Anti-Obesity and Weight Management-Related Antioxidant Potential Properties of Calabrian Pine Extracts: Pinus nigra Subsp. laricio (Poir.) Maire
Abstract
1. Introduction
2. Results
2.1. Phytochemical Profile
2.2. Antioxidant Activity
2.3. Inhibition of Nitric Oxide Production
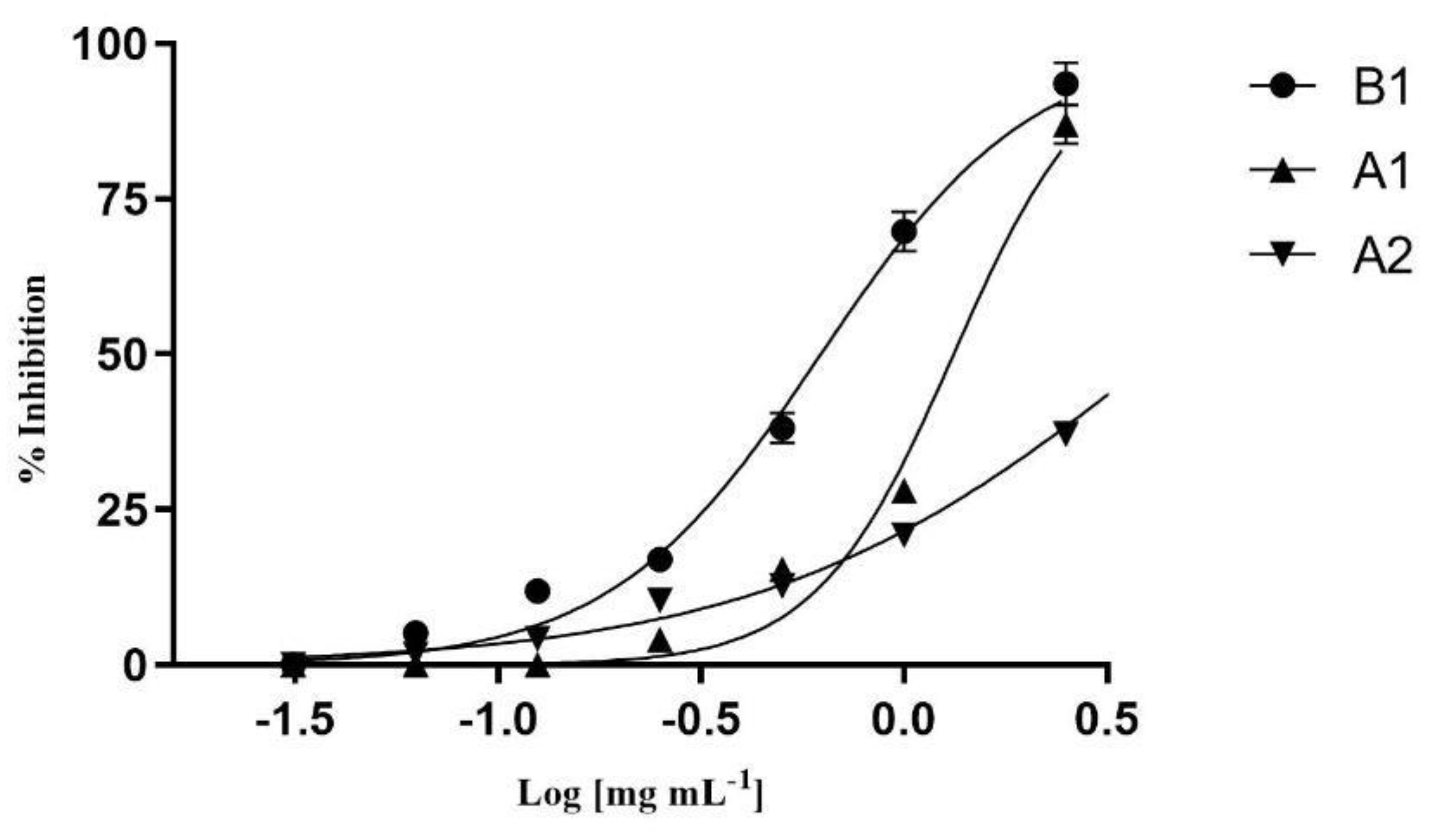
2.4. Pancreatic Lipase and α-Amylase Inhibition
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Sample Preparation
4.3. Total Phenolic and Flavonoid Content
4.4. GC-MS Analysis
4.5. In Vitro Methods for Antioxidant Activity Assessment
4.6. Cell Culture
In Vitro Evaluation of the Inhibitory Properties on Nitric Oxide (NO) Production
4.7. Measurement of Pancreatic Lipase and α-Amylase Activities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pignatti, S. Flora Europea; Edizioni Edagricole-New Business S.r.l.: Bologna, Italia, 2017–2019; Volume 1, p. 77. [Google Scholar]
- Meschini, A.; Longhi, G. Le pinete di pino laricio. Loro conservazione e loro miglioramento. In Atti del Congresso Nazionale di Selvicoltura per il Miglioramento e la Conservazione dei Boschi Italiani; Accademia Italiana di Scienze Forestali: Firenze, Italia, 1955; Volume 14, pp. 199–226. [Google Scholar]
- Lupia, A.; Lupia, C.; Lupia, R. Etnobotanica in Calabria: Viaggio Alla Scoperta di Antichi Saperi Intorno al Mondo Delle Piante; Rubbettino: Soveria Mannelli, Italy, 2017. [Google Scholar]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Yang, J.-H.; Byeon, E.-H.; Kim, W.; Kang, D.; Han, J.; Hong, S.-G.; Kim, D.-R.; Park, S.-J.; Huh, J.-W.; et al. Anti-obesity effect of pine needle extract on high-fat diet-induced obese mice. Plants 2021, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Fkiri, S.; Ghazghazi, H.; Rigane, G.; Ben Salem, R.; Mezni, F.; Khaldi, A.; Khouja, M.L.; Nasr, Z. Chemical compositions and biological activities essential oil from the needles of North African Pinus Pinaster Var. Rev. Roum. Chim. 2019, 64, 511–517. [Google Scholar] [CrossRef]
- Sak, K.; Jürisoo, K.; Raal, A. Estonian folk traditional experiences on natural anticancer remedies: From past to the future. Pharm. Biol. 2014, 52, 855–866. [Google Scholar] [CrossRef]
- Politeo, O.; Skocibusic, M.; Maravic, A.; Ruscic, M.; Milos, M. Chemical composition and antimicrobial activity of the essential oil of endemic Dalmatian black pine (Pinus nigra ssp. dalmatica). Chem. Biodivers. 2011, 8, 540–547. [Google Scholar] [CrossRef]
- Apetrei, C.L.; Tuchilus, C.; Aprotosoaie, A.C.; Oprea, A.; Malterud, K.E.; Miron, A. Chemical, antioxidant and antimicrobial investigations of Pinus cembra L. bark and needles. Molecules 2011, 16, 7773–7788. [Google Scholar] [CrossRef]
- Packer, L.; Rimbach, G.; Virgili, F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (pinus maritima) bark, pycnogenol. Free. Radic. Biol. Med. 1999, 27, 704–724. [Google Scholar] [CrossRef]
- Perri, M.R.; Pellegrino, M.; Marrelli, M.; Aquaro, S.; Cavaliere, F.; Grande, F.; Occhiuzzi, M.A.; Lupia, C.; Toma, C.-C.; Conforti, F.; et al. Identification of Pinosylvin in Pinus nigra subsp. laricio: A Naturally Occurring Stilbenoid Suppressing LPS-Induced Expression of Pro-Inflammatory Cytokines and Mediators and Inhibiting the JAK/STAT Signaling Pathway. Pharmaceuticals 2023, 16, 718. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in Inflammation. Curr. Drug Target Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative study regarding the chemical composition and biological activity of pine (Pinus nigra and P. sylvestris) bark extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How does the phenol structure influence the results of the Folin-Ciocalteu assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Cho, S.-M.; Lee, M.-H.; Lee, E.-O.; Kim, S.-H.; Lee, H.-J. Ethanol extract of Pinus koraiensis leaves containing lambertianic acid exerts anti-obesity and hypolipidemic effects by activating adenosine monophosphate-activated protein kinase (AMPK). BMC Complement. Altern. Med. 2016, 16, 51. [Google Scholar] [CrossRef]
- Won, S.B.; Jung, G.-Y.; Kim, J.; Chung, Y.S.; Hong, E.K.; Kwon, Y.H. Protective effect of Pinus koraiensis needle water extract against oxidative stress in HepG2 cells and obese mice. J. Med. Food 2013, 16, 569–576. [Google Scholar] [CrossRef]
- Wang, M.; Gu, D.; Li, H.; Wang, Q.; Kang, J.; Chu, T.; Guo, H.; Yang, Y.; Tian, J. Rapid prediction and identification of lipase inhibitors in volatile oil from Pinus massoniana L. needles. Phytochemistry 2017, 141, 114–120. [Google Scholar] [CrossRef]
- Lim, W.X.J.; Gammon, C.S.; von Hurst, P.; Chepulis, L.; Page, R.A. The Inhibitory Effects of New Zealand Pine Bark (Enzogenol®) on α-Amylase, α-Glucosidase, and Dipeptidyl Peptidase-4 (DPP-4) Enzymes. Nutrients 2022, 14, 1596. [Google Scholar] [CrossRef]
- Lee, M.-H.; Park, S.; Xu, Y.; Kim, J.-E.; Han, H.; Lee, J.-H.; Paik, J.K.; Lee, H.-J. Ethanol Extract of Pinus koraiensis Leaves Mitigates High Fructose-Induced Hepatic Triglyceride Accumulation and Hypertriglyceridemia. Appl. Sci. 2022, 12, 6745. [Google Scholar] [CrossRef]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of Apple Pomace Flour Obtained Industrially by Dehydration as a Source of Biomolecules with Antioxidant, Antidiabetic and Antiobesity Effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Craciunescu, O.; Seciu-Grama, A.-M.; Mihai, E.; Utoiu, E.; Negreanu-Pirjol, T.; Lupu, C.E.; Artem, V.; Ranca, A.; Negreanu-Pirjol, B.-S. The Chemical Profile, Antioxidant, and Anti-Lipid Droplet Activity of Fluid Extracts from Romanian Cultivars of Haskap Berries, Bitter Cherries, and Red Grape Pomace for the Management of Liver Steatosis. Int. J. Mol. Sci. 2023, 24, 16849. [Google Scholar] [CrossRef]
- Al Mamun, A.; Rakib, A.; Mandal, M.; Kumar, S.; Singla, B.; Singh, U.P. Polyphenols: Role in Modulating Immune Function and Obesity. Biomolecules 2024, 14, 221. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-Phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Menichini, G.; Alfano, C.; Marrelli, M.; Toniolo, C.; Provenzano, E.; Statti, G.A.; Nicoletti, M.; Menichini, F.; Conforti, F. Hypericum perforatum L. subsp. perforatum induces inhibition of free radicals and enhanced phototoxicity in human melanoma cells under ultraviolet light. Cell Prolif. 2013, 46, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Marrelli, M.; Statti, G.; Menichini, F.; Uzunov, D.; Solimene, U.; Menichini, F. Comparative chemical composition and antioxidant activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Nyman and Calamintha grandiflora (L.) Moench (Labiatae). Nat. Prod. Res. 2012, 26, 91–97. [Google Scholar] [CrossRef]
- Grande, F.; Marrelli, M.; Amodeo, V.; Occhiuzzi, M.A.; Pinzaru, I.; Fucile, M.; Dehelean, C.A.; Alexa, E.; Conforti, F.; Statti, G. Molecular Docking Studies and In Vitro Activity of Paliurus spina-christi Mill Extracts as Pancreatic Lipase Inhibitors. Antioxidants 2024, 13, 160. [Google Scholar] [CrossRef]
| Sample | Yield (%) | TPC 1 | TFC 2 |
|---|---|---|---|
| Apical shoots | 10.88 | 20.66 ± 0.27 a | 0.40 ± 0.01 a |
| Branches | 6.40 | 18.10 ± 0.70 b | 0.24 ± 0.01 b |
| Compound 1 | Rt 2 | RAP 3 | |
|---|---|---|---|
| Apical Shoots | Branches | ||
| Borneol | 9.98 | Tr 4 | - |
| 2-Decenal | 10.95 | Tr | - |
| Bornyl acetate | 11.21 | Tr | - |
| Cyclotetradecane | 12.12 | Tr | - |
| Phenethyl isovalerate | 13.03 | Tr | - |
| Hexadecene | 13.73 | Tr | - |
| Pristanic acid | 15.52 | 0.1 | - |
| Pentadecanoic acid, 14 methyl-, methyl ester | 16.02 | 0.7 | - |
| Palmitic acid | 16.07 | 1.1 | 1.0 |
| Manoyl oxide | 16.69 | 0.5 | - |
| Margaric acid | 16.93 | 0.3 | 0.3 |
| 10,13-Octadecadienoic acid, methyl ester | 17.07 | 0.3 | - |
| Vaccenic acid | 17.14 | - | 3.7 |
| Ethyl linoleate | 17.52 | 0.6 | 2.5 |
| Elaidic acid | 17.55 | - | 6.2 |
| Linoleic acid | 17.91 | 0.4 | 2.0 |
| 3-(4-N, N-dimethylaminophenyl) propenoic acid 2-(diethoxyphosphinyl)-ethyl ester | 19.60 | 1.8 | 2.9 |
| Cyclotetracosane | 20.61 | - | 1.0 |
| 9-Octadecenal | 20.67 | - | 5.2 |
| γ-Sitosterol | 22.94 | 0.26 | 2.0 |
| Compound 1 | Rt 2 | RAP 3 | |
|---|---|---|---|
| Apical Shoots | Branches | ||
| Benzoic acid | 10.25 | - | 0.6 |
| 1-Tetradecene | 12.13 | 0.18 | - |
| Vanillyl alcohol | 12.84 | - | 0.1 |
| 1-Hexadecene | 13.74 | 0.37 | - |
| 1-Octadecene | 15.15 | 0.24 | - |
| Cetene | 15.79 | - | 0.2 |
| Hexadecanoic acid, methyl ester | 16.02 | 1.17 | - |
| Hexadecanoic acid, ethyl ester | 16.33 | 0.99 | - |
| 5-Octadecene | 17.00 | 1.91 | - |
| Isomaturnin | 17.06 | - | 0.4 |
| Decanedioic acid, dibutyl ester | 17.50 | 1.33 | - |
| 2(1H)-Naphthalenone,octahydro-4a-phenyl | 17.78 | - | 0.4 |
| Pinocembrin | 19.60 | - | 0.5 |
| Stigmast-3-en-6-ol | 22.66 | 3.2 | - |
| Sample | Fraction | IC50 (µg/mL) | ||
|---|---|---|---|---|
| DPPH Test | β-Carotene Bleaching Test | |||
| 30 min | 60 min | |||
| Apical shoots | Raw extract | 326.20 ± 1.10 f | 35.27 ± 1.32 d,e | 46.49 ± 1.10 f |
| n-Hexane | >1000 | >100 | >100 | |
| CH2Cl2 | >1000 | 92.32 ± 1.58 i | >100 | |
| AcOEt | 196.10 ± 3.50 d | 10.69 ± 0.26 b | 23.51 ± 0.85 c | |
| H2O | >1000 | >100 | >100 | |
| Branches | Raw extract | 26.14 ± 0.90 b | 3.33 ± 0.16 a | 5.07 ± 0.15 a |
| n-Hexane | 666.73 ± 9.90 g | 60.25 ± 0.63 g | 88.20 ± 1.96 i | |
| CH2Cl2 | 271.27 ± 8.23 e | 38.27 ± 0.78 e | 68.66 ± 1.83 h | |
| AcOEt | 15.67 ± 0.16 a,b | 11.35 ± 0.15 b | 33.54 ± 1.26 d | |
| H2O | 60.72 ± 1.78 c | 36.39 ± 0.84 d,e | 92.26 ± 3.35 i | |
| Ascorbic acid * | 2.00 ± 0.01 a | - | - | |
| Propyl gallate * | - | 1.00 ± 0.02 a | 1.00 ± 0.02 a | |
| Sample | Fraction | IC50 (μg/mL) |
|---|---|---|
| Apical shoots | Raw extract | n.a. |
| n-Hexane | 84.40 ± 1.54 b | |
| CH2Cl2 | n.a. | |
| AcOEt | n.a. | |
| H2O | n.a. | |
| Branches | Raw extract | 213.20 ± 6.14 c |
| n-Hexane | 43.52 ± 2.34 a | |
| CH2Cl2 | 50.68 ± 3.63 a | |
| AcOEt | n.a. | |
| H2O | n.a. | |
| Indomethacin * | 53.00 ± 0.81 a | |
| L-NAME * | 45.86 ± 0.46 a |
| Sample | Fraction | IC50 (mg/mL) |
|---|---|---|
| Apical shoots | Raw extract | 1.33 ± 0.05 c |
| H2O | 4.20 ± 0.18 d | |
| Branches | Raw extract | 0.62 ± 0.02 b |
| H2O | n.a. | |
| Orlistat * | 0.018 ± 0.001 a |
| Sample | Fraction | IC50 (µg/mL) |
|---|---|---|
| Apical shoots | Raw extract | 139.00 ± 1.50 d |
| n-Hexane | 198.17 ± 2.15 f | |
| CH2Cl2 | 161.90 ± 1.61 e | |
| AcOEt | 22.05 ± 0.29 b | |
| H2O | 47.32 ± 1.43 c | |
| Acarbose * | - | 12.68 ± 0.54 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fucile, M.; Lupia, C.; Armentano, M.; Marrelli, M.; Zicarelli, L.; Toma, C.-C.; Statti, G.; Conforti, F. Anti-Obesity and Weight Management-Related Antioxidant Potential Properties of Calabrian Pine Extracts: Pinus nigra Subsp. laricio (Poir.) Maire. Plants 2025, 14, 851. https://doi.org/10.3390/plants14060851
Fucile M, Lupia C, Armentano M, Marrelli M, Zicarelli L, Toma C-C, Statti G, Conforti F. Anti-Obesity and Weight Management-Related Antioxidant Potential Properties of Calabrian Pine Extracts: Pinus nigra Subsp. laricio (Poir.) Maire. Plants. 2025; 14(6):851. https://doi.org/10.3390/plants14060851
Chicago/Turabian StyleFucile, Mary, Carmine Lupia, Martina Armentano, Mariangela Marrelli, Ludovica Zicarelli, Claudia-Crina Toma, Giancarlo Statti, and Filomena Conforti. 2025. "Anti-Obesity and Weight Management-Related Antioxidant Potential Properties of Calabrian Pine Extracts: Pinus nigra Subsp. laricio (Poir.) Maire" Plants 14, no. 6: 851. https://doi.org/10.3390/plants14060851
APA StyleFucile, M., Lupia, C., Armentano, M., Marrelli, M., Zicarelli, L., Toma, C.-C., Statti, G., & Conforti, F. (2025). Anti-Obesity and Weight Management-Related Antioxidant Potential Properties of Calabrian Pine Extracts: Pinus nigra Subsp. laricio (Poir.) Maire. Plants, 14(6), 851. https://doi.org/10.3390/plants14060851









