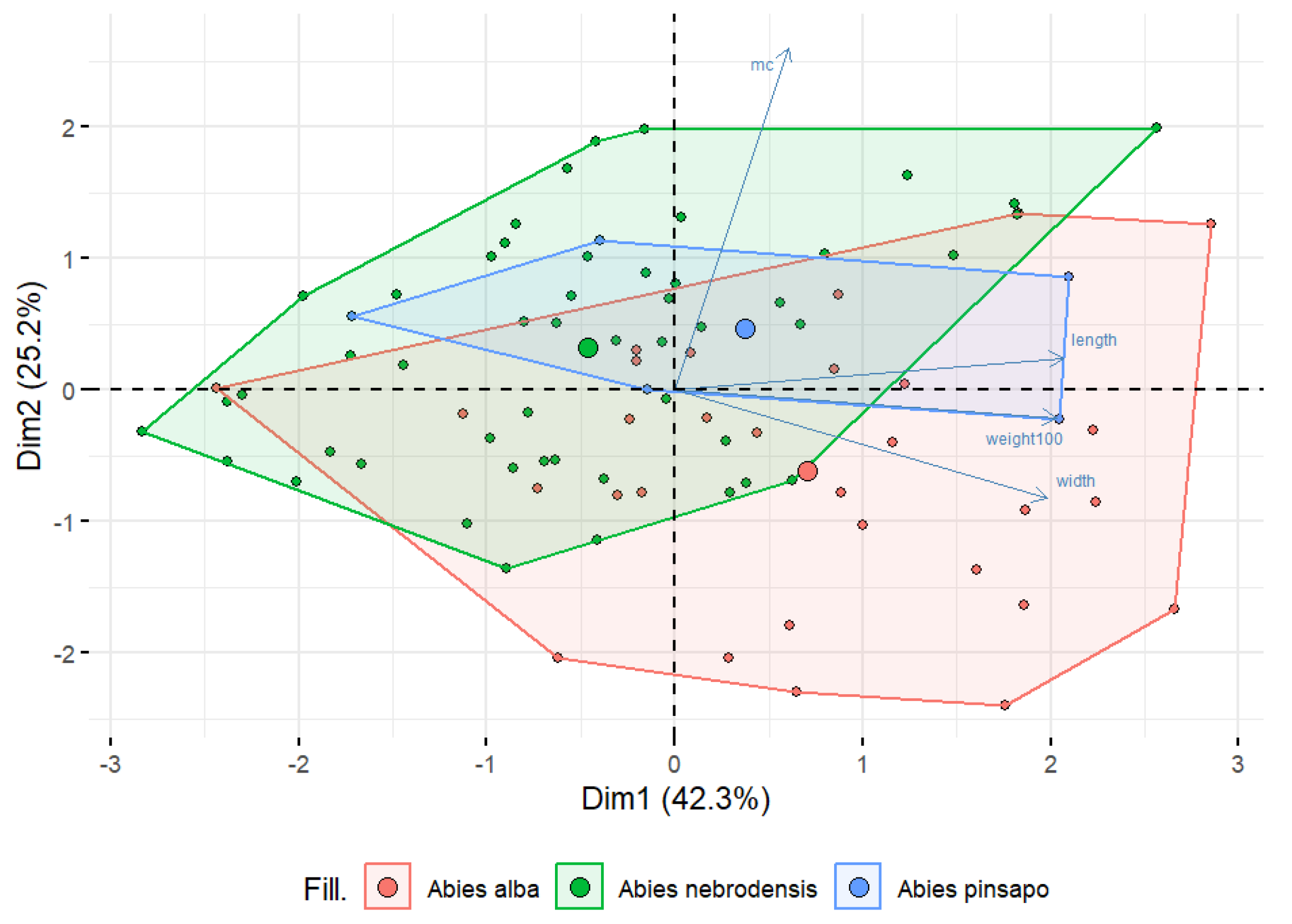
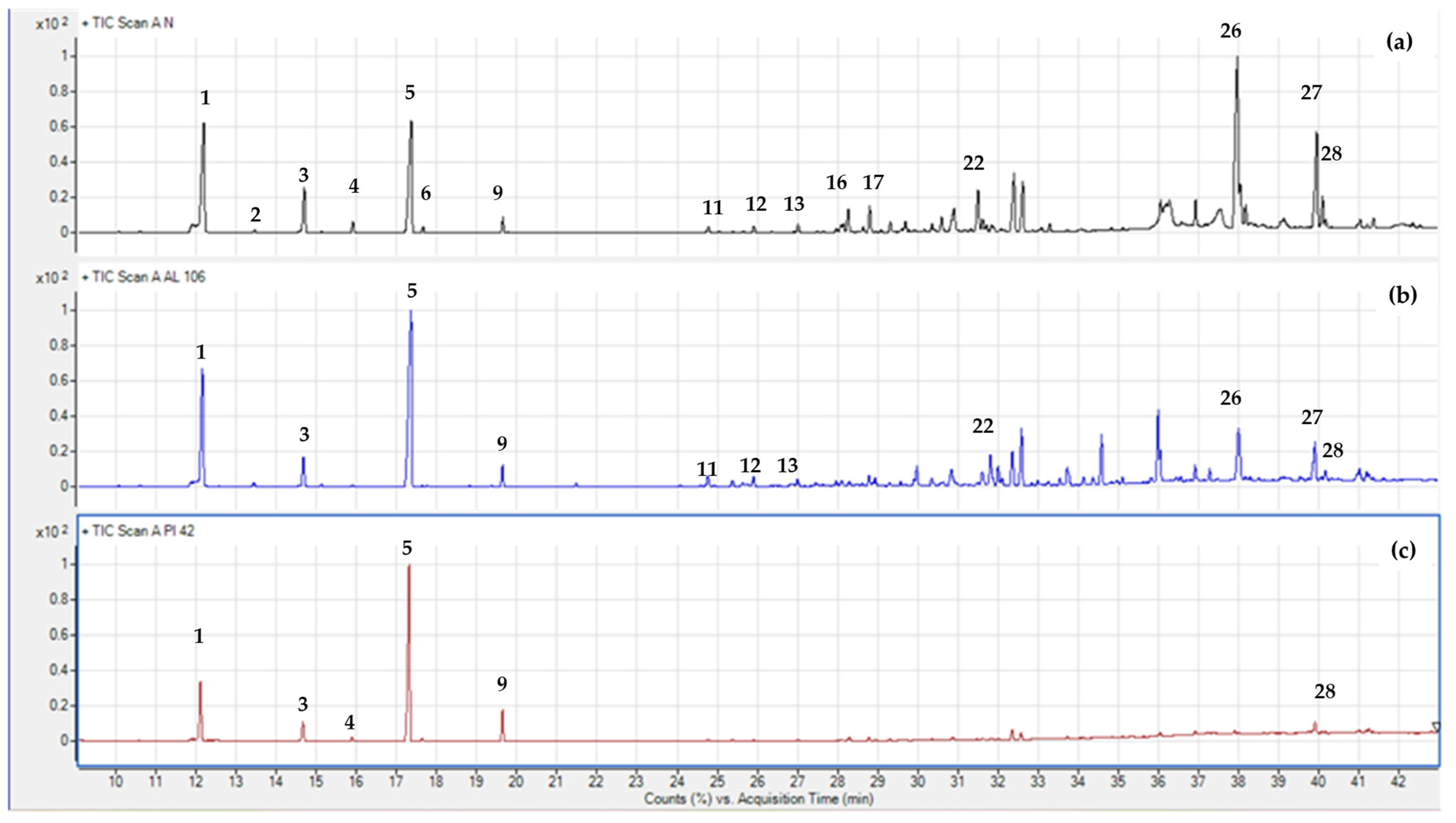



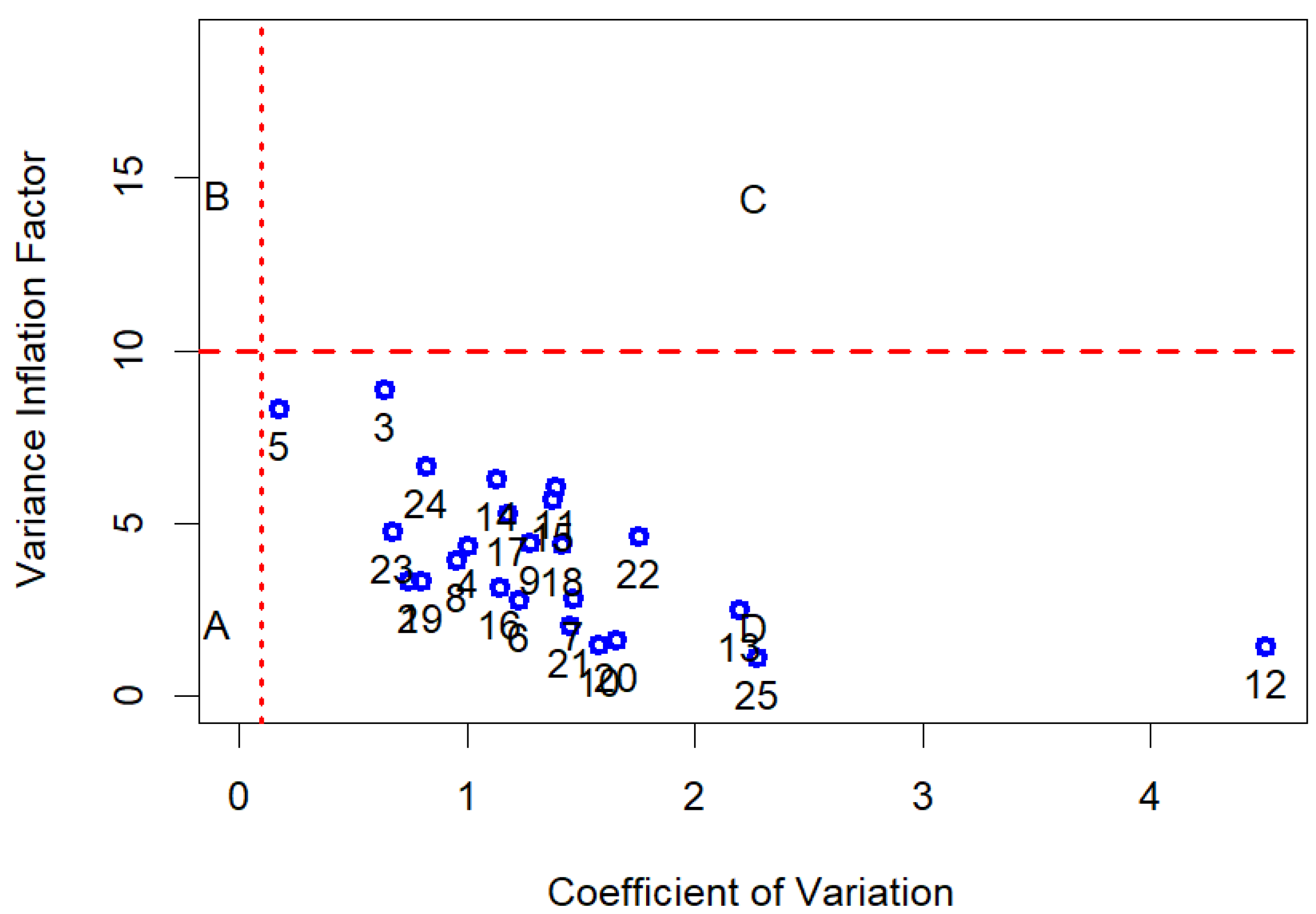
| Species | Population ID | MC (%) | Weight of 100 Seeds (g) | Length (mm) | Width (mm) |
|---|---|---|---|---|---|
| A. alba | AA39 | 7.06 ± 2.23 e | 5.27 ± 1.25 b | 11.5 ± 1.8 ab | 0.7 ± 0.18 ab |
| A. alba | AA64 | 9.34 ± 3.59 de | 3.73 ± 0.20 bdc | 10.3 ± 1.3 abc | 0.55 ± 0.13 ab |
| A. alba | AA70 | 6.66 ± 3.74 e | 4.46 ± 0.78 bcd | 10.7 ± 0.7 ab | 0.63 ± 0.07 ab |
| A. alba | AA106 | 12.95 ± 1.92 abcde | 5.13 ± 0.5 b | 11.5 ± 0.9 ab | 0.62 ± 0.09 ab |
| A. alba | AA107 | 13.47 ± 6.42 abcde | 6.42 ± 1.41 a | 11.4 ± 1.4 ab | 0.67 ± 0.14 ab |
| A. alba | AA120 | 11.04 ± 1.69 bcde | 4.18 ± 0.15 bcd | 10.8 ± 0.7 ab | 0.72 ± 0.07 a |
| A. pinsapo | AP42 | 14.57 ± 2.05 abcd | 4.79 ± 1.56 bc | 10.8 ± 0.9 ab | 0.59 ± 0.09 ab |
| A. nebrodensis | AN6 | 13.11 ± 3.71 abcde | 3.06 ± 0.25 d | 11.1 ± 0.6 ab | 0.57 ± 0.06 ab |
| A. nebrodensis | AN7 | 10.08 ± 3.03 cde | 3.97 ± 0.39 bcd | 9.9 ± 0.5 abc | 0.54 ± 0.05 ab |
| A. nebrodensis | AN8 | 12.34 ± 2.07 abcde | 3.11 ± 0.51 d | 8.7 ± 0.3 c | 0.49 ± 0.03 b |
| A. nebrodensis | AN10 | 12.43 ± 3.13 abcde | 3.79 ± 0.22 bcd | 10.3 ± 0.7 abc | 0.58 ± 0.07 ab |
| A. nebrodensis | AN12 | 18.99 ± 3.35 a | 4.93 ± 1.45 b | 12 ± 1.2 a | 0.62 ± 0.12 ab |
| A. nebrodensis | AN13 | 12.69 ± 2.62 abcde | 3.97 ± 0.32 bcd | 9.8 ± 0.7 bc | 0.64 ± 0.07 ab |
| A. nebrodensis | AN19 | 7.20 ± 1.61 e | 3.23 ± 0.53 cd | 9.6 ± 0.5 bc | 0.52 ± 0.05 ab |
| A. nebrodensis | AN21 | 17.36 ± 3.9 abc | 3.89 ± 0.29 bcd | 10.4 ± 0.2 abc | 0.64 ± 0.02 ab |
| A. nebrodensis | AN22 | 16.97 ± 4.35 abc | 2.93 ± 0.07 d | 11.5 ± 1 ab | 0.65 ± 0.1 ab |
| A. nebrodensis | AN27 | 14.98 ± 3.7 abcd | 3.81 ± 0.26 bcd | 9.7 ± 0.7 bc | 0.62 ± 0.07 ab |
| p-value | 0.0000 *** | 0.0000 *** | 0.0000 *** | 0.0109 * | |
| C.V% | 26.85% | 18.51% | 9.12% | 15.10% | |
| L.S.D 0.05 | 4.21 | 0.97 | 0.12 | 0.12 |
| No. | Terpene Compound | A. nebrodensis | A. pinsapo | A. alba | H Statistic | p-Value | Level of Significance |
|---|---|---|---|---|---|---|---|
| n = 33 | n = 20 | n = 117 | n = 170 | ||||
| 1 | α-Pinene | 11.09 ± 0.92 | 18.85 ± 0.96 | 15.43 ± 0.76 | 3.45 | 0.18 | ns |
| 2 | Camphene | 0.24 ± 0.03 | 0.13 ± 0.01 | 0.39 ± 0.02 | 33.05 | 0.00 | *** |
| 3 | β-Pinene | 3.05 ± 0.31 | 5.89 ± 0.32 | 3.21 ± 0.19 | 24.45 | 0.00 | *** |
| 4 | Myrcene | 1.77 ± 0.13 | 0.91 ± 0.09 | 0.66 ± 0.08 | 64.66 | 0.00 | *** |
| 5 | Limonene | 49.57 ± 4.14 | 66.03 ± 1.99 | 62.42 ± 1.3 | 0.80 | 0.67 | ns |
| 6 | β-Phellandrene | 0.61 ± 0.08 | 0.41 ± 0.04 | 0.22 ± 0.03 | 51.26 | 0.00 | *** |
| 7 | γ-Terpinene | tr | tr | tr | |||
| 8 | p-Cymene | tr | 0.09 ± 0.01 | 0.18 ± 0.01 | 56.58 | 0.00 | *** |
| 9 | Terpinolene | 0.05 ± 0.01 | tr | tr | |||
| 10 | Limonene oxide cis | 0.14 ± 0.03 | 0.22 ± 0.04 | 0.44 ± 0.06 | 9.93 | 0.01 | ** |
| 11 | Pinocarvone | 0.12 ± 0.07 | 0.27 ± 0.09 | 0.62 ± 0.06 | 29.65 | 0.00 | *** |
| 12 | Bornyl acetate | 0 ± 0 | 0 ± 0 | 0.05 ± 0.02 | 4.78 | 0.09 | ns |
| 13 | Terpinen-4-ol | tr | 0 ± 0 | tr | |||
| 14 | Trans-verbenol | 1.65 ± 0.32 | 0.88 ± 0.16 | 1.62 ± 0.13 | 10.04 | 0.01 | ** |
| 15 | Verbenone | 0.43 ± 0.25 | tr | 2.16 ± 0.2 | 54.88 | 0.00 | *** |
| 16 | β-Elemene | 0.8 ± 0.15 | 0 ± 0 | 0.07 ± 0.02 | - | - | |
| 17 | Sesquiterpene.1 | 0.27 ± 0.05 | 0.05 ± 0.02 | 0.16 ± 0.01 | 12.62 | 0.00 | ** |
| 18 | Sesquiterpene.2 | 2.5 ± 0.26 | 1.11 ± 0.15 | 0.78 ± 0.11 | 41.27 | 0.00 | *** |
| 19 | α-Humulene | 1.07 ± 0.17 | 0.23 ± 0.05 | 0.32 ± 0.04 | 11.62 | 0.00 | ** |
| 20 | Germacrene D | 2.52 ± 0.44 | 0.18 ± 0.06 | 0.37 ± 0.08 | - | - | |
| 21 | Sesquiterpene.3 | 0.72 ± 0.14 | 0.79 ± 0.19 | 2.33 ± 0.13 | 42.97 | 0.00 | *** |
| 22 | β-Caryophyllene oxide | 0.3 ± 0.2 | 0 ± 0 | 1.02 ± 0.12 | 31.13 | 0.00 | *** |
| 23 | Germacrene D-4-ol | 9.27 ± 1.68 | 0.16 ± 0.09 | 0.69 ± 0.24 | - | - | |
| 24 | α-epi-cadinol | 1.2 ± 0.34 | 0.17 ± 0.08 | 0.97 ± 0.11 | 10.94 | 0.00 | ** |
| 25 | Selina-6-en-4-ol | 9.83 ± 2.59 | 2.55 ± 0.67 | 3.16 ± 0.33 | 13.04 | 0.00 | *** |
| 26 | α-Cubebene | 0.65 ± 0.09 | 0.42 ± 0.07 | 1.07 ± 0.06 | 28.00 | 0.00 | *** |
| 27 | α-Copaene | 0.48 ± 0.07 | 0.22 ± 0.05 | 0.79 ± 0.05 | 26.53 | 0.00 | *** |
| 28 | β-Copaene | 1.58 ± 0.74 | 0.4 ± 0.07 | 0.75 ± 0.07 | 18.71 | 0.00 | *** |
| Monoterpenes hydrocarbons | 66.52 | 92.53 | 82.95 | ||||
| Oxygenated monoterpenes | 2.20 | 1.14 | 4.47 | ||||
| Sesquiterpene hydrocarbons | 20.59 | 2.88 | 5.84 | ||||
| Oxygenated sesquiterpenes | 10.61 | 3.40 | 6.65 | ||||
| Total identified [%] | 99.92 | 99.95 | 99.91 | ||||
| Compound/Species | A. nebrodensis vs. A. alba | A. alba vs. A. pinsapo | A. nebrodensis vs. A. pinsapo |
|---|---|---|---|
| α-Pinene | 0.0072228 ** | 0.009428 ** | 1.08 × 10−5 *** |
| Camphene | 0.0039926 ** | 3.212 × 10−8 *** | 2.41 × 10−2 * |
| β-Pinene | 1 | 4.142 × 10−8 *** | 1.92 × 10−6 *** |
| Myrcene | 1.59 × 10−8 *** | 0.020705 * | 3.83 × 10−2 * |
| Limonene | 0.036737 * | 0.921246 | 2.68 × 10−2 * |
| β-Phellandrene | 1.113 × 10−8 *** | 0.000128 *** | 1 |
| γ-Terpinene | 0.13746 | 1 | 0.4414 |
| p-Cymene | 6.92 × 10−14 *** | 0.010214 * | 1.50 × 10−2 * |
| Terpinolene | 0.72723 | 1 | 4.41 × 10−1 |
| Limonene oxide cis | 0.0004826 *** | 1 | 1.05 × 10−1 |
| Pinocarvone | 7.108 × 10−7 *** | 0.058621 | 3.28 × 10−1 |
| Bornyl acetate | 0.1989 | 0.40403 | 1.00 |
| Terpinen-4-ol | 0.5432794 | 0.0108495 * | 1.91 × 10−3 ** |
| Trans-verbenol | 1 | 0.052235 | 2.09 × 10−1 |
| Verbenone | 3.691 × 10−8 *** | 4.016 × 10−8 *** | 1 |
| β-Elemene | 2.97 × 10−7 *** | 0.38228 | 1.65 × 10−6 *** |
| Sesquiterpene.1 | 0.8285204 | 0.006418 ** | 2.18 × 10−3 ** |
| Sesquiterpene.2 | 1.99 × 10−6 *** | 0.023123 * | 1.30 × 10−1 |
| α-Humulene | 0.0040646 ** | 1 | 4.67 × 10−2 * |
| Germacrene D | 3.811 × 10−5 *** | 1 | 1.81 × 10−2 * |
| Sesquiterpene.3 | 2.31 × 10−9 *** | 6.179 × 10−6 *** | 1 |
| β-Caryophyllene oxide | 8.853 × 10−5 *** | 3.206 × 10−5 *** | 1 |
| Germacrene D-4-ol | 3.516 × 10−8 *** | 1 | 2.00 × 10−4 *** |
| α-epi-cadinol | 1 | 0.0027414 ** | 4.72 × 10−2 * |
| Selina-6-en-4-ol | 0.0040008 ** | 1 | 9.92 × 10−3 ** |
| α-Cubebene | 0.0013365 ** | 8.4424 × 10−7 *** | 1.56 × 10−1 |
| α-Copaene | 0.011587 * | 6.545 × 10−7 *** | 4.71 × 10−2 * |
| β-Copaene | 0.2532145 | 0.0040231 ** | 2.46 × 10−4 *** |
| Variable | LD1 | LD2 |
|---|---|---|
| α-Pinene | −0.004277661 | −0.085274092 |
| Camphene | 0.143528773 | −2.932279547 |
| β-Pinene | −0.11926859 | 0.793177699 |
| Myrcene | 1.293628722 | −0.247687015 |
| Limonene | −0.01197199 | 0.024988731 |
| β-Phellandrene | 1.473550949 | −0.437051156 |
| γ-Terpinene | −1.69750848 | 0.410111115 |
| p-Cymene | 2.112028567 * | 1.848104132 * |
| Terpinolene | −20.41296551 | −4.298545602 |
| Limonene-oxide-cis | −0.075998513 | −0.608828993 |
| Pinocarvone | −0.66069519 | 1.797811335 * |
| Bornyl acetate | 0.840008653 | 0.478153842 |
| Terpinen-4-ol | −0.29230847 | 0.134471116 |
| Trans-verbenol | 0.436510957 | −0.321339896 |
| Verbenone | −0.018931995 | −0.166428292 |
| Sesquiterpene.1 | 2.365264726 * | −1.590761873 |
| Sesquiterpene.2 | 0.293305148 | 0.895506246 |
| α-Humulene | 1.283763095 | −1.696687733 |
| Sesquiterpene.3 | −0.191231726 | −0.506511034 |
| β-Caryophyllene-oxide | 0.071466501 | −0.056933295 |
| α-epi-cadinol | 0.041705135 | −0.009005742 |
| Selina-6-en-4-ol | −0.040522488 | 0.04171892 |
| α-Cubebene | −0.624629032 | −0.323550455 |
| α-Copaene | 0.215010018 | −0.019346339 |
| β-Copaene | 0.076285341 | −0.025504718 |
| A. alba | A. nebrodensis | A. pinsapo | |
|---|---|---|---|
| A. alba | 23 (95.83%) | 1 (4.17%) | - |
| A. nebrodensis | - | 5 (100%) | - |
| A. pinsapo | - | - | 4 (100%) |
| Total samples = 33 Total correct classifications: 32 samples = 96.97% | |||
| Plant N° | Species | Population ID | Collection Site | Province | Altitude (m a.s.l.) | Latitude N | Longitude E | Region | Country |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A. alba | AA39 | Serra San Bruno-Monte Pecoraro | Vibo Valentia | 1100–1400 | 38°31′59″ | 16°19′59″ | Calabria | Italy |
| 2 | A. alba | AA107 | Serra S. Bruno S. Maria | Vibo Valentia | 800–1100 | 38°33′39″ | 16°18′58″ | Calabria | Italy |
| 3 | A. alba | AA70 | Vallombrosa | Florence | 800–1200 | 43°44′58″ | 11°33′21″ | Tuscany | Italy |
| 4 | A. alba | AA64 | Abetone | Pistoia | 1200–1500 | 44°08′02″ | 10°40′17″ | Tuscany | Italy |
| 5 | A. alba | AA106 | Serra San Bruno-Archiforo | Vibo Valentia | 900–1300 | 38°31′59″ | 16°19′59″ | Calabria | Italy |
| 6 | A. alba | AA120 | Gariglione | Catanzaro | 1400–1700 | 39°08′12″ | 16°38′34″ | Calabria | Italy |
| 7 | A. pinsapo | AP42 | Unknown | Spain | |||||
| 8 | A. nebrodensis | AN6 | Monte Scalone | Palermo | 1644 | 37°50′25″ | 14°01′22″ | Sicily | Italy |
| 9 | A. nebrodensis | AN7 | Pendici di Monte Scalone | Palermo | 1587.07 | 37°50′27″ | 14°01′30″ | Sicily | Italy |
| 10 | A. nebrodensis | AN8 | Pendici di Monte Scalone | Palermo | 1562.33 | 37°50′28″ | 14°01′29″ | Sicily | Italy |
| 11 | A. nebrodensis | AN10 | Monte Scalone | Palermo | 1509.05 | 37°50′34″ | 14°01′6″ | Sicily | Italy |
| 12 | A. nebrodensis | AN12 | Pendici di Monte Scalone | Palermo | 1587.12 | 37°50′26″ | 14°01′25″ | Sicily | Italy |
| 13 | A. nebrodensis | AN13 | Pendici di Monte Scalone | Palermo | 1561.5 | 37°50′29″ | 14°01′30″ | Sicily | Italy |
| 14 | A. nebrodensis | AN19 | Vallone Madonna degli Angeli e Monte Scalone | Palermo | 1468.74 | 37°50′35″ | 14°01′19″ | Sicily | Italy |
| 15 | A. nebrodensis | AN21 | Vallone Madonna degli Angeli | Palermo | 1402.3 | 37°50′50″ | 14°01′20″ | Sicily | Italy |
| 16 | A. nebrodensis | AN22 | Vallone Madonna degli Angeli | Palermo | 1390.99 | 37°50′43″ | 14°01′15″ | Sicily | Italy |
| 17 | A. nebrodensis | AN27 | Vallone Madonna degli Angeli | Palermo | 1579.08 | 37°50′33″ | 14°01′37″ | Sicily | Italy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarraf, W.; İzgü, T.; Benelli, C.; Cencetti, G.; Michelozzi, M.; Crisci, A. Seed Characteristics and Terpene Variability of Mediterranean Fir Species (Abies nebrodensis, A. pinsapo, and A. alba). Plants 2025, 14, 892. https://doi.org/10.3390/plants14060892
Tarraf W, İzgü T, Benelli C, Cencetti G, Michelozzi M, Crisci A. Seed Characteristics and Terpene Variability of Mediterranean Fir Species (Abies nebrodensis, A. pinsapo, and A. alba). Plants. 2025; 14(6):892. https://doi.org/10.3390/plants14060892
Chicago/Turabian StyleTarraf, Waed, Tolga İzgü, Carla Benelli, Gabriele Cencetti, Marco Michelozzi, and Alfonso Crisci. 2025. "Seed Characteristics and Terpene Variability of Mediterranean Fir Species (Abies nebrodensis, A. pinsapo, and A. alba)" Plants 14, no. 6: 892. https://doi.org/10.3390/plants14060892
APA StyleTarraf, W., İzgü, T., Benelli, C., Cencetti, G., Michelozzi, M., & Crisci, A. (2025). Seed Characteristics and Terpene Variability of Mediterranean Fir Species (Abies nebrodensis, A. pinsapo, and A. alba). Plants, 14(6), 892. https://doi.org/10.3390/plants14060892











