Influence of Plant Growth-Promoting Rhizobacteria (PGPR) Inoculation on Phenolic Content and Key Biosynthesis-Related Processes in Ocimum basilicum Under Spodoptera frugiperda Herbivory
Abstract
1. Introduction
2. Results
2.1. Total Phenolic Content (TPC)
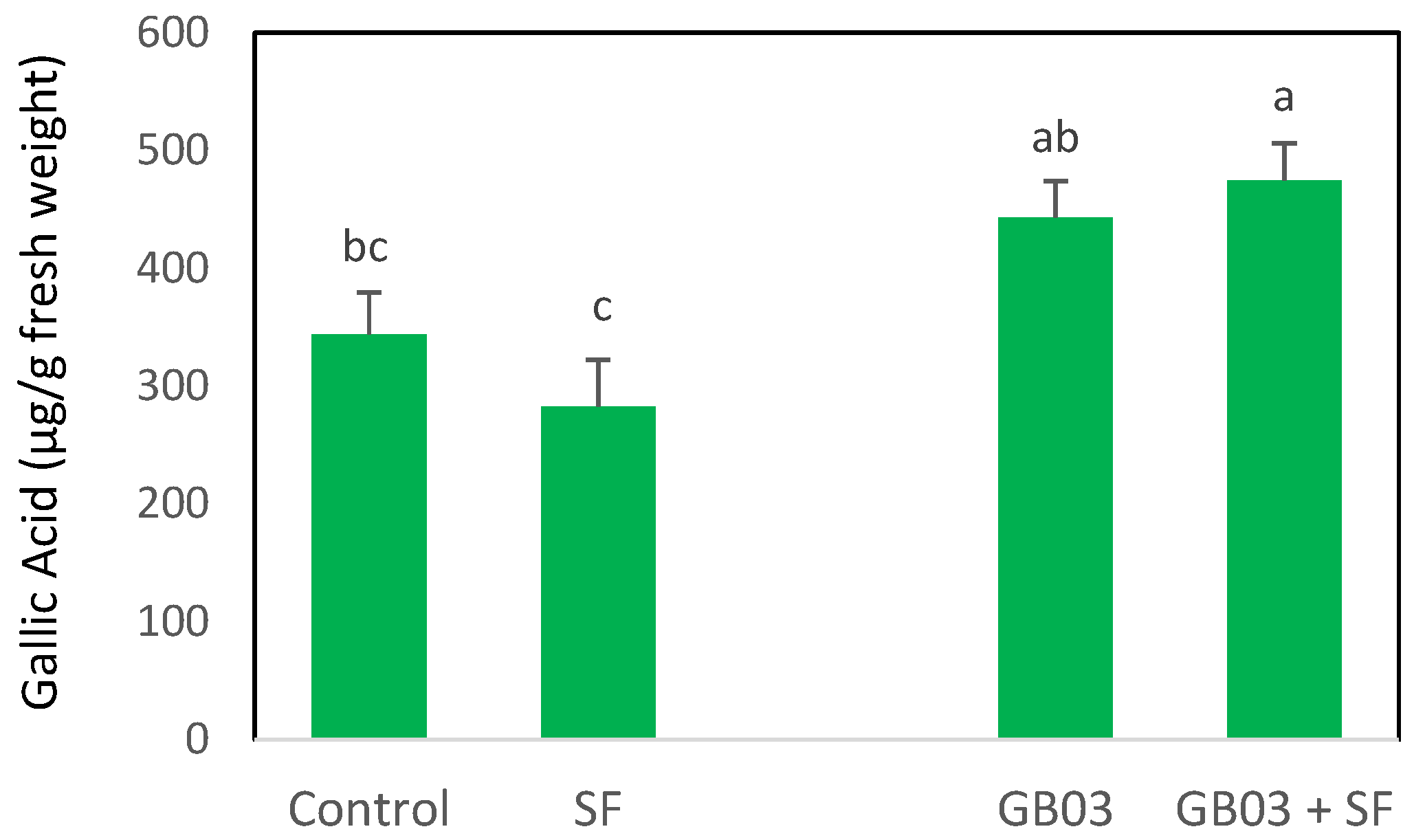
2.2. Phenylalanine Ammonia-Lyase Activity (PAL)
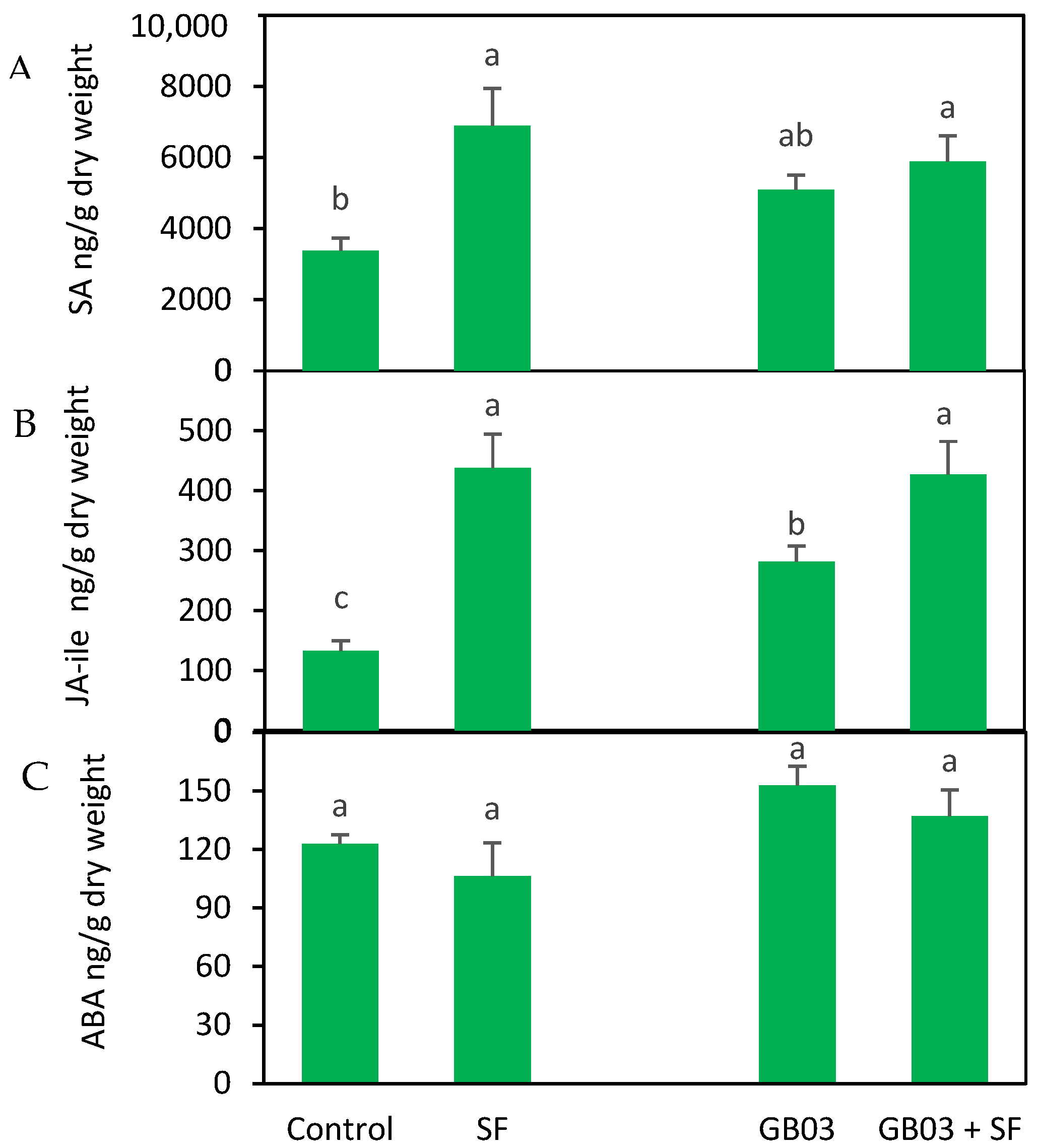
2.3. Endogenous Phytohormones
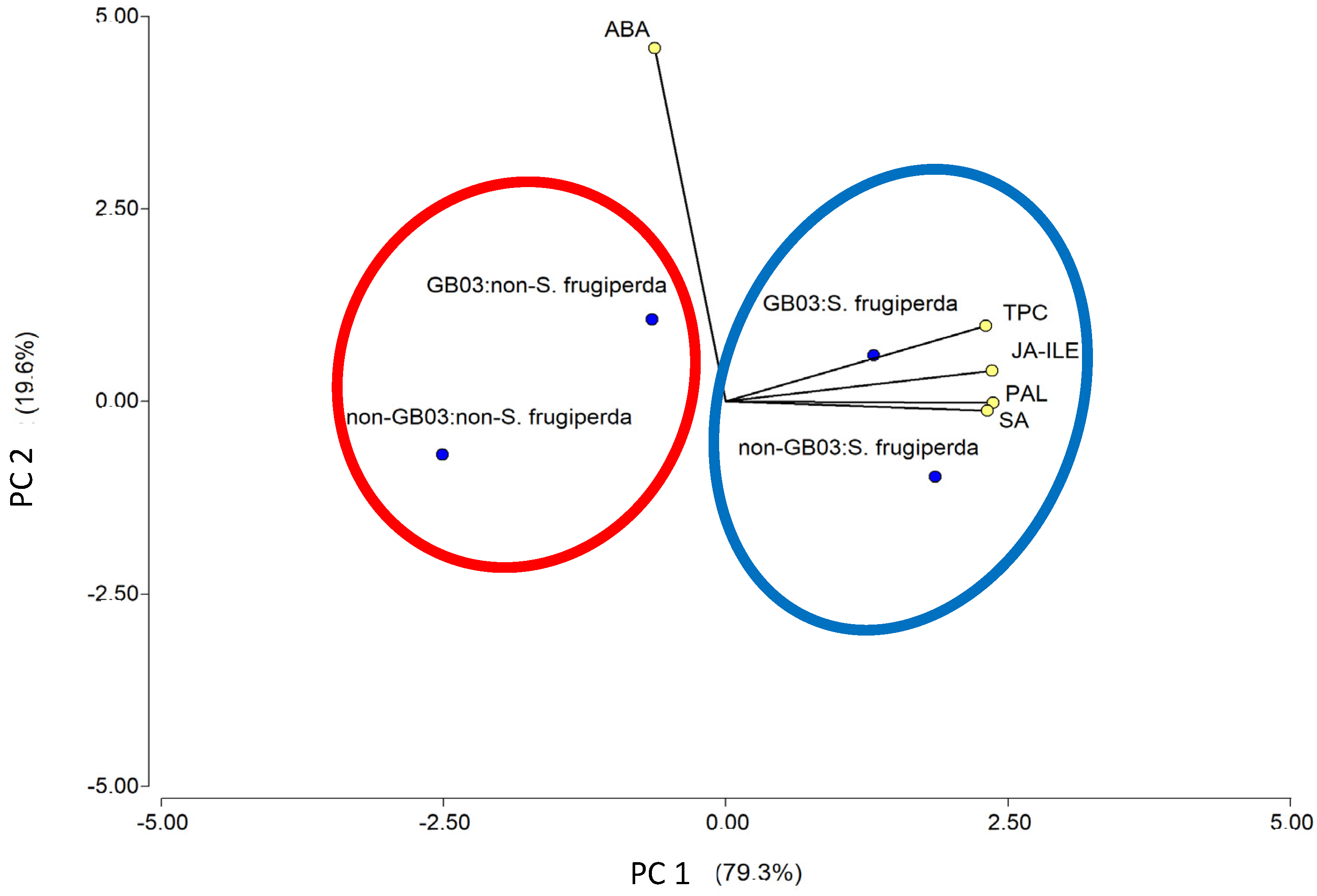
2.4. Principal Component Analysis
3. Discussion
3.1. Rhizobacteria-Mediated Plant Defense and Stress Response
3.2. Phenolic Compound Accumulation and Biosynthesis Pathways
3.3. Role of Phytohormones in Plant Defense Modulation
3.4. Interactions Between PGPR Inoculation and Herbivory in Defense Mechanisms
4. Materials and Methods
4.1. Bacterial Strains, Culture Conditions and Media
4.2. Insects Culture
4.3. Seed Sterilization and Plant Cultivation
4.4. Bioassays and Treatments
4.5. Determination of Total Phenolic Content (TPC)
4.6. Determination of PAL Enzyme Activity
4.7. Hormone Extraction
4.8. SPE-UPLC-MS/MS Phytohormone Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoffmann, A.A.; Hercus, M.J. Environmental stress as an evolutionary force. Bioscience 2000, 50, 217–226. [Google Scholar] [CrossRef]
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laidò, G.; Russo, M.A.; Ficco, D.B.M. Specialized Metabolites: Physiological and Biochemical Role in Stress Resistance, Strategies to Improve Their Accumulation, and New Applications in Crop Breeding and Management. Plant Physiol. Biochem. 2022, 172, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.; Urwin, P.E. The Interaction of Plant Biotic and Abiotic Stresses: From Genes to the Field. J. Exp. Bot. 2012, 63, 3523–3544. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Weng, J.K.; Lynch, J.H.; Matos, J.O.; Dudareva, N. Adaptive Mechanisms of Plant Specialized Metabolism Connecting Chemistry to Function. Nat. Chem. Biol. 2021, 17, 1037–1045. [Google Scholar] [CrossRef]
- Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 2023, 8, 100154. [Google Scholar]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Wallis, C.M.; Galarneau, E.R.A. Phenolic Compound Induction in Plant-Microbe and Plant-Insect Interactions: A Meta-Analysis. Front. Plant Sci. 2020, 11, 580753. [Google Scholar] [CrossRef]
- Saini, N.; Anmol, A.; Kumar, S.; Bakshi, M.; Dhiman, Z. Exploring Phenolic Compounds as Natural Stress Alleviators in Plants—A Comprehensive Review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Plant Secondary Metabolites as a Tool to Investigate Biotic Stress Tolerance in Plants: A Review. Gesunde Pflanz. 2022, 74, 771–790. [Google Scholar] [CrossRef]
- Tak, Y.; Kumar, M. Phenolics: A Key Defence Secondary Metabolite to Counter Biotic Stress. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; pp. 309–329. [Google Scholar] [CrossRef]
- Mushtaq, W.; Fauconnier, M.L. Phenolic Profiling Unravelling Allelopathic Encounters in Agroecology. Plant Stress 2024, 13, 100523. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, and Ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tak, Y.; Potkule, J.; Choyal, P.; Tomar, M.; Meena, N.L.; Kaur, C. Phenolics as Plant Protective Companion Against Abiotic Stress. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; Volume 1, pp. 277–308. [Google Scholar]
- Misra, D.; Dutta, W.; Jha, G.; Ray, P. Interactions and Regulatory Functions of Phenolics in Soil-Plant-Climate Nexus. Agronomy 2023, 13, 280. [Google Scholar] [CrossRef]
- Kim, D.S.; Hwang, B.K. An Important Role of the Pepper Phenylalanine Ammonia-Lyase Gene (PAL1) in Salicylic Acid-Dependent Signalling of the Defence Response to Microbial Pathogens. J. Exp. Bot. 2014, 65, 2295–2306. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- MacDonald, M.J.; D’Cunha, G.B. A Modern View of Phenylalanine Ammonia Lyase. Biochem. Cell Biol. 2007, 85, 273–282. [Google Scholar] [CrossRef]
- Cass, C.L.; Peraldi, A.; Dowd, P.F.; Mottiar, Y.; Santoro, N.; Karlen, S.D.; Bukhman, Y.V.; Foster, C.E.; Thrower, N.; Bruno, L.C.; et al. Effects of Phenylalanine Ammonia Lyase (PAL) Knockdown on Cell Wall Composition, Biomass Digestibility, and Biotic and Abiotic Stress Responses in Brachypodium. J. Exp. Bot. 2015, 66, 4317–4335. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.J. Multifaceted Regulations of Gateway Enzyme Phenylalanine Ammonia-Lyase in the Biosynthesis of Phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef]
- Rasool, F.; Uzair, M.; Naeem, M.K.; Rehman, N.; Afroz, A.; Shah, H.; Khan, M.R. Phenylalanine Ammonia-Lyase (PAL) Genes Family in Wheat (Triticum aestivum L.): Genome-Wide Characterization and Expression Profiling. Agronomy 2021, 11, 2511. [Google Scholar] [CrossRef]
- Kisa, D.; İmamoğlu, R.; Genç, N.; Şahin, S.; Muhammad, A.; Elmastaş, M. The Interactive Effect of Aromatic Amino Acid Composition on the Accumulation of Phenolic Compounds and the Expression of Biosynthesis-Related Genes in Ocimum basilicum. Physiol. Mol. Biol. Plants 2021, 27, 2057–2069. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics Composition and Antioxidant Activity of Sweet Basil (Ocimum basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Scagel, C.F. Chicoric Acid Levels in Commercial Basil (Ocimum basilicum) and Echinacea purpurea products. J. Funct. Foods 2010, 2, 77–84. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaâl, M.; Navari-Izzo, F.; Ouerghi, Z. Changes in the antioxidative systems of Ocimum basilicum L. (cv. Fine) Under Different Sodium Salts. Acta Physiol. Plant 2012, 34, 1873–1881. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.M. Chatting with a Tiny Belowground Member of the Holobiome: Communication Between Plants and Growth-Promoting Rhizobacteria. Adv. Bot. Res. 2017, 82, 135–160. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Gange, A.C. Interactions involving rhizobacteria and foliar-feeding insects. In Aboveground–Belowground Community Ecology; Springer: Cham, Switzerland, 2018; pp. 117–133. [Google Scholar]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and Plants—With special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Rashid, M.H.; Chung, Y.R. Induction of Systemic Resistance Against Insect Herbivores in Plants by Beneficial Soil Microbes. Front. Plant Sci. 2017, 8, 1816. [Google Scholar] [CrossRef]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria Activates (+)-δ-cadinene Synthase Genes and Induces Systemic Resistance in Cotton Against Beet Armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 39, 935–943. [Google Scholar] [CrossRef]
- Bell, K.; Naranjo-Guevara, N.; Santos, R.C.d.; Meadow, R.; Bento, J.M.S. Predatory Earwigs are Attracted by Herbivore-Induced Plant Volatiles Linked with Plant Growth-Promoting Rhizobacteria. Insects 2020, 11, 271. [Google Scholar] [CrossRef]
- Friman, J.; Pineda, A.; van Loon, J.J.; Dicke, M. Bidirectional Plant-Mediated Interactions Between Rhizobacteria and Shoot-Feeding Herbivorous Insects: A Community Ecology Perspective. Ecol. Entomol. 2021, 46, 110. [Google Scholar] [CrossRef]
- Choi, S.K.; Jeong, H.; Kloepper, J.W.; Ryu, C.M. Genome Sequence of Bacillus amyloliquefaciens GB03, an Active Ingredient of the First Commercial Biological Control Product. Genome Announc. 2014, 2, e01092-14. [Google Scholar] [CrossRef]
- Cappellari, L.R.; Santoro, M.; Reinoso, H.; Travaglia, C.; Giordano, W.; Banchio, E. Anatomical, Morphological, and Phytochemical Effects of Inoculation with Plant Growth Promoting Rhizobacteria on Peppermint (Mentha piperita). J. Chem. Ecol. 2015, 41, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Banchio, E.; Xie, X.; Zhang, H.; Paré, P.W. Soil Bacteria Elevate Essential Oil Accumulation and Emissions in Sweet Basil. J. Agric. Food Chem. 2009, 5, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogin, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant Growth Promotion in Cereal and Leguminous Agricultural Important Plants: From Microorganism Capacities to Crop Production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant Growth Promoting Rhizobacteria (PGPR): Current and Future Prospects for Development of Sustainable Agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The Multifarious PGPR Serratia marcescens CDP-13 Augments Induced Systemic Resistance and Enhanced Salinity Tolerance of Wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen Biocontrol Using Plant Growth-Promoting Bacteria (PGPR): Role of Bacterial Diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef]
- Banchio, E.; Bogino, P.; Santoro, M.V.; Torres, L.; Zygadlo, J.; Giordano, W. Systemic Induction of Monoterpene Biosynthesis in Origanum x majoricum by Soil Bacteria. J. Agric. Food Chem. 2010, 58, 650–654. [Google Scholar] [CrossRef]
- Santoro, M.V.; Zygadlo, J.; Giordano, W.; Banchio, E. Volatile Organic Compounds from Rhizobacteria Increase Biosynthesis of Essential Oils and Growth Parameters in Peppermint (Mentha piperita). Plant Physiol. Biochem. 2011, 49, 1177–1182. [Google Scholar] [CrossRef]
- Cappellari, L.R.; Santoro, M.V.; Nievas, F.; Giordano, W.; Banchio, E. Increase of Secondary Metabolite Content in Marigold by Inoculation with Plant Growth-Promoting Rhizobacteria. Appl. Soil Ecol. 2013, 70, 16–22. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Nagargade, M.; Tyagi, V.; Singh, M.K. Plant growth-promoting rhizobacteria: A biological approach toward the production of sustainable agriculture. In Role of Rhizospheric Microbes in Soil; Springer: Singapore, 2018; Volume 1, pp. 205–223. [Google Scholar]
- Disi, J.; Simmons, J.; Zebelo, S. Plant Growth-Promoting Rhizobacteria Induced Defense Against Insect Herbivores. In Field Crops: Sustainable Management by PGPR; Sustainable Development and Biodiversity; Springer: Berlin/Heidelberg, Germany, 2019; Volume 23, pp. 385–410. [Google Scholar] [CrossRef]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcantara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Huang, J.; Lu, X.; Zhou, C. Development of Plant Systemic Resistance by Beneficial Rhizobacteria: Recognition, Initiation, Elicitation and Regulation. Front. Plant Sci. 2022, 13, 952397. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef]
- Cappellari, L.R.; Chiappero, J.; Santoro, M.; Giordano, W.; Banchio, E. Inducing Phenolic Production and Volatile Organic Compounds Emission by Inoculating Mentha piperita with Plant Growth-Promoting Rhizobacteria. Sci. Hort. 2017, 220, 193–198. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.R.; Sosa Alderete, L.G.; Palermo, T.B.; Banchio, E. Plant Growth Promoting Rhizobacteria Improve the Antioxidant Status in Mentha piperita Grown under Drought Stress Leading to an Enhancement of Plant Growth and Total Phenolic Content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Cappellari, L.; Chiappero, J.; Palermo, T.B.; Walter, G.; Banchio, E. Impact of Soil Rhizobacteria Inoculation and Leaf-Chewing Insect Herbivory on Mentha piperita Leaf Secondary Metabolites. J. Chem. Ecol. 2020, 46, 619–630. [Google Scholar] [CrossRef]
- Niveyro, S.L.; Mortensen, A.G.; Fomsgaard, I.S. Differences Among Five Amaranth Varieties (Amaranthus spp.) Regarding Secondary Metabolites and Foliar Herbivory by Chewing Insects in the Field. Arthropod Plant Interact. 2013, 7, 235–245. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Maggi, F.; Wandjou, J.G.N.; Koné-Bamba, D.; Sagratini, G.; Vittori, S.; Caprioli, G. Insecticidal activity of the essential oil and polar extracts from Ocimum gratissimum grown in Ivory Coast: Efficacy on insect pests and vectors and impact on non-target species. Ind. Crops Prod. 2019, 132, 377–385. [Google Scholar] [CrossRef]
- Palermo, T.B.; Cappellari, L.R.; Palermo, J.S.; Giordano, W.; Banchio, E. Simultaneous Impact of Rhizobacteria Inoculation and Leaf-Chewing Insect Herbivory on Essential Oil Production and VOC Emissions in Ocimum basilicum. Plants 2024, 13, 932. [Google Scholar] [CrossRef]
- Palermo, T.B.; Cappellari, L.R.; Chiappero, J.; Giordano, W.; Banchio, E. Beneficial Rhizobacteria Inoculation on Ocimum basilicum Reduces the Growth Performance and Nutritional Value of Spodoptera frugiperda. Pest. Manag. Sci. 2021, 78, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Joni, F.R.; Hamid, H.; Yanti, Y. Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Increasing the Activity of Defense Enzymes in Tomato Plants. Int. J. Environ. Agric. Biotechnol. IJEAB 2020, 5, 1474–1479. [Google Scholar] [CrossRef]
- Rani, P.U.; Pratyusha, S. Defensive Role of Gossypium hirsutum L. Anti-Oxidative Enzymes and Phenolic Acids in Response to Spodoptera litura F. feeding. J. Asia. Pac. Entomol. 2013, 16, 131–136. [Google Scholar] [CrossRef]
- Kovalikova, Z.; Kubes, J.; Skalicky, M.; Kuchtickova, N.; Maskova, L.; Tuma, J.; Vachova, P.; Hejnak, V. Changes in Content of Polyphenols and Ascorbic Acid in Leaves of White Cabbage After Pest Infestation. Molecules 2019, 24, 2622. [Google Scholar] [CrossRef]
- Sandhyarani, K.; Rani, P.U. Insect Herbivory Induced Foliar Oxidative Stress: Changes in Primary Compounds, Secondary Metabolites and Reactive Oxygen Species in Sweet Potato Ipomoea batata (L.). Allelopath. J. 2013, 31, 157–168. [Google Scholar]
- Sambangi, P.; Usha Rani, P. Induction of Phenolic Acids and Metals in Arachis hypogaea L. Plants Due to Feeding of Three Lepidopteran Pests. Arthropod-Plant Interact. 2013, 7, 517–525. [Google Scholar] [CrossRef]
- Kumari, R.; Pandey, E.; Bushra, S.; Faizan, S.; Pandey, S. Plant Growth Promoting Rhizobacteria (PGPR) Induced Protection: A Plant Immunity Perspective. Physiol. Plant. 2024, 176, e14495. [Google Scholar] [CrossRef]
- Khan, V.; Jha, A.; Seth, T.; Iqbal, N.; Umar, S. Exploring the role of jasmonic acid in boosting the production of secondary metabolites in medicinal plants: Pathway for future research. Ind. Crops Prod. 2024, 220, 119227. [Google Scholar] [CrossRef]
- Pineda, A.; Soler, R.; Pozo, M.J.; Rasmann, S.; Turlings, T.C.J. Above-Belowground Interactions Involving Plants, Microbes and Insects. Front. Plant Sci. 2015, 6, 318. [Google Scholar] [CrossRef]
- Serteyn, L.; Quaghebeur, C.; Ongena, M.; Cabrera, N.; Barrera, A.; Molina-Montenegro, M.A.; Francis, F.; Ramírez, C.C. Induced Systemic Resistance by a Plant Growth-Promoting Rhizobacterium Impacts Development and Feeding Behavior of Aphids. Insects 2020, 11, 234. [Google Scholar] [CrossRef]
- Pangesti, N.; Reichelt, M.; van de Mortel, J.E.; Kapsomenou, E.; Gershenzon, J.; van Loon, J.J.; Dicke, M.; Pineda, A. Jasmonic Acid and Ethylene Signaling Pathways Regulate Glucosinolate Levels in Plants During Rhizobacteria-Induced Systemic Resistance Against a Leaf-Chewing Herbivore. J. Chem. Ecol. 2016, 42, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Reymond, P.; Weber, H.; Damond, M.; Farmer, E.E. Differential Gene Expression in Response to Mechanical Wounding and Insect Feeding in Arabidopsis. Plant Cell 2000, 12, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Kousar, B.; Bano, A.; Khan, N. PGPR modulation of secondary metabolites in tomato infested with Spodoptera litura. Agronomy 2020, 10, 778. [Google Scholar] [CrossRef]
- Walters, D.R. Plant Defense: Warding Off Attack by Pathogens, Herbivores and Parasitic Plants; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Park, W.T.; Kim, Y.B.; Seo, J.M.; Kim, S.J.; Chung, E.; Lee, J.H.; Park, S.U. Accumulation of anthocyanin and associated gene expression in radish sprouts exposed to light and methyl jasmonate. J. Agric. Food Chem. 2013, 61, 4127–4132. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, J.K.; Uddin, R.; Xu, H.; Park, W.T.; Tuan, P.A.; Li, X.; Chung, E.; Lee, J.-H.; Park, S.U. Metabolomics Analysis and Biosynthesis of Rosmarinic Acid in Agastache rugosa Kuntze Treated with Methyl Jasmonate. PLoS ONE 2013, 8, e64199. [Google Scholar] [CrossRef]
- Men, L.; Yan, S.; Liu, G. De Novo Characterization of Larix gmelinii (Rupr.) Rupr. Transcriptome and Analysis of its Gene Expression Induced by Jasmonates. BMC Genom. 2014, 14, 548. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Joseph, C.; Hagege, D. Jasmonic Acid Elicitation of Hypericum perforatum L. Cell Suspensions and Effects on the Production of Phenylpropanoids and Naphtodianthrones. Plant Cell Tissue Organ. Cult. 2007, 89, 1–13. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Kim, S.-H.; Chung, I.-M. Influence of amphetamine, γ-aminobutyric acid, and fosmidomycin on metabolic, transcriptional variations and determination of their biological activities in turnip (Brassica rapa ssp. rapa). S. Afr. J. Bot. 2016, 103, 181–192. [Google Scholar] [CrossRef]
- López-Orenes, A.; Martínez-Moreno, J.M.; Calderón, A.A.; Ferrer, M.A. Changes in Phenolic Metabolism in Salicylic Acid-Treated Shoots of Cistus heterophyllus. Plant Cell Tiss. Organ Cult. PCTOC 2013, 113, 417–427. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Wen, P.-F.; Kong, W.-F.; Pan, Q.-H.; Zhan, J.-C.; Li, J.-M.; Wan, S.-B.; Huang, W.-D. Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Postharvest Biol. Technol. 2006, 40, 64–72. [Google Scholar] [CrossRef]
- Dong, J.; Wan, G.; Liang, Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotechnol. 2010, 148, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Sun, Y.; Su, J.; Ren, Q.; Li, C.; Ge, F. Elevated O3 reduces the fitness of Bemisia tabaci via enhancement of the SA-dependent defense of the tomato plant. Arthropod-Plant Interact. 2012, 6, 425–437. [Google Scholar] [CrossRef]
- Taguchi, G.; Yoshizawa, K.; Kodaira, R.; Hayashida, N.; Okazaki, M. Plant Hormone Regulation on Scopoletin Metabolism from Culture Medium into Tobacco Cells. Plant Sci. 2001, 160, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.C.G.L.; Leppla, W.A.; Dickerson, V. Caterpillar: A Rearing Procedure and Artificial Medium. J. Econ. Entomol. 1976, 69, 487–488. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Bradford, M.A. Rapid and Sensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Balcke, G.U.; Handrick, V.; Bergau, N.; Fichtner, M.; Henning, A.; Stellmach, H.; Tissier, A.; Hause, B.; Frolov, A. An UPLC-MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods 2012, 8, 47. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palermo, J.S.; Palermo, T.B.; Cappellari, L.d.R.; Balcke, G.U.; Tissier, A.; Giordano, W.; Banchio, E. Influence of Plant Growth-Promoting Rhizobacteria (PGPR) Inoculation on Phenolic Content and Key Biosynthesis-Related Processes in Ocimum basilicum Under Spodoptera frugiperda Herbivory. Plants 2025, 14, 857. https://doi.org/10.3390/plants14060857
Palermo JS, Palermo TB, Cappellari LdR, Balcke GU, Tissier A, Giordano W, Banchio E. Influence of Plant Growth-Promoting Rhizobacteria (PGPR) Inoculation on Phenolic Content and Key Biosynthesis-Related Processes in Ocimum basilicum Under Spodoptera frugiperda Herbivory. Plants. 2025; 14(6):857. https://doi.org/10.3390/plants14060857
Chicago/Turabian StylePalermo, Jimena Sofía, Tamara Belén Palermo, Lorena del Rosario Cappellari, Gerd Ulrich Balcke, Alain Tissier, Walter Giordano, and Erika Banchio. 2025. "Influence of Plant Growth-Promoting Rhizobacteria (PGPR) Inoculation on Phenolic Content and Key Biosynthesis-Related Processes in Ocimum basilicum Under Spodoptera frugiperda Herbivory" Plants 14, no. 6: 857. https://doi.org/10.3390/plants14060857
APA StylePalermo, J. S., Palermo, T. B., Cappellari, L. d. R., Balcke, G. U., Tissier, A., Giordano, W., & Banchio, E. (2025). Influence of Plant Growth-Promoting Rhizobacteria (PGPR) Inoculation on Phenolic Content and Key Biosynthesis-Related Processes in Ocimum basilicum Under Spodoptera frugiperda Herbivory. Plants, 14(6), 857. https://doi.org/10.3390/plants14060857





