Effects of Salt Stress at the Booting Stage of Grain Development on Physiological Responses, Starch Properties, and Starch-Related Gene Expression in Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Results
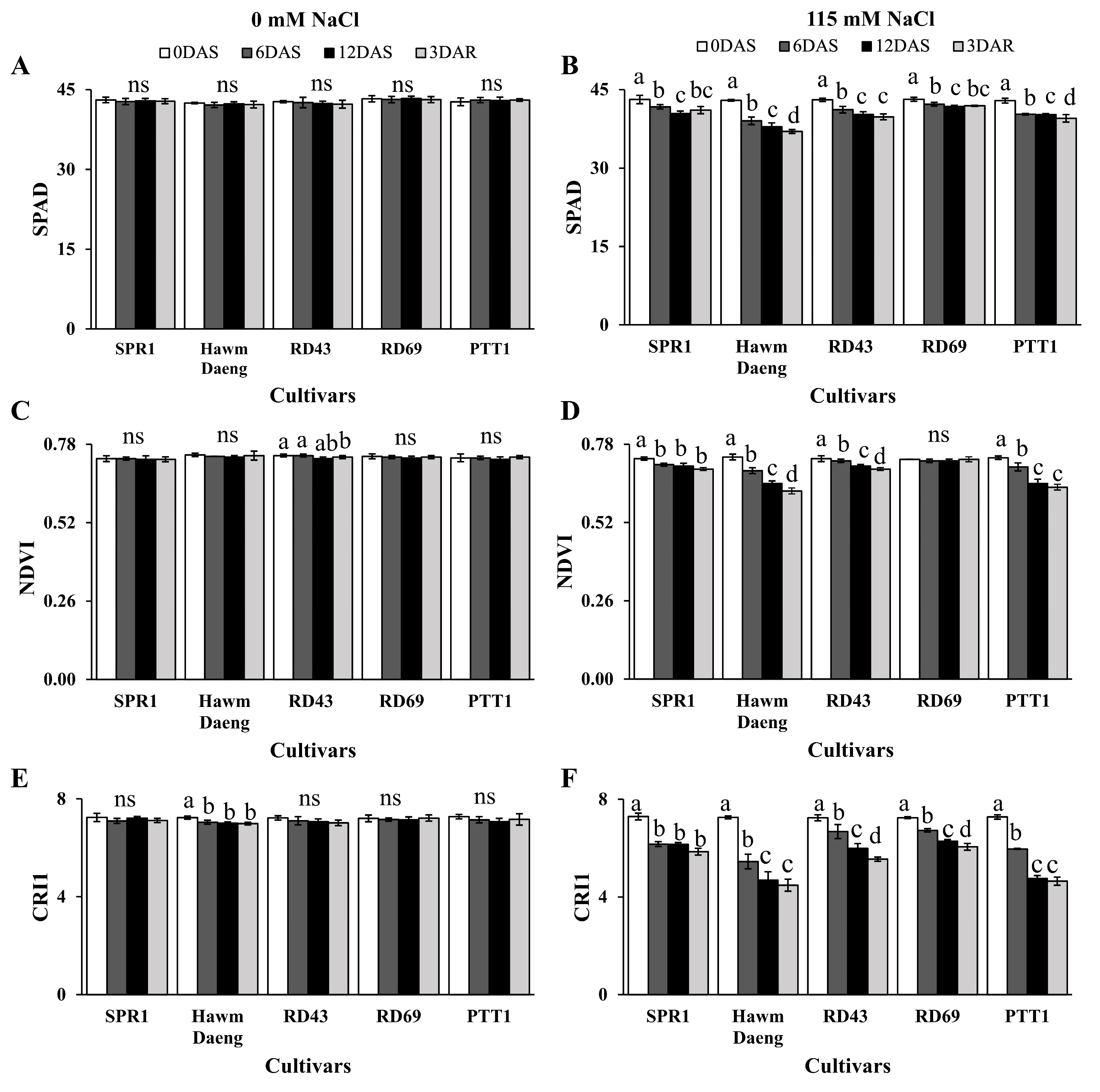
2.1. Different Physiological Responses to Salt Stress Were Detected in Five Rice (Oryza sativa L.) Cultivars at the Booting Stage of Development
2.2. Effect of Salt Stress on Yield Components of Rice
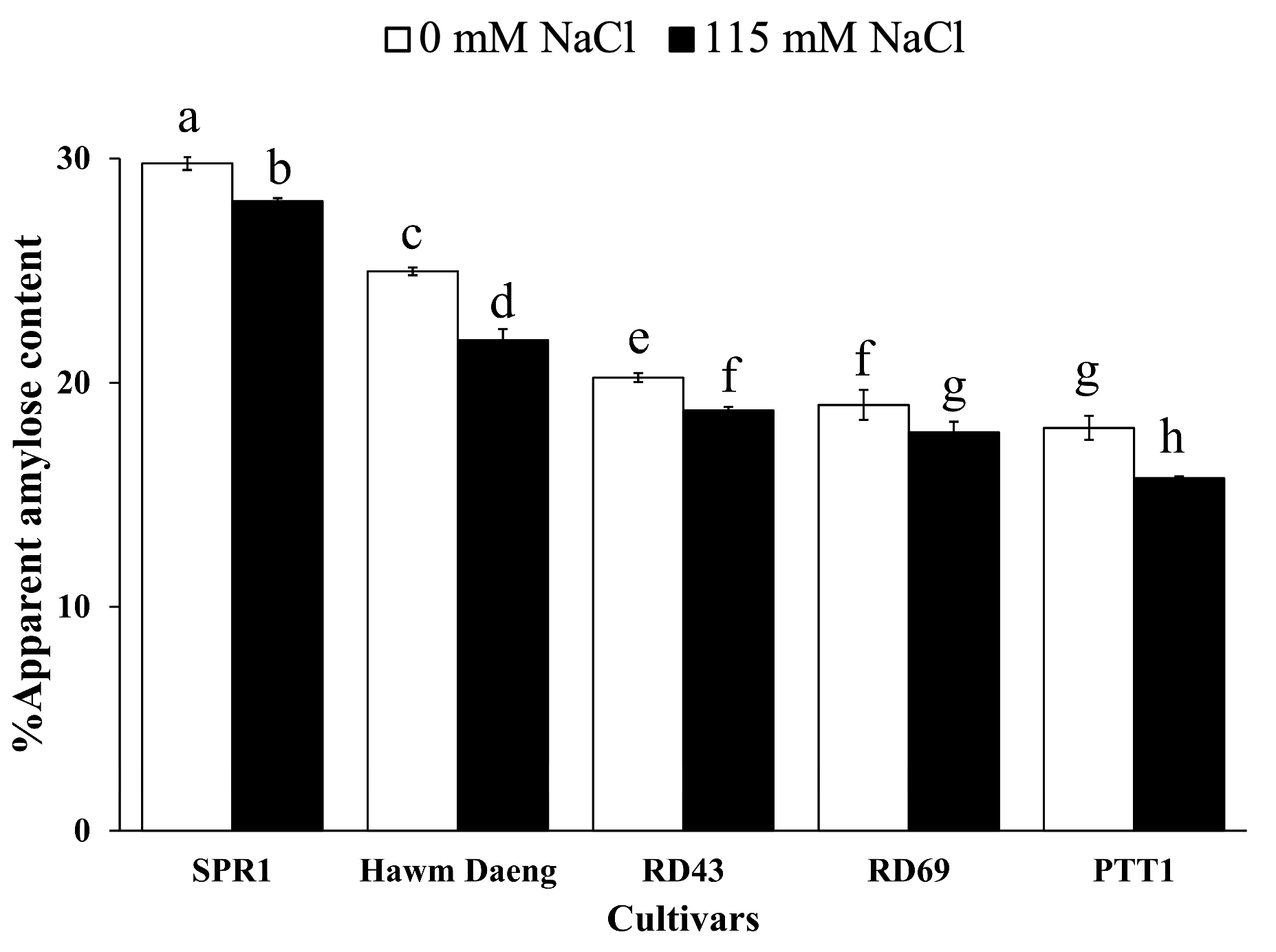
2.3. Effects of Salt Stress on the Starch Properties of Rice
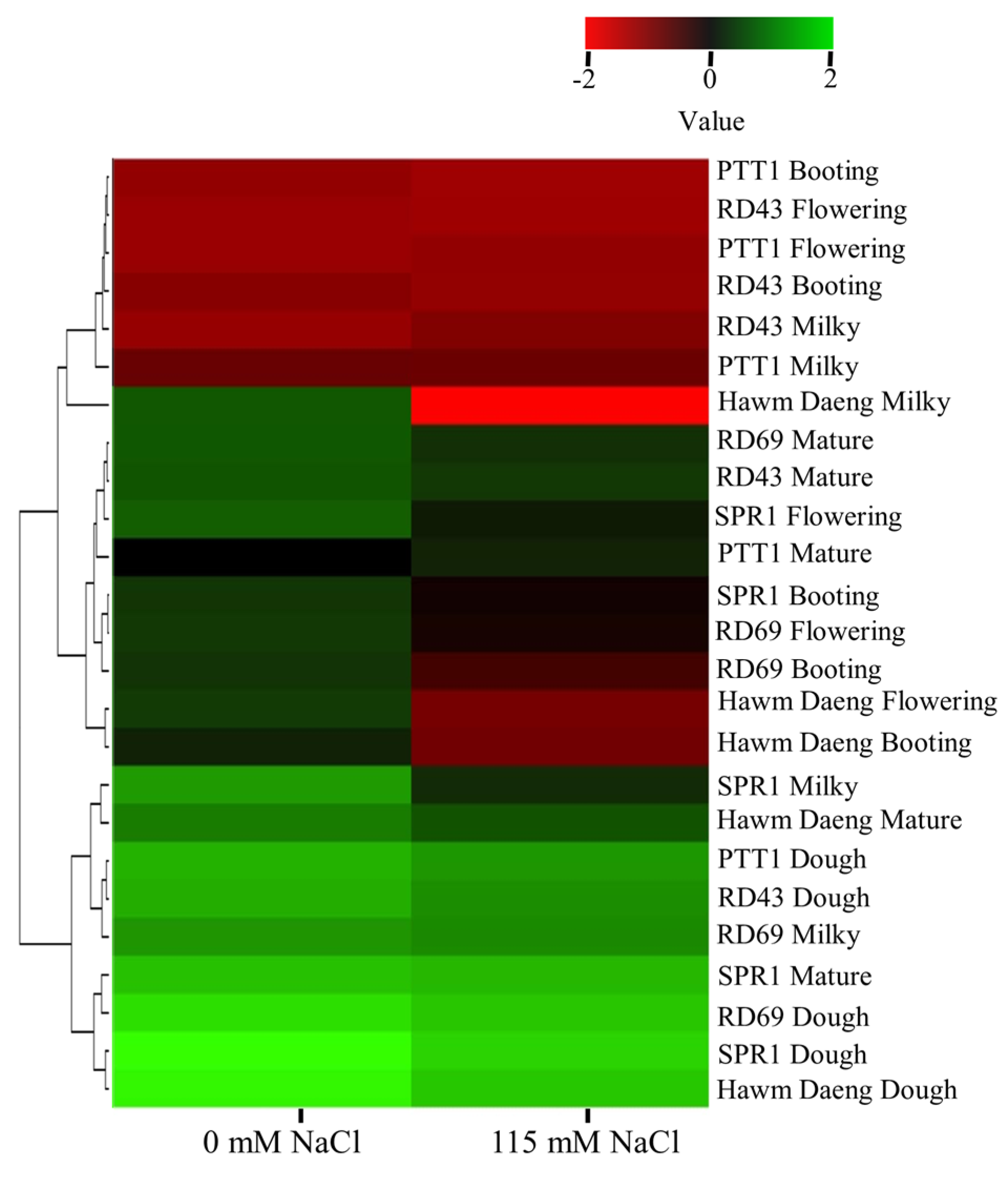
2.4. Expression Analysis of Gene Regulating Starch Properties
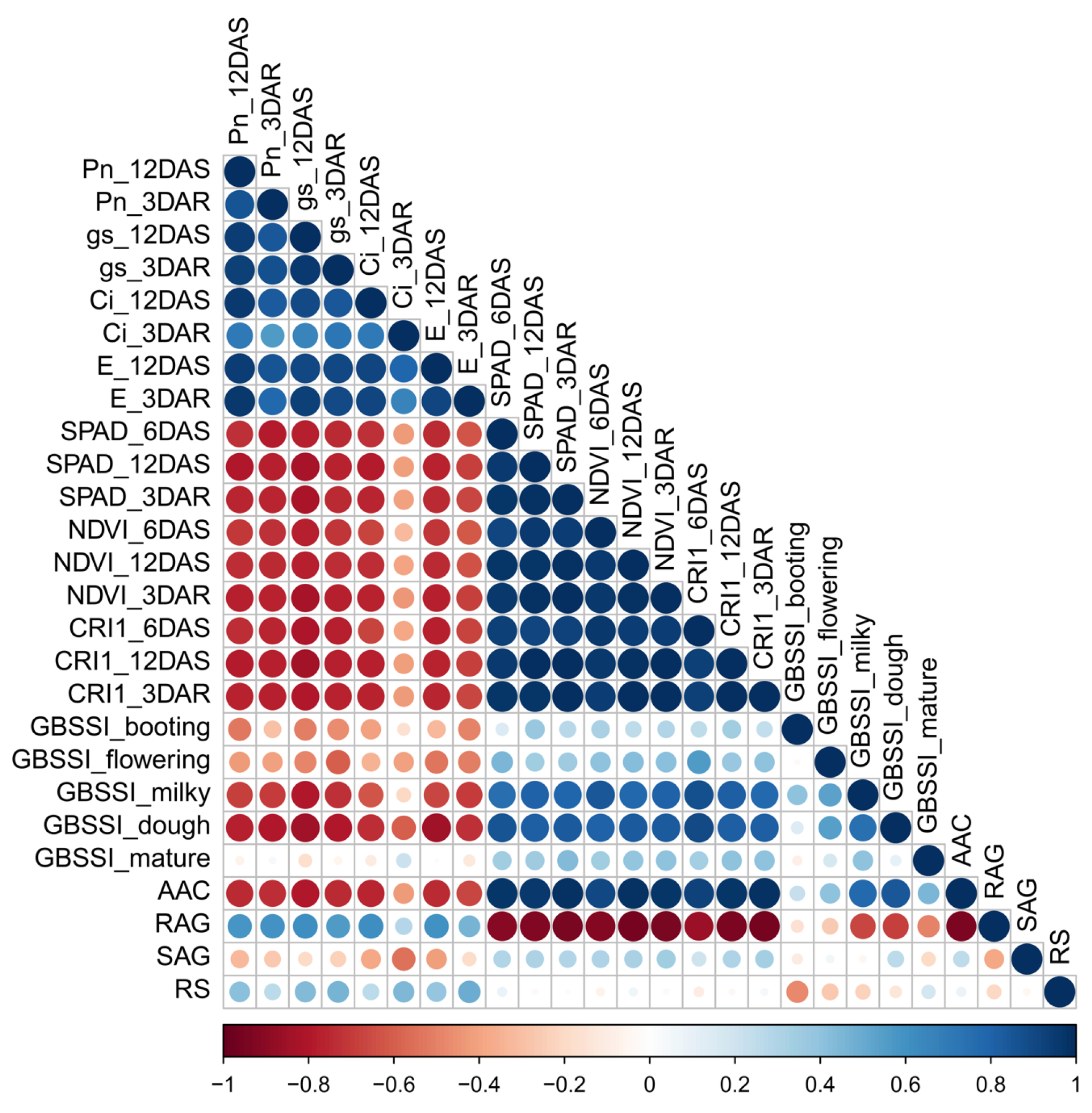
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Growth Conditions and Experimental Design
4.3. Measurement of Physiological Parameters
4.4. Measurement of Yield Components
4.5. Measurement of Starch Properties
4.6. Gene Expression Analysis
4.7. Experimental Design and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAC | Apparent amylose content |
| Ci | Intercellular CO2 concentration |
| CRI1 | Carotenoid reflectance index 1 |
| DAR | Days after recovery |
| DAS | Days after salt treatment |
| E | Transpiration rate |
| GBSSI | Granule-bound starch synthase I |
| gs | Stomatal conductance |
| NDVI | Normalized difference vegetation index |
| PI | Performance index |
| Pn | Net photosynthetic rate |
| PSI | Photosystem I |
| PSII | Photosystem II |
| RAG | Rapidly available glucose |
| RC | Reaction center |
| RS | Resistant starch |
| SAG | Slowly available glucose |
| SPAD | Leaf greenness |
References
- Zeng, D.; Tian, Z.; Rao, Y.; Dong, G.; Yang, Y.; Huang, L.; Leng, Y.; Xu, J.; Sun, C.; Zhang, G.; et al. Design of high-yield and superior-quality rice. Nat. Plants 2017, 3, 17031. [Google Scholar] [CrossRef] [PubMed]
- Hizukuri, S.; Takeda, Y.; Maruta, N.; Juliano, B.O. Molecular structures of rice starch. Carbohydr. Res. 1989, 189, 227–235. [Google Scholar] [CrossRef]
- Tao, K.; Yu, W.; Prakash, S.; Gilbert, R.G. High-amylose rice: Starch molecular structural features controlling cooked rice texture and preference. Carbohydr. Polym. 2019, 219, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Julaino, B.O. Rice in Human Nutrition; FAO Food and Nutrition Series, No.26; The International Rice Research Institute (IRRI): Los Baños, Philippines, 1993; pp. 43–44. [Google Scholar]
- Bao, J.S. Toward understanding the genetic and molecular bases of the eating and cooking qualities of rice. Cereal Food World 2012, 57, 148. [Google Scholar] [CrossRef]
- Pandey, M.K.; Rani, N.S.; Madhav, M.S.; Sundaram, R.M.; Varaprasad, G.S.; Sivaranjani, A.K.; Bohra, A.; Kumar, G.R.; Kumar, A. Different isoforms of starch-synthesizing enzymes controlling amylose and amylopectin content in rice (Oryza sativa L.). Biotechnol. Adv. 2012, 30, 1697–1706. [Google Scholar]
- Phing Lau, W.C.; Latif, M.A.; Rafii, M.Y.; Ismail, M.R.; Puteh, A. Advances to improve the eating and cooking qualities of rice by marker-assisted breeding. Crit. Rev. Biotechnol. 2016, 36, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Zhang, J.; Wei, W.; Zhu, T.; Qu, H.; Liu, Y.; Xu, G. Improving rice eating and cooking quality by enhancing endogenous expression of a nitrogen-dependent floral regulator. Plant Biotechnol. J. 2023, 21, 2654–2670. [Google Scholar] [CrossRef]
- Englyst, H.; Kingman, S.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), S33–S50. [Google Scholar]
- Englyst, H.N.; Veenstra, J.; Hudson, G.J. Measurement of rapidly available glucose (RAG) in plant foods: A potential in vitro predictor of the glycaemic response. Br. J. Nutr. 1996, 75, 327–337. [Google Scholar] [CrossRef]
- Bao, J.; Xu, F.; Park, Y.; Zhou, X.; He, Q. Genome-wide association study of the resistant starch content in rice grains. Starch 2017, 69, 1600343. [Google Scholar] [CrossRef]
- Biselli, C.; Volante, A.; Desiderio, F.; Tondelli, A.; Gianinetti, A.; Finocchiaro, F.; Taddei, F.; Gazza, L.; Sgrulletta, D.; Cattivelli, L.; et al. GWAS for starch-related parameters in Japonica rice (Oryza sativa L.). Plants 2019, 8, 292. [Google Scholar] [CrossRef]
- Dobo, M.; Ayres, N.; Walker, G.; Park, W.D. Polymorphism in the GBSS gene affects amylose content in US and European rice germplasm. J. Cereal Sci. 2010, 52, 450–456. [Google Scholar] [CrossRef]
- Wang, X.; Feng, B.; Xu, Z.; Sestili, F.; Zhao, G.; Xiang, C.; Lafiandra, D.; Wang, T. Identification and characterization of granule bound starch synthase I (GBSSI) gene of tartary buckwheat (Fagopyrum tataricum Gaertn.). Gene 2014, 534, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sahoo, U.; Baisakha, B.; Okpani, O.A.; Ngangkham, U.; Parameswaran, C.; Basak, N.; Kumar, G.; Sharma, S.G. Resistant starch could be decisive in determining the glycemic index of rice cultivars. J. Cereal Sci. 2018, 79, 348–353. [Google Scholar] [CrossRef]
- Wei, C.; Jiang, J.; Liu, C.; Fang, X.; Zhou, T.; Xue, Z.; Wang, W.; Zhang, W.; Zhang, H.; Liu, L.; et al. Effects of source strength and sink size on starch metabolism, starch properties and grain quality of rice (Oryza sativa L.). Agronomy 2023, 13, 1288. [Google Scholar] [CrossRef]
- Jeng, T.L.; Wang, C.S.; Tseng, T.H.; Sung, J.M. Expression of granule-bound starch synthase in developing rice grain. J. Sci. Food Agric. 2007, 87, 2456–2463. [Google Scholar] [CrossRef]
- Hussain, M.; Ahmad, S.; Hussain, S.; Lal, R.; Ul-Allah, S.; Nawaz, A. Rice in saline soils: Physiology, biochemistry, genetics, and management. Adv. Agron. 2018, 148, 231–287. [Google Scholar]
- Grattan, S.R.; Zeng, L.; Shannon, M.C.; Roberts, S.R. Rice is more sensitive to salinity than previously thought. Calif. Agric. 2002, 56, 189–198. [Google Scholar] [CrossRef]
- Yeo, A.R.; Yeo, M.E.; Flowers, S.A.; Flowers, T.J. Screening of rice (Oryza sativa L.) genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance. Theor. Appl. Genet. 1990, 79, 377–384. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef]
- Rao, P.S.; Mishra, B.; Gupta, S.R. Effects of soil salinity and alkalinity on grain quality of tolerant, semi-tolerant and sensitive rice genotypes. Rice Sci. 2013, 20, 284–291. [Google Scholar] [CrossRef]
- Yao, D.; Wu, J.; Luo, Q.; Zhang, D.; Zhuang, W.; Xiao, G.; Deng, Q.; Bai, B. Effects of salinity stress at reproductive growth stage on rice (Oryza sativa L.) composition, starch structure, and physicochemical properties. Front. Nutr. 2022, 9, 926217. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.A.; Juraimi, A.S.; Begum, M.; Hanafi, M.M.; Ismail, M.R.; Selamat, A. Effect of salt stress on germination and early seedling growth of rice (Oryza sativa L.). Afr. J. Biotechnol. 2010, 9, 1911–1918. [Google Scholar]
- Gerona, M.E.B.; Deocampo, M.P.; Egdane, J.A.; Ismail, A.M.; Dionisio-Sese, M.L. Physiological responses of contrasting rice genotypes to salt stress at reproductive stage. Rice Sci. 2019, 26, 207–219. [Google Scholar] [CrossRef]
- Aghakouchak, A.; Chiang, F.; Huning, L.S.; Love, C.A.; Mallakpour, I.; Mazdiyasni, O.; Moftakhari, H.; Papalexiou, S.M.; Ragno, E.; Sadegh, M. Climate Extremes and Compound Hazards in a Warming World. Ann. Rev. Earth Planet Sci. 2020, 48, 519–548. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Y.; Han, J.; Yang, Q.; Liang, J.; Liu, X.; Wang, Y.; Huang, Z. Impacts of future climate change on rice yield based on crop model simulation—A meta-analysis. Sci. Total Environ. 2024, 949, 175038. [Google Scholar] [CrossRef]
- Negacz, K.; Malek, Ž.; de Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J. Exp. Bot. 1995, 46, 1843–1852. [Google Scholar] [CrossRef]
- Singh, R.K.; Gregorio, G.B.; Ismail, A.M. Breeding rice varieties with tolerance to salt stress. J. Indian Soc. Coastal Agric. Res. 2008, 26, 16–21. [Google Scholar]
- Singh, R.K.; Flowers, T.J. The physiology and molecular biology of the effects of salinity on rice. In Handbook of Plant and Crop Stress, 3rd ed.; Taylor and Francis: Boca Raton, FL, USA, 2010; pp. 904–905. [Google Scholar]
- Hoang, T.M.L.; Tran, T.N.; Nguyen, T.K.T.; Williams, B.; Wurm, P.; Bellairs, S.; Mundree, S. Improvement of salinity stress tolerance in rice: Challenges and opportunities. Agronomy 2016, 6, 54. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.; Vispo, N.A.; Calapit-Palao, C.D.O.; Pangaan, I.D.; Viña, C.D.; Singh, R.K. Reproductive stage salinity tolerance in rice: A complex trait to phenotype. Indian J. Plant Physiol. 2016, 21, 528–536. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, T.; Zhu, K.; Wang, W.; Zhang, W.; Zhang, H.; Liu, L.; Zhang, Z.; Wang, Z.; Wang, B.; et al. Effects of salt stress on grain yield and quality parameters in rice cultivars with differing salt tolerance. Plants 2023, 12, 3243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Yang, J.; Jin, Y.; Wu, J.; Xu, H.; Xiao, Y.; Lai, Y.; Guo, Z.; Wang, J.; et al. Comparative effects of heat stress at booting and grain-filling stage on yield and grain quality of high-quality hybrid rice. Foods 2023, 12, 4093. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dash, G.K.; Barik, M.; Panda, P.A.; Lal, M.K.; Baig, M.J.; Swain, P. Effect of drought stress on resistant starch content and glycemic index of rice (Oryza sativa L.). Starch-Stärke 2020, 72, 1900229. [Google Scholar] [CrossRef]
- Chen, G.; Peng, L.; Gong, J.; Wang, J.; Wu, C.; Sui, X.; Tian, Y.; Hu, M.; Li, C.; He, X.; et al. Effects of water stress on starch synthesis and accumulation of two rice cultivars at different growth stages. Front. Plant Sci. 2023, 14, 1133524. [Google Scholar] [CrossRef]
- Habila, S.; Khunpolwattana, N.; Chantarachot, T.; Buaboocha, T.; Comai, L.; Chadchawan, S.; Pongpanich, M. Salt stress responses and SNP-based phylogenetic analysis of Thai rice cultivars. Plant Genome 2022, 15, e20189. [Google Scholar] [CrossRef]
- Lekklar, C.; Pongpanich, M.; Suriya-arunroj, D.; Chinpongpanich, A.; Tsai, H.; Comai, L.; Chadchawan, S.; Buaboocha, T. Genome-wide association study for salinity tolerance at the flowering stage in a panel of rice accessions from Thailand. BMC Genom. 2019, 20, 76. [Google Scholar] [CrossRef]
- Tiwari, K.; Tiwari, S.; Kumar, N.; Sinha, S.; Krishnamurthy, S.L.; Singh, R.; Kalia, S.; Singh, N.K.; Rai, V. QTLs and Genes for Salt Stress Tolerance: A Journey from Seed to Seed Continued. Plants 2024, 13, 1099. [Google Scholar] [CrossRef]
- Muthuramalingam, P.; Jeyasri, R.; Rakkammal, K.; Satish, L.; Shamili, S.; Karthikeyan, A.; Valliammai, A.; Priya, A.; Selvaraj, A.; Gowri, P.; et al. Multi-Omics and Integrative Approach towards Understanding Salinity Tolerance in Rice: A Review. Biology 2022, 11, 1022. [Google Scholar] [CrossRef]
- Ullah, M.A.; Abdullah-Zawawi, M.-R.; Zainal-Abidin, R.-A.; Sukiran, N.L.; Uddin, M.I.; Zainal, Z. A Review of Integrative Omic Approaches for Understanding Rice Salt Response Mechanisms. Plants 2022, 11, 1430. [Google Scholar] [CrossRef]
- Liang, X.; Li, J.; Yang, Y.; Jiang, C.; Guo, Y. Designing salt stress-resilient crops: Current progress and future challenges. J. Integr. Plant Biol. 2024, 66, 303–329. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Li, X.; Han, S.; Wang, G.; Liu, X.; Amombo, E.; Xie, Y.; Fu, J. The fungus Aspergillus aculeatus enhances salt-stress tolerance, metabolite accumulation, and improves forage quality in perennial ryegrass. Front. Microbiol. 2017, 8, 1664. [Google Scholar] [CrossRef] [PubMed]
- Praphasanobol, P.; Purnama, P.R.; Junbuathong, S.; Chotechuen, S.; Moung-Ngam, P.; Kasettranan, W.; Paliyavuth, C.; Comai, L.; Pongpanich, M.; Buaboocha, T.; et al. Genome-wide association study of starch properties in local Thai rice. Plants 2023, 12, 3290. [Google Scholar] [CrossRef]
- Xu, C.; Li, Q.; Liu, X.; Wang, H.; Ling, F. Effects of nitrogen supply level on photosynthesis and chlorophyll fluorescence characteristics of rice under salt stress. Emir. J. Food Agric. 2019, 31, 741–751. [Google Scholar] [CrossRef]
- Trotti, J.; Trapani, I.; Gulino Aceto, M.; Minio, M.; Gerotto, C.; Mica, E.; Valè, G.; Barbato, R.; Pagliano, C. Physiological responses to salt stress at the seedling stage in wild (Oryza rufipogon Griff.) and cultivated (Oryza sativa L.) rice. Plants 2024, 13, 369. [Google Scholar] [CrossRef]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Jia, B.; Zhou, G. Elevated-CO2 response of stomata and its dependence on environmental factors. Front. Plant Sci. 2016, 7, 657. [Google Scholar] [CrossRef]
- Kumar, R.K.S.V.; Kumar, S.; Choudhary, B.L. High light stress response and tolerance mechanism in plant. Interdiscip. J. Contemp. Res. 2017, 4, 1. [Google Scholar]
- Zuo, G.; Huo, J.; Yang, X.; Mei, W.; Zhang, R.; Khan, A.; Feng, N.; Zheng, D. Photosynthetic mechanisms underlying NaCl-induced salinity tolerance in rice (Oryza sativa). BMC Plant Biol. 2024, 24, 41. [Google Scholar] [CrossRef]
- Hu, C.; Rao, J.; Song, Y.; Chan, S.A.; Tohge, T.; Cui, B.; Lin, H.; Fernie, A.R.; Zhang, D.; Shi, J. Dissection of flag leaf metabolic shifts and their relationship with those occurring simultaneously in developing seed by application of non-targeted metabolomics. PLoS ONE 2020, 15, e0227577. [Google Scholar] [CrossRef]
- Zeng, L.; Shannon, M.C. Effects of salinity on grain yield and yield components of rice at different seeding densities. Agron. J. 2000, 92, 418–423. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Hussain, S.; Yang, S.; Li, R.; Liu, S.; Chen, Y.; Wei, H.; Dai, Q.; Hou, H. Study on the effect of salt stress on yield and grain quality among different rice varieties. Front. Plant Sci. 2022, 13, 918460. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, C.; Liu, L.; Tan, Y.; Sheng, X.; Yu, D.; Sun, Z.; Sun, X.; Chen, J.; Yuan, D.; et al. Effect of salinity stress on rice yield and grain quality: A meta-analysis. Eur. J. Agron. 2023, 144, 126765. [Google Scholar] [CrossRef]
- Chung, H.J.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Chen, M.H.; Bett-Garber, K.; Lea, J.; McClung, A.; Bergman, C. High resistant starch rice: Variation in starch related SNPs, and functional, and sensory properties. Foods 2021, 11, 94. [Google Scholar] [CrossRef]
- Luo, M.; Gong, W.; Zhang, S.; Xie, L.; Shi, Y.; Wu, D.; Shu, X. Discrepancies in resistant starch and starch physicochemical properties between rice mutants similar in high amylose content. Front. Plant Sci. 2023, 14, 1267281. [Google Scholar] [CrossRef]
- Zheng, C.; Niu, S.; Yan, Y.; Zhou, G.; Peng, Y.; He, Y.; Zhou, J.; Li, Y.; Xie, X. Moderate salinity stress affects rice quality by influencing expression of amylose- and protein-content-associated genes. Int. J. Mol. Sci. 2024, 25, 4042. [Google Scholar] [CrossRef]
- Watling, K. Measuring Salinity, in Facts, Land Series, L137; Queensland Government Natural Resources and Water: Brisbane city, QLD, Australia, 2007; pp. 418–420. [Google Scholar]
- Juliano, B.O. A simplified assay for milled-rice amylose. Cereal Sci. Today 1971, 16, 334–360. [Google Scholar]
- Vennapusa, A.R.; Somayanda, I.M.; Doherty, C.J.; Jagadish, S.K. A universal method for high-quality RNA extraction from plant tissues rich in starch, proteins and fiber. Sci. Rep. 2020, 10, 16887. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]




| Yield Component | Condition | Rice Cultivar | ||||
|---|---|---|---|---|---|---|
| SPR1 | Hawm Daeng | RD43 | RD69 | PTT1 | ||
| Tiller number per plant | 0 mM NaCl | 14.69 ± 1.40 ab | 14.67 ± 1.12 ab | 15.42 ± 0.83 a | 12.58 ± 0.87 b | 14.58 ± 1.66 ab |
| 115 mM NaCl | 12.58 ± 1.53 b | 15.67 ± 0.86 a | 15.92 ± 1.03 a | 12.58 ± 2.13 b | 14.67 ± 1.83 ab | |
| Panicle number per plant | 0 mM NaCl | 13.75 ± 1.10 a | 14.42 ± 1.32 a | 14.25 ± 1.52 a | 10.42 ± 0.50 c | 14.42 ± 1.45 a |
| 115 mM NaCl | 12.83 ± 1.14 a | 15.00 ± 0.82 a | 13.92 ± 1.07 a | 9.84 ± 0.69 c | 13.58 ± 1.10 a | |
| Panicle length (cm) | 0 mM NaCl | 26.99 ± 0.58 a | 25.70 ± 1.17 bc | 24.56 ± 0.70 c | 26.33 ± 0.39 ab | 26.50 ± 1.05 ab |
| 115 mM NaCl | 26.56 ± 0.57 ab | 25.59 ± 0.82 bc | 25.30 ± 0.25 bc | 27.09 ± 1.41 a | 25.33 ± 0.52 bc | |
| Filled grains per panicle | 0 mM NaCl | 116.50 ± 15.80 b | 112.20 ± 12.80 b | 107.10 ± 5.37 b | 149.17 ± 8.02 a | 114.24 ± 11.03 b |
| 115 mM NaCl | 75.14 ± 4.32 c | 50.59 ± 2.44 e | 66.16 ± 4.15 cd | 108.86 ± 8.85 b | 55.56 ± 8.13 de | |
| Unfilled grains per panicle | 0 mM NaCl | 25.81 ± 1.83 def | 24.84 ± 3.17 ef | 21.81 ± 2.79 f | 23.20 ± 2.51 f | 29.06 ± 4.76 de |
| 115 mM NaCl | 34.16 ± 2.52 bc | 38.29 ± 2.37 b | 30.22 ± 1.81 cd | 29.49 ± 2.19 de | 44.55 ± 3.52 a | |
| Total seeds per plant | 0 mM NaCl | 1589.00 ± 92.89 ab | 1604.92 ± 46.64 ab | 1521.50 ± 121.44 b | 1552.09 ± 73.64 ab | 1635.00 ± 20.52 a |
| 115 mM NaCl | 965.09 ± 22.45 d | 757.42 ± 17.39 e | 918.08 ± 44.38 d | 1066.33 ± 38.37 c | 748.92 ± 69.42 e | |
| Seed weight per plant | 0 mM NaCl | 39.54 ± 2.44 a | 37.37 ± 0.67 b | 37.32 ± 2.00 b | 37.39 ± 0.99 b | 41.32 ± 1.83 a |
| 115 mM NaCl | 24.77 ± 0.27 c | 19.15 ± 0.49 d | 23.30 ± 0.99 c | 23.35 ± 0.50 c | 16.84 ± 0.81 e | |
| 1000–seed weight | 0 mM NaCl | 25.17 ± 0.31 a | 23.35 ± 0.59 cd | 24.87 ± 0.67 ab | 24.16 ± 0.67 bc | 24.95 ± 0.63 ab |
| 115 mM NaCl | 23.08 ± 0.98 de | 21.34 ± 0.27 g | 22.21 ± 0.36 f | 21.97 ± 0.53 fg | 22.27 ± 0.40 ef | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praphasanobol, P.; Chokwiwatkul, R.; Habila, S.; Chantawong, Y.; Buaboocha, T.; Comai, L.; Chadchawan, S. Effects of Salt Stress at the Booting Stage of Grain Development on Physiological Responses, Starch Properties, and Starch-Related Gene Expression in Rice (Oryza sativa L.). Plants 2025, 14, 885. https://doi.org/10.3390/plants14060885
Praphasanobol P, Chokwiwatkul R, Habila S, Chantawong Y, Buaboocha T, Comai L, Chadchawan S. Effects of Salt Stress at the Booting Stage of Grain Development on Physiological Responses, Starch Properties, and Starch-Related Gene Expression in Rice (Oryza sativa L.). Plants. 2025; 14(6):885. https://doi.org/10.3390/plants14060885
Chicago/Turabian StylePraphasanobol, Parama, Ratchata Chokwiwatkul, Susinya Habila, Yosita Chantawong, Teerapong Buaboocha, Luca Comai, and Supachitra Chadchawan. 2025. "Effects of Salt Stress at the Booting Stage of Grain Development on Physiological Responses, Starch Properties, and Starch-Related Gene Expression in Rice (Oryza sativa L.)" Plants 14, no. 6: 885. https://doi.org/10.3390/plants14060885
APA StylePraphasanobol, P., Chokwiwatkul, R., Habila, S., Chantawong, Y., Buaboocha, T., Comai, L., & Chadchawan, S. (2025). Effects of Salt Stress at the Booting Stage of Grain Development on Physiological Responses, Starch Properties, and Starch-Related Gene Expression in Rice (Oryza sativa L.). Plants, 14(6), 885. https://doi.org/10.3390/plants14060885





