The Relationship Between Phenological Characteristics and Life Forms Within Temperate Semi-Natural Grassland Ecosystems in the Central Himalaya Region of India
Abstract
:1. Introduction
2. Results
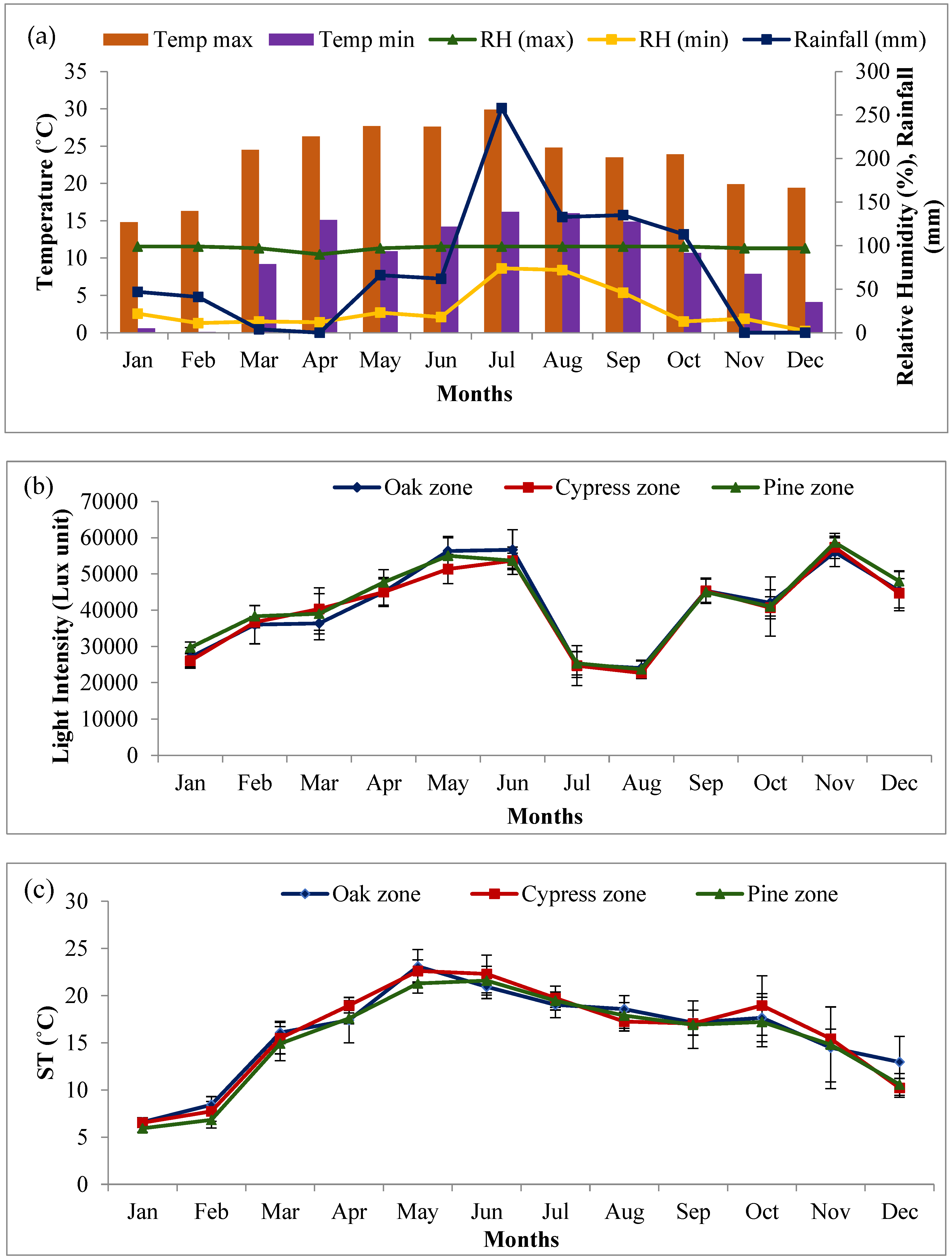
2.1. Site Characteristics
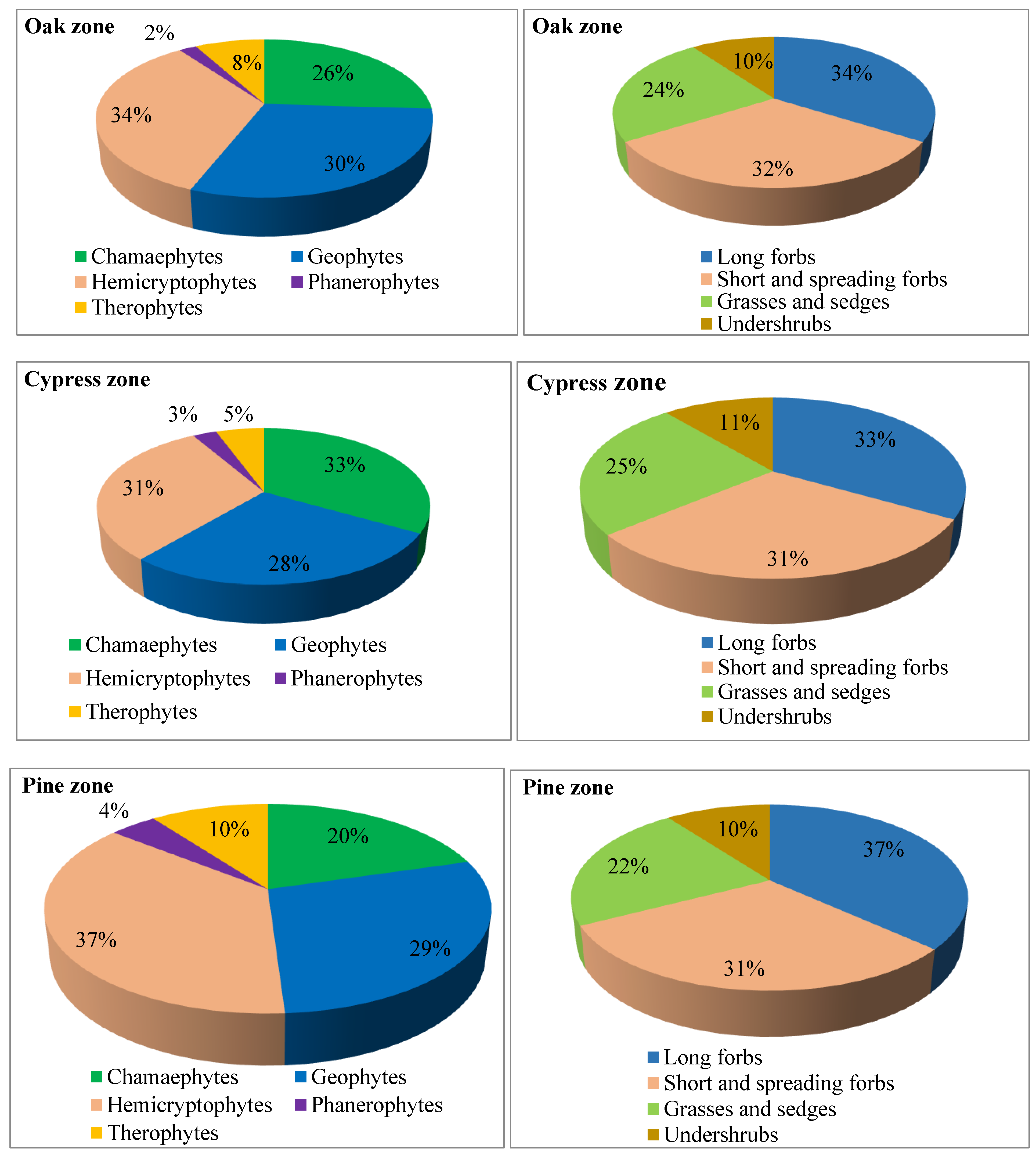
2.2. Floristic Composition and Life Forms
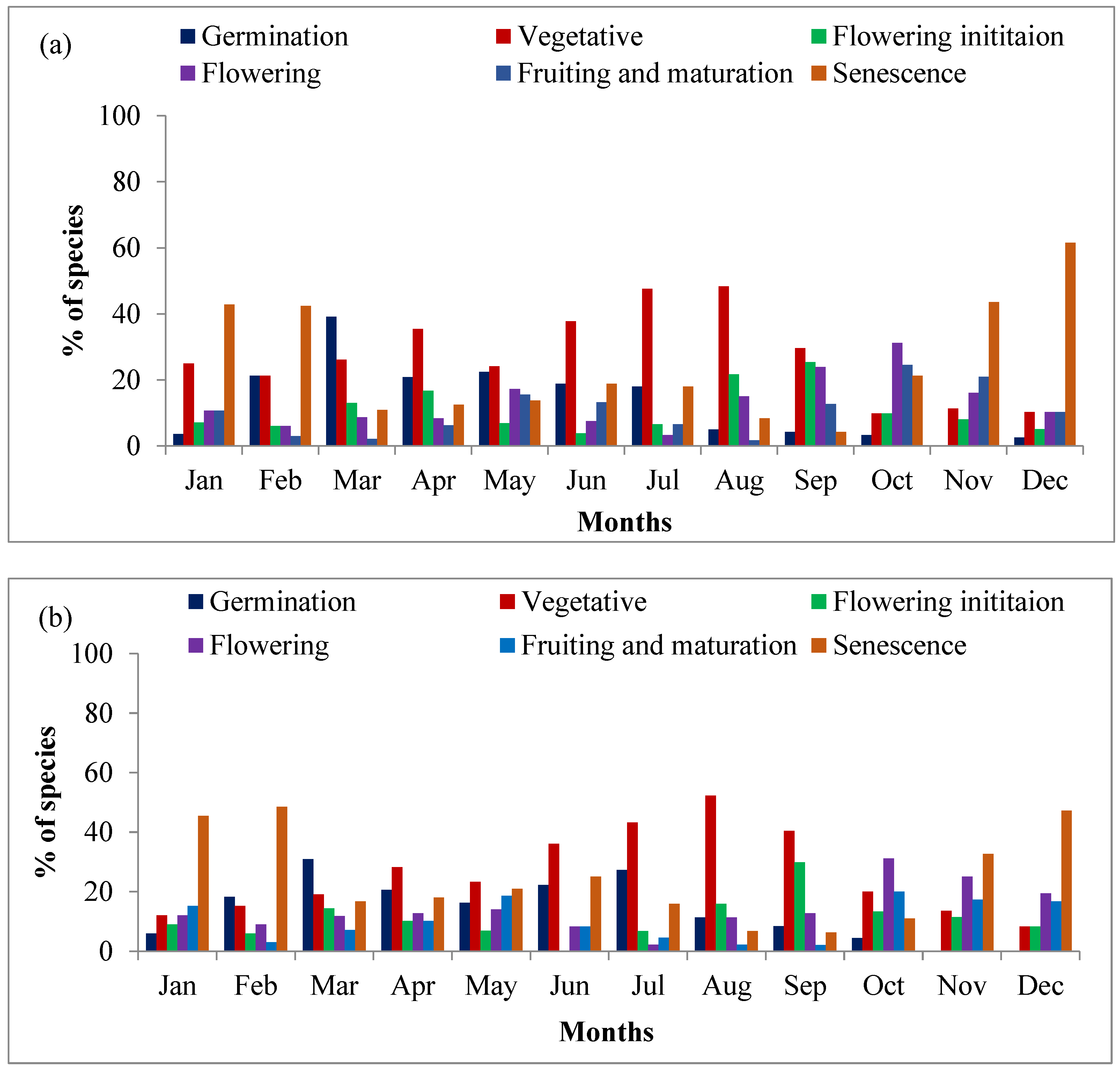
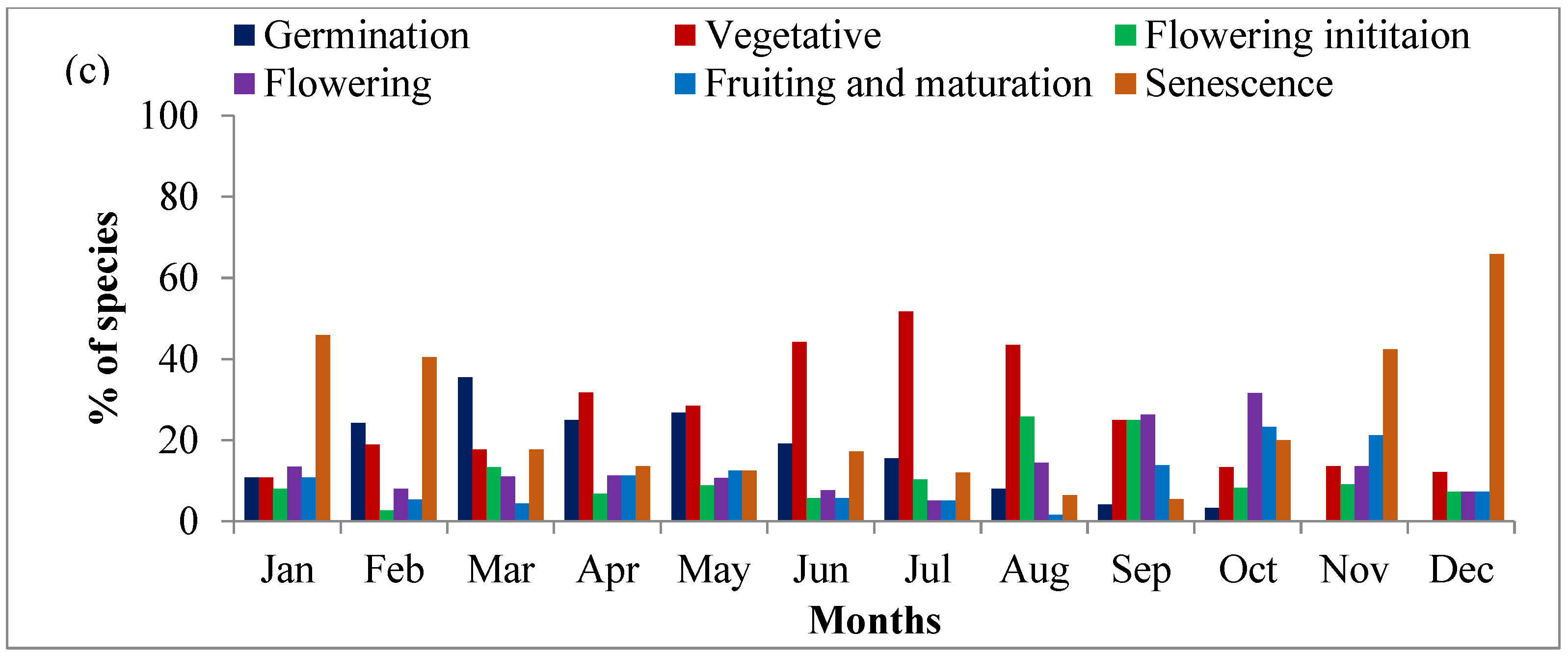
2.3. Community Level Phenology
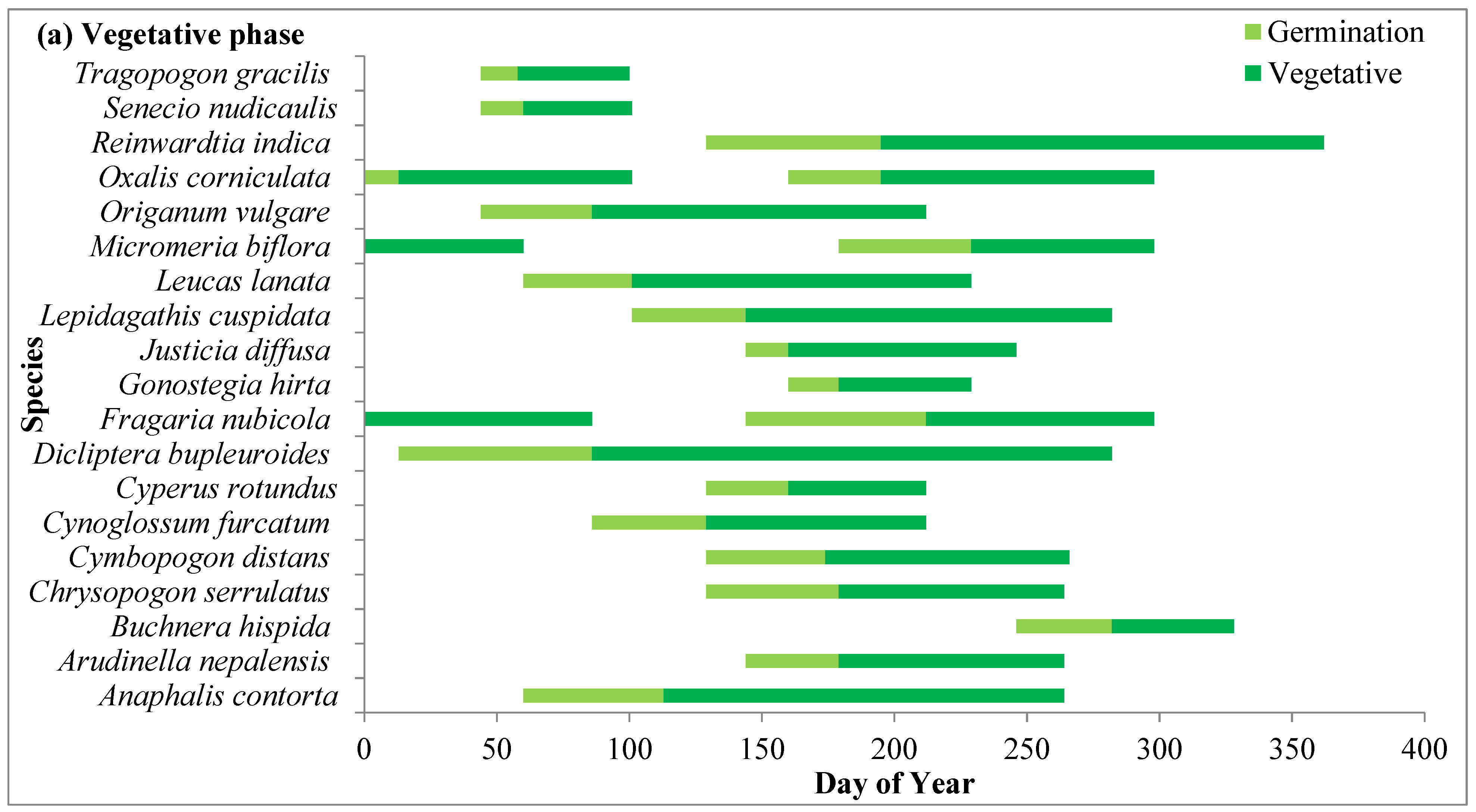
2.4. Phenological Attributes of Species
2.4.1. Vegetative Phase
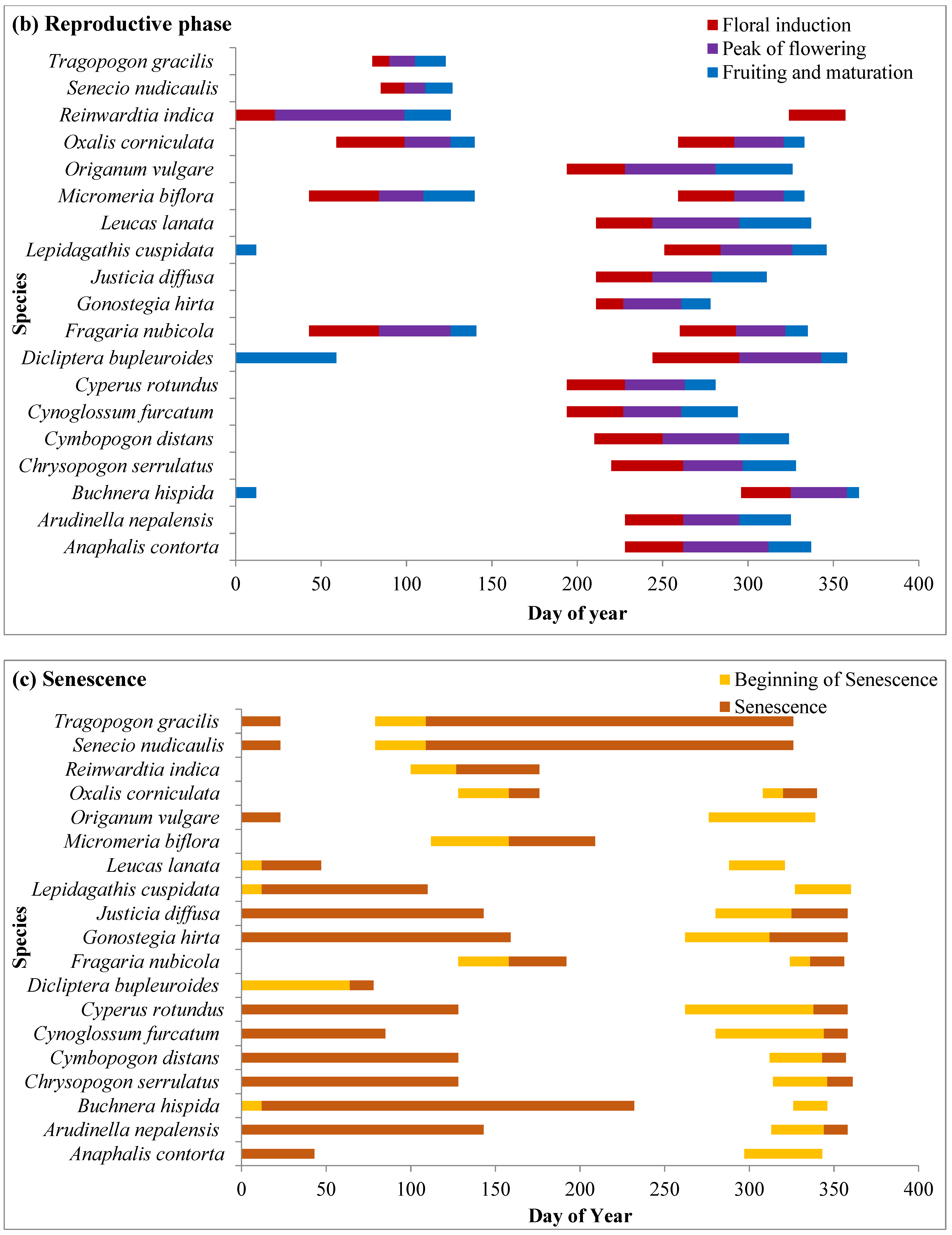
2.4.2. Reproductive Phase
2.4.3. Senescence Phase
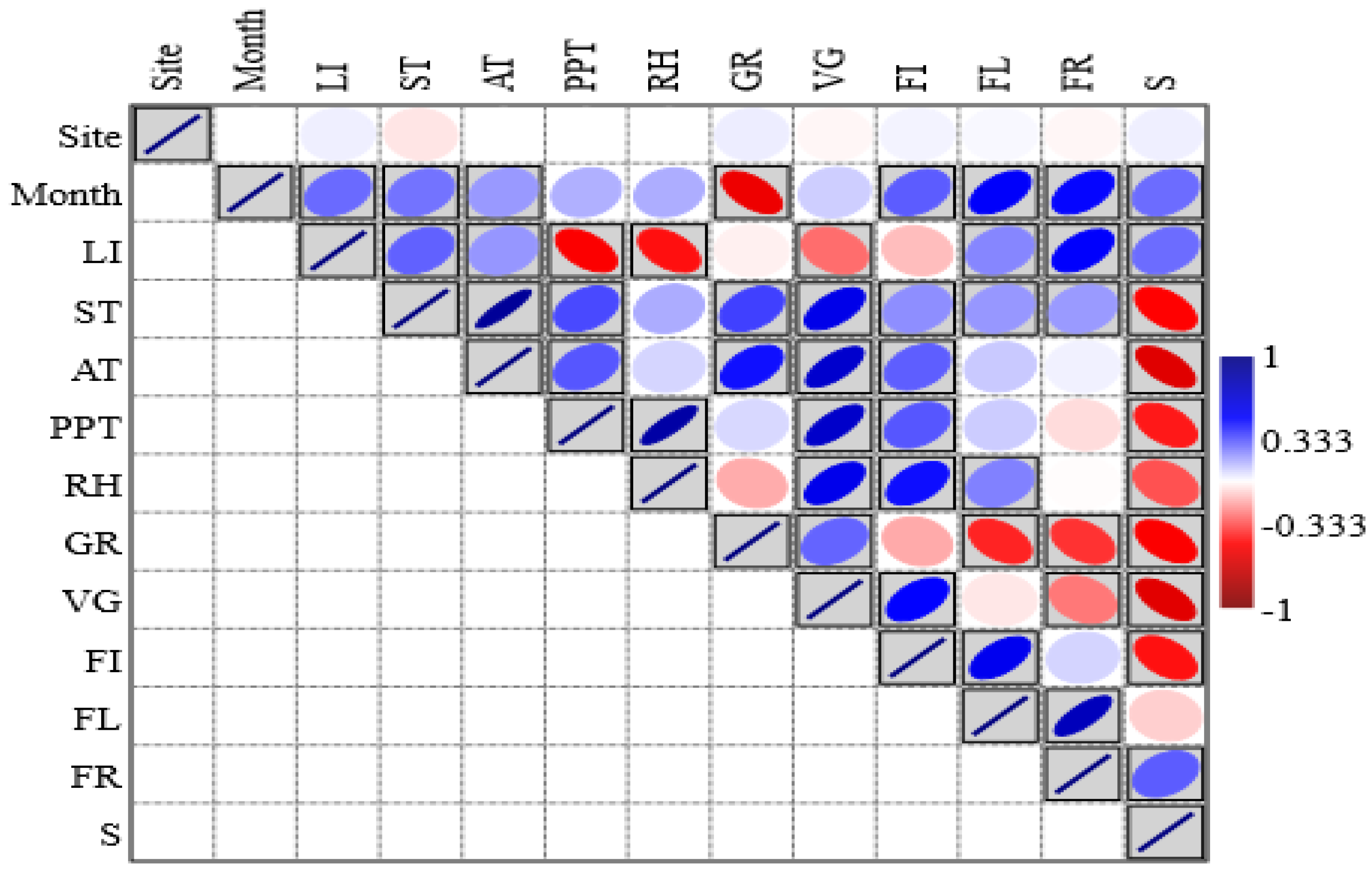
2.5. Relationship Between Phenological Attributes and Growth Forms
2.6. Relationship Between Phenological Attributes and Climatic Conditions
3. Discussion
4. Materials and Methods
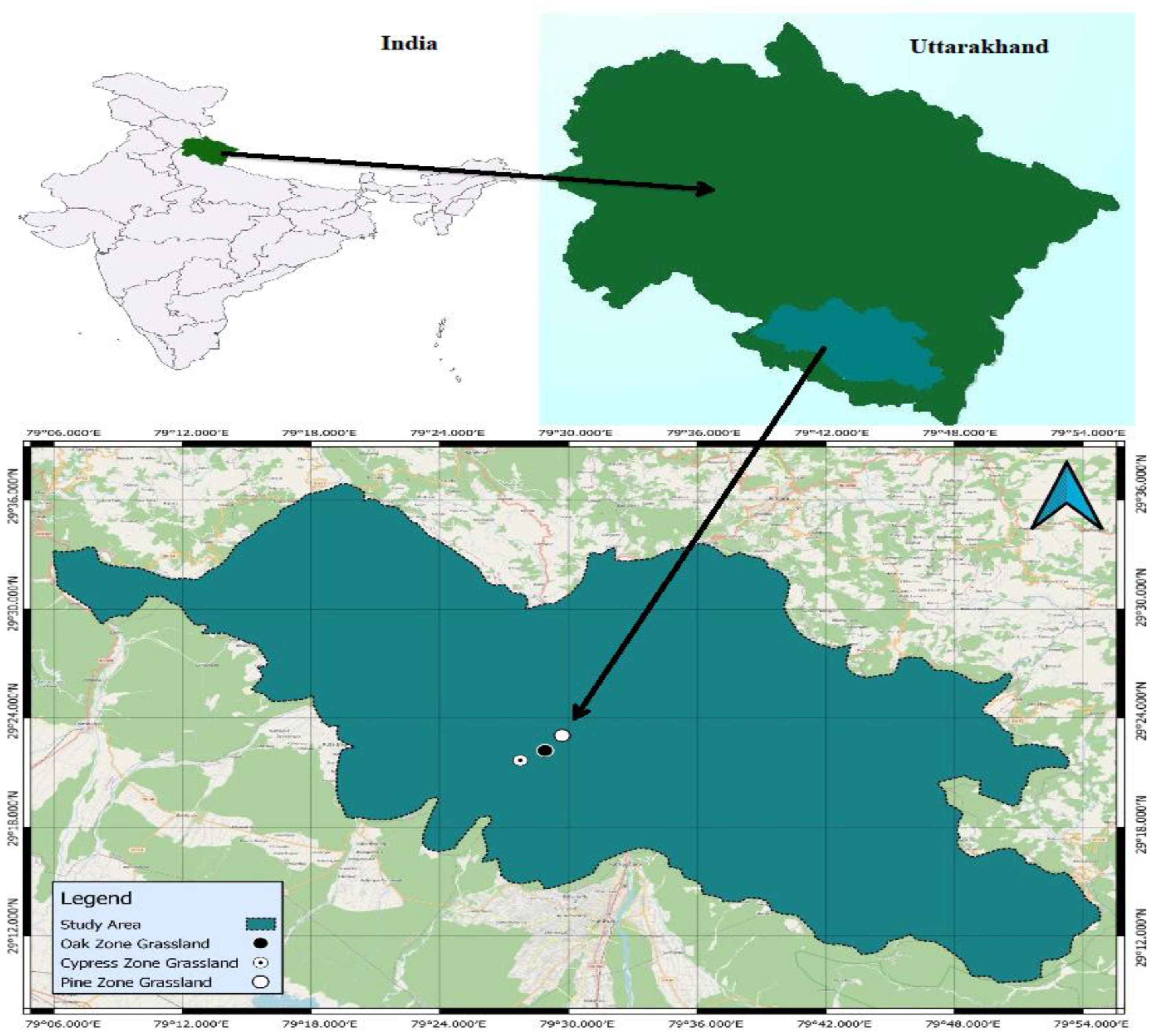
4.1. Study Area
4.2. Data Collection and Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, N.; Tewari, A.; Shah, S.; Mittal, A. Phenology and water relations of tree line species of Western Himalaya, India. Braz. J. Bot. 2024, 47, 343–354. [Google Scholar] [CrossRef]
- Hussain, M.; Alam, J.; Shah, G.; Majid, A.; Shaheen, H.; Habib, T.; Khan, S. Floristic inventory, biological spectra and phenology of monocots of the Musk Deer National Park, Azad Jammu and Kashmir, Pakistan. Sylwan 2021, 165, 164–183. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University press: Oxford, UK, 1934. [Google Scholar]
- Duparc, A.; Redjadj, C.; Viard-Crétat, F.; Lavorel, S.; Austrheim, G.; Loison, A. Co-variation between plant above-ground biomass and phenology in sub-alpine grasslands. Appl. Veg. Sci. 2013, 16, 305–316. [Google Scholar] [CrossRef]
- Shen, X.; Liu, B.; Henderson, M.; Wang, L.; Wu, Z.; Wu, H.; Lu, X. Asymmetric effects of day time and night time warming on spring phenology in the temperate grasslands of China. Agric. For. Meteorol. 2018, 259, 240–249. [Google Scholar] [CrossRef]
- Ruml, M.; Vulić, T. Importance of phenological observations and predictions in agriculture. J. Agric. Sci. 2005, 50, 217–225. [Google Scholar] [CrossRef]
- Richardson, A.D.; Anderson, R.S.; Arain, M.A.; Barr, A.G.; Bohrer, G.; Chen, G.; Xue, Y. Terrestrial biosphere models need better representation of vegetation phenology: Results from the North American Carbon Program Site Synthesis. Glob. Change Biol. 2012, 18, 566–584. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chiariello, N.R.; Loarie, S.R.; Mooney, H.A.; Field, C.B. Diverse responses of phenology to global changes in a grassland ecosystem. Proc. Natl. Acad. Sci. USA 2006, 103, 13740–13744. [Google Scholar] [CrossRef] [PubMed]
- Morisette, J.T.; Richardson, A.D.; Knapp, A.K.; Fisher, J.I.; Graham, E.A.; Abatzoglou, J.; Liang, L. Tracking the rhythm of theseasons in the face of global change: Phenological research in the 21st century. Front. Ecol. Environ. 2009, 7, 253–260. [Google Scholar] [CrossRef]
- Tang, J.; Körner, C.; Muraoka, H.; Piao, S.; Shen, M.; Thackeray, S.J.; Yang, X. Emerging opportunities and challenges in phenology: A review. Ecosphere 2016, 7, e01436. [Google Scholar] [CrossRef]
- Hussain, F.; Shah, S.M.; Badshah, L.; Durrani, M.J. Diversity and ecological characteristics of flora of Mastujvalley, district Chitral, Hindukush range, Pakistan. Pak. J. Bot. 2015, 47, 495–510. [Google Scholar]
- Surmal, O.; Singh, B.; Musarella, C.M. Understanding species diversity, phenology and environmental implications of different life forms in coniferous forests: A case study from Bhallesa Hills of Pir Panjal Mountain, Western Himalaya, India. Forests 2022, 13, 2050. [Google Scholar] [CrossRef]
- Gillison, A.N. Plant functional indicators of vegetation response to climate change, past present and future: I. Trends, emerging hypotheses and plant functional modality. Flora 2019, 254, 12–30. [Google Scholar] [CrossRef]
- Saini, A.; Singh, B. Tribal Knowledge on Ethnobotanical Plants of Uttarakhand Himalaya: Review Research Documentary. In Plants for Novel Drug Molecules-Ethnobotany to Ethnopharmacology; Singh, B., Sharma, Y.P., Eds.; New India Publishing Agency: New Delhi, India, 2021; pp. 91–145. [Google Scholar]
- Rana, T.S.; Datt, B.; Rao, R.R. Life-forms and biological spectrum of the flora of Tons valley, Garhwal Himalaya (Uttaranchal), India. Taiwania 2002, 47, 164–169. [Google Scholar]
- AbdEl-Ghani, M.M.; Amer, W.M. Soil–vegetation relationships in a coastal desert plain of southern Sinai, Egypt. J. Arid. Environ. 2003, 55, 607–628. [Google Scholar] [CrossRef]
- Haq, S.M.; Malik, A.H.; Khuroo, A.A.; Rashid, I. Floristic composition and biological spectrum of Keran-a remote valley of north western Himalaya. Acta Ecol. Sin. 2019, 39, 372–379. [Google Scholar] [CrossRef]
- Nautiyal, M.C.; Nautiyal, B.P.; Prakash, V. Phenology and growth form distribution in an alpine pasture at Tungnath, Garhwal, Himalaya. Mt. Res. Dev. 2001, 21, 168–174. [Google Scholar] [CrossRef]
- Badshah, L.; Hussain, F.; Sher, Z. Floristic inventory, ecological characteristics and biological spectrum of rangeland, districtTank, Pakistan. Pak. J. Bot. 2013, 45, 1159–1168. [Google Scholar]
- Al-Yemini, M.; Sher, H. Biological spectrum with some other ecological attributes of the flora and vegetation of the Asir Mountain of South West, Saudi Arabia. Afr. J. Biotechnol. 2010, 9, 5550–5559. [Google Scholar]
- Pandit, B.R.; Pahurkar, A.J. Vegetational studies in Piram Island near Bhavnagar (Gujarat). Flora Fauna 1998, 4, 79–85. [Google Scholar]
- Thakur, A.S. Floristic composition, life-forms and biological spectrum of tropical dry deciduous forest in Sagar District, Madhya Pradesh, India. Trop. Plant Res. 2015, 2, 112–119. [Google Scholar]
- Saha, G. Spermatophytic flora of Gorumara National Park in the Duars of West Bengal, India. Ph.D. Thesis, University of North Bengal, West Bengal, India, 2017. [Google Scholar]
- Sahoo, A.K.; Barik, K.L. Anthropogenic active grassland communities: Floral diversity and biological spectrum analysis in Odisha. Ecol. Front. 2024, 44, 673–683. [Google Scholar] [CrossRef]
- Bernier, G. The control of floral evocation and morphogenesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 175–219. [Google Scholar] [CrossRef]
- Ansquer, P.; AlHaj-Khaled, R.; Cruz, P.; Theau, J.P.; Therond, O.; Duru, M. Characterizing and predicting plant phenology in species-rich grasslands. Grass Forage Sci. 2009, 64, 57–70. [Google Scholar] [CrossRef]
- Gremer, J.R.; Bradford, J.B.; Munson, S.M.; Duniway, M.C. Desert grassland responses to climate and soil moisture suggest divergent vulnerabilities across the southwestern United States. Glob. Change Biol. 2015, 21, 4049–4062. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D.; Hanes, J.M.; Liang, L. Comparing carbon flux and high-resolution spring phenological measurements in a northern mixed forest. Agric. For. Meteorol. 2013, 169, 136–147. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Renner, S.S.; Chmielewski, F.M. The International Phenological Garden network (1959 to 2021): Its 131 gardens, cloned study species, data archiving, and future. Int. J. Biometeorol. 2022, 66, 35–43. [Google Scholar] [CrossRef]
- Chang, J.; Ciais, P.; Viovy, N.; Soussana, J.F.; Klumpp, K.; Sultan, B. Future productivity and phenology changes in European grasslands for different warming levels: Implications for grassland management and carbon balance. Carbon Balance Manag. 2017, 12, 11. [Google Scholar] [CrossRef]
- Fartyal, A.; Bargali, S.S.; Bargali, K. The effect of different slope aspects on plant diversity and soil characteristics in a temperate grassland of Kumaun Himalaya. Vegetos 2024, 37, 286–295. [Google Scholar] [CrossRef]
- Tiscornia, G.; Jaurena, M.; Baethgen, W. Drivers, process, and consequences of native grass land degradation: Insights from a literature review and a Survey in Ríodel a Plata grasslands. Agronomy 2019, 9, 239. [Google Scholar] [CrossRef]
- Kalakoti, B.S. Flora of Nainital District; Avichal Prakashan: Haldwani, India, 2022. [Google Scholar]
- Gupta, R.K. Flora Nainitalensis; Navyug Traders: New Delhi, India, 1968. [Google Scholar]
- Ralhan, P.K.; Khanna, R.K.; Singh, S.P.; Singh, J.S. Phenological characteristics of the tree layer of Kumaun Himalayan forests. Vegetatio 1985, 60, 91–101. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Zhou, L.; Ciais, P.; Zhu, B. Variations in satellite-derived phenology in China’s temperate vegetation. Glob. Chang. Biol. 2006, 12, 672–685. [Google Scholar] [CrossRef]
- Piao, S.; Mohammat, A.; Fang, J.; Cai, Q.; Feng, J. NDVI-based increase in growth of temperate grasslands and its responses to climate changes in China. Glob. Environ. Chang. 2006, 16, 340–348. [Google Scholar] [CrossRef]
- Cong, N.; Piao, S.L.; Chen, A.P.; Wang, X.H.; Lin, X.; Chen, S.P.; Han, S.J.; Zhou, G.S.; Zhang, X.P. Spring vegetationgreen-update in China inferred from SPOTNDVI data: A multiple model analysis. Agric. For. Meteorol. 2012, 165, 104–113. [Google Scholar] [CrossRef]
- Archibold, O.W. Ecology of World Vegetation; Chapman and Hall: London, UK, 1995; p. 510. [Google Scholar]
- Jayanthi, J.; Jalal, J.S. Life-forms and Biological spectrum of semi-arid grassland: Great Indian Bustard Wildlife Sanctuary, Maharashtra. Phytotaxonomy 2015, 14, 44–60. [Google Scholar]
- Shimwell, D.W. The Description and Classification of Vegetation; University of Washington Press: Seattle, WA, USA, 1971; p. 322. [Google Scholar]
- Cain, S.A.; Castro, G.D. Manual of Vegetation Analysis; Harper and Brothers: New York, NY, USA, 1959. [Google Scholar]
- Horbach, S.; Rauschkolb, R.; Römermann, C. Flowering and leaf phenology are more variable and stronger associated to functional traits in herbaceous compared to tree species. Flora 2023, 300, 152218. [Google Scholar] [CrossRef]
- Singh, U.N.; Ambasht, R.S. Biotic stress and variability in structure and organic (net primary) production of grassland communities at Varanasi, India. Trop. Ecol. 1975, 16, 86–95. [Google Scholar]
- Singh, M.P.; Joshi, M.C. Concentration, standing state and cycling of nitrogen insoil-vegetation components of sand dune herbaceous vegetation around Pilani, Rajasthan, India. Canad. J. Bot. 1985, 63, 2350–2356. [Google Scholar] [CrossRef]
- Singh, J.S.; Yadava, P.S. Seasonal variation in composition, plant biomass, and net primary productivity of a tropical grassland at Kurukshetra, India. Ecol. Monogr. 1974, 44, 351–376. [Google Scholar] [CrossRef]
- Rout, P.K.; Barik, K.L. Life forms and biological spectrum of a grassland community of Bangiriposi in Odisha. Int. J. Sci. Res. Sci. Technol. 2015, 1, 90–94. [Google Scholar]
- Singh, J.S. Seasonal Variation in Composition, Plant Biomass and net Community Production in the Grassland at Varanasi. Ph.D. Thesis, Banaras Hindu University, India, 1967. [Google Scholar]
- Rao, C.C. Biological Spectrum of Karamnasa Watershed flora (Varanasi, India). In Proceedings of the Symposium on Recent Advances in Tropical Ecology (Part-II); Misra, R., Gopal., B., Eds.; ISTE, BHU: Varanasi, India, 1968. [Google Scholar]
- Misra, M.K.; Misra, B.N. Biological spectrum of a tropical grassland community at Berhampur. Indian J. For. 1979, 2, 313–315. [Google Scholar]
- Malana, M.; Misra, B.N. Effect of burning on biological spectrum of tropical grassland. Geobios 1980, 7, 293–295. [Google Scholar]
- Rath, S.P.; Misra, B.N. Effect of grazing on the floristic composition and life form of species in the grassland of Berhampur. Indian J. For. 1980, 3, 336–339. [Google Scholar]
- Naik, B.K. Phytosociology and Primary Production of a Natural Grassland Community of Western Orissa. Ph.D. Thesis, Sambalpur University, Sambalpur, Orissa, India, 1985. [Google Scholar]
- Patnaik, S.K. Ecological Studies of an Upland Coastal Grassland of South Orissa. Ph.D. Thesis, Berhampur University, Berhampur, Orissa, India, 1993. [Google Scholar]
- Behera, R.K.; Misra, B.N. Biological spectrum of a grassland community of Phulbani (India). Mendel 1993, 10, 59–61. [Google Scholar]
- Pradhan, D. Primary Production and Phytosociology of a Grassland Community of Bhubaneswar. Ph.D. Thesis, Berhampur University, Berhampur, Orissa, India, 1994. [Google Scholar]
- Barik, K.L.; Misra, B.N. Biological spectrum of a grassland ecosystem of south Orissa. Ecoprint 1998, 5, 73–77. [Google Scholar]
- Devi, A.R.; Sharma, B.M.; Sharma, H.M. Analysis of life-forms and biological spectrum of the grassland communities in Thoubal district, Manipur. Indian J. Environ. Ecoplann. 2004, 8, 97–101. [Google Scholar]
- Xie, J.; Jonas, T.; Rixen, C.; de Jong, R.; Garonna, I.; Notarnicola, C.; Asam, S.; Michael, E.; Schaepmana, M.E.; Kneubühler, M. Land surface phenology and greenness in Alpine grasslands driven by seasonal snow and meteorological factors. Sci. Total Environ. 2020, 725, 138380. [Google Scholar] [CrossRef]
- Blois, J.L.; Zarnetske, P.L.; Fitzpatrick, M.C.; Finnegan, S. Climate change and the past, present, and future of biotic interactions. Science 2013, 341, 499–504. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Upadhaya, K.; Mir, A.H.; Iralu, V. Reproductive phenology and germination behavior of some important tree species of North east India. Proc. Natl. Acad. Sci. India Sec. B Biol. Sci. 2018, 88, 1033–1041. [Google Scholar] [CrossRef]
- Upadhaya, K.; Barik, S.K.; Adhikari, D.; Baishya, R.; Lakadong, N.J. Regeneration ecology and population status of acritically endangered and endemic tree species (Ilex khasiana Purk.) inNorth-eastern India. J. For. Res. 2009, 20, 223–228. [Google Scholar]
- Currier, C.M.; Sala, O.E. Precipitation versus temperature as phenology controls in drylands. Ecology 2022, 103, e3793. [Google Scholar] [CrossRef]
- Ram, J.; Singh, S.P.; Singh, J.S. Community level phenology of grassland above treeline in central Himalaya, India. Arct. Alp. Res. 1988, 20, 325–332. [Google Scholar] [CrossRef]
- Kala, C.P. Ecology and Conservation of Alpine Meadows in the Valley of Flowers National Park, Garhwal Himalaya; Wildlife Institute of India: Dehradun, India, 1998; p. 99. [Google Scholar]
- Pangtey, Y.P.S.; Rawal, R.S.; Bankoti, N.; Samant, S.S. Phenology of high-altitude plants of Kumaun in Central Himalaya, India. Int. J. Biometeorol. 1990, 34, 122–127. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of Low-Temperature Stress on Germination, Growth, and Phenology of Plants: A Review. In Physiological Processes in Plants Under Low Temperature Stress; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Negi, B.; Khatri, K.; Bargali, S.S.; Bargali, K.; Fartyal, A. Phenological behaviour of Ageratina adenophora compared with native herb species across varied habitats in the Kumaun Himalaya. Plant Ecol. 2025. [Google Scholar] [CrossRef]
- Abu-Asab, M.S.; Peterson, P.M.; Shetler, S.G.; Orli, S.S. Earlier plant flowering in spring as a response to global warming in the Washington, DC, area. Biodivers. Conserv. 2001, 10, 597–612. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dimmitt, M.A. Flowering phenology of six woody plants in the northern Sonoran Desert. Bull. Torrey Bot. Club 1994, 121, 215–229. [Google Scholar] [CrossRef]
- Ahmad, M.; Uniyal, S.K.; Batish, D.R.; Rathee, S.; Sharma, P.; Singh, H.P. Flower phenological events and duration patternis influenced by temperature and elevation in Dhauladhar mountain range of Lesser Himalaya. Ecol. Indic. 2021, 129, 107902. [Google Scholar] [CrossRef]
- Knapp, A.K.; McCarron, J.K.; Silletti, A.M.; Hoch, G.A.; Heisler, J.C.; Lett, M.S.; Smith, M.D. Ecological Consequences of the Replacement of Native Grassland by Juniperus virginiana and Other Woody Plants. In Western North American Juniperus Communities: A Dynamic Vegetation Type; Springer: New York, NY, USA, 2008; pp. 156–169. [Google Scholar]
- Knapp, A.K.; Chen, A.; Griffin-Nolan, R.J.; Baur, L.E.; Carroll, C.J.; Gray, J.E.; Smith, M.D. Resolving the Dust Bowl paradox of grassland responses to extreme drought. Proc. Natl. Acad. Sci. USA 2020, 117, 22249–22255. [Google Scholar] [CrossRef]
- Zelikova, T.J.; Williams, D.G.; Hoenigman, R.; Blumenthal, D.M.; Morgan, J.A.; Pendall, E. Seasonality of soil moisture mediates responses of ecosystem phenology to elevated CO2 and warming in a semi-arid grassland. J. Ecol. 2015, 103, 1119–1130. [Google Scholar] [CrossRef]
- Castillioni, K.; Newman, G.S.; Souza, L.; Iler, A.M. Effects of drought on grassland phenology depend on functional types. New Phytol. 2022, 236, 1558–1571. [Google Scholar] [CrossRef] [PubMed]
- Manila-Fajardo, A.C.; Tubale, R.A.; Estrada, J.L. Floral and Fruiting Phenology in the Lowland Forests of Palanan, Isabela, Philippines. In Plant Diversity in Biocultural Landscapes; Springer Nature: Singapore, 2023; pp. 277–299. [Google Scholar]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Bairlein, F. Ecological responses to recent climatechange. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sah, N.; Kumar, M.; Upadhyay, R.; Dutt, S. Hill slope instability of Nainital City, Kumaun Lesser Himalaya, Uttarakhand, India. J. Rock. Mech. Geotech. Eng. 2018, 10, 280–289. [Google Scholar] [CrossRef]
- Singh, S.P. Structure and Function of the Low and High Altitude Grazing-Land Ecosystems and the Impact of Livestock Component in the Central Himalaya. In Final report (Department of Environment and Forests, Government of India, Ref. No. 14/5/87-ER); Kumaun University: Nainital, India, 1991. [Google Scholar]
- Joshi, M. Seasonal changes in species diversity, phytomass and net primary production in a temperate grassland in Kumaun, central Himalaya, India. J. Trop. For. Sci. 1995, 7, 521–531. [Google Scholar]
- Champion, H.G.; Seth, S.K. A Revised Survey of the Forest Types of India; Government of India Publications: New Delhi, India, 1968. [Google Scholar]
- Rawat, G.S. Temperate and alpine grasslands of the Himalaya: Ecology and conservation. Parks 1998, 8, 27–36. [Google Scholar]
- Manral, V.; Bargali, K.; Bargali, S.S.; Shahi, C. Changes in soil biochemical properties following replacement of Banj oak forest with Chir pine in Central Himalaya, India. Ecol. Process. 2020, 9, 30. [Google Scholar] [CrossRef]
- Negi, B.; Khatri, K.; Bargali, S.S.; Bargali, K.; Fartyal, A.; Chaturvedi, R.K. Impact of invasive Ageratina adenophora on relative performance of woody vegetation in different forest ecosystems of Kumaun Himalaya, India. J. Mt. Sci. 2023, 20, 2557–2579. [Google Scholar] [CrossRef]
- Pilar, C.D.; Gabriel, M.M. Phenological pattern of fifteen Mediterranean phanaerophytes from shape Quercus ilex communities of NE-Spain. Plant Ecol. 1998, 139, 103–112. [Google Scholar] [CrossRef]




| Scientific Name | Family | Habit | Life Form | Oak Zone | Cypress Zone | Pine Zone | Sources Used for Identification |
|---|---|---|---|---|---|---|---|
| TALL FORB | |||||||
| Achyranthes bidentata Blume | Amaranthaceae | A/P | Th | − | − | + | FON, FN |
| Ageratina adenophora (Spreng.) King & H. Rob. | Asteraceae | P | Ph | + | + | + | FN |
| Anaphalis contorta (D. Don) Hook.f. | Asteraceae | P | Ch | + | + | + | FON, FN |
| Artemisia annua L. | Asteraceae | A | Th | + | + | + | FON |
| Bidens pilosa L. | Asteraceae | A | Th | − | + | + | FN |
| Campanula spp. | Campanulaceae | A/B/P | He | − | − | + | FON, FN |
| Cynoglossum furcatum Wall. | Boraginaceae | A/B | He | + | + | + | FN |
| Dicliptera bupleuroides Nees | Acanthaceae | P | Ch | + | + | + | FON, FN |
| Gonostegia hirta Turcz. | Urticaceae | P | Ge | + | + | + | FON, FN |
| Leucas lanata Benth. | Lamiaceae | P | Ch | + | + | + | FON, FN |
| Nepeta leucophylla Benth. | Lamiaceae | P | Th | − | − | + | FN |
| Nepeta racemosa Lam. | Lamiaceae | P | Th | + | − | − | FON |
| Origanum vulgare L. | Lamiaceae | P | Ch | + | + | + | FON, FN |
| Plectranthus japonicus (Burm.f.) Koidz. | Lamiaceae | P | Ch | + | − | − | FN |
| Plectranthus rugosus Wall. ex Benth. | Lamiaceae | P | Ch | − | + | + | FN |
| Polygonum capitatum Buch.-Ham. ex D. Don. | Polygonaceae | A | Ch | + | − | − | FN |
| Polygonum nepalense Meisn. | Polygonaceae | A | Th | + | − | + | FN |
| Reinwardtia indica Dumort. | Linaceae | P | Ge | + | + | + | FN |
| Roscoea purpurea Sm. | Zingiberaceae | P | Ge | + | − | + | FON, FN |
| Senecio nudicaulis Buch.-Ham. ex D.Don | Asteraceae | P | Ch | + | + | − | FON, FN |
| Swertia chirayita (Roxb.) H. Karst. | Gentinaceae | A | Ch | − | − | + | FN |
| Tragopogon gracilis D. Don | Asteraceae | P/B | Ch | + | − | − | FON, FN |
| Verbascum thapsus L. | Scrophulariaceae | B | He | + | − | + | FON, FN |
| GRASSES AND SEDGES | |||||||
| Arthraxon lanceolatus (Roxb.) Hochst. | Poaceae | P | He | + | + | + | FN |
| Arudinella nepalensis Trin. | Poaceae | P | He | + | + | + | FON, FN |
| Chrysopogon serrulatus Trin. | Poaceae | P | He | + | + | + | FON |
| Cymbopogon distans Nees ex Steud. | Poaceae | P | He | + | + | + | FON, FN |
| Cyperus niveus Retz. | Cyperaceae | P | He | − | − | + | FN |
| Cyperus rotundus L. | Cyperaceae | P | He | + | − | − | FON, FN |
| Digitaria cruciata (Nees) A. Camus | Poaceae | A | He | + | − | + | FON, FN |
| Eulalia mollis (Griseb.) Kuntze | Poaceae | P | He | − | + | − | FON, FN |
| Heteropogon contortus (L.) P. Beauv. ex Roem. & Schult. | Poaceae | P | He | + | + | + | FON |
| Koeleria macrantha (Ledeb.) Schult. | Poaceae | P | He | + | + | − | FN |
| Oplismenus compositus (L.) P. Beauv. | Poaceae | P | He | + | − | + | FON, FN |
| Paspalum dilatatum Poir. | Poaceae | P | He | + | − | − | FON |
| Paspalum distichum L. | Poaceae | P | He | − | + | + | FON |
| Saccharum rufipilum Steud. | Poaceae | P | He | − | + | + | FN |
| Sporobolus diander (Retz.) Beauv. | Poaceae | P | He | + | − | − | FON, FN |
| Sporobolus fertilis (Steud.) W.D.Clayt | Poaceae | P | He | + | − | + | FON |
| SHORT FORBS | |||||||
| Arenaria serpyllifolia L. | Caryophyllaceae | A/P | Ch | − | + | − | FON |
| Buchnera hispida Buch.-Ham. ex D.Don | Orobanchaceae | A | Ch | + | + | + | FON |
| Commelina benghalensis L. | Commelinaceae | A | Ge | + | − | + | FON |
| Curculigo orchioides Gaertn. | Hypoxidaceae | A | Ge | + | − | − | FON |
| Cyanotis vaga D.Don | Commelinaceae | P | Ge | + | + | + | FON |
| Desmodium microphyllum (Thunb.)DC. | Fabaceae | P | He | + | − | + | FON |
| Flemingia bracteata Roxb. | Fabaceae | P | Ge | − | − | + | FN |
| Flemingia procumbens Roxb. | Fabaceae | P | Ge | − | − | + | FON |
| Fragaria vesca L. | Rosaceae | P | Ge | + | + | − | FN |
| Galium aparine L. | Rubiaceae | A | Ch | + | + | − | FON |
| Geranium ocellatum Jacq. ex Cambess. | Geraniaceae | A | He | − | + | + | FN |
| Gerbera gossypina (Royle) Beauverd | Asteraceae | P | Ch | + | − | − | FON |
| Himalaiella heteromallla D. Don | Asteraceae | P | Ch | − | + | + | FON |
| Hypoxis aurea Lour. | Hypoxidaceae | P | Ge | + | − | − | FON |
| Justicia diffusa Willd. | Acanthaceae | P | He | + | + | + | FON |
| Micromeria biflora (Buch.-Ham. ex D.Don) Benth. | Lamiaceae | P | Ge | + | + | + | FON |
| Mimosa spp | Fabaceae | A/P | Ch | + | − | − | FON |
| Nicandra physalodes (L.) Gaertn. | Solanaceae | A | Th | − | − | + | FON |
| Oreocome candollei (Wall. ex DC.) | Apiaceae | P | Th | + | + | − | http://www.flowersofindia.net/ |
| Oxalis corniculata L. | Oxalidaceae | A | Ge | + | + | + | FON, FN |
| Polygala persicariifolia DC. | Polygalaceae | A | He | + | − | + | FON, FN |
| Salvia lanata Roxb. | Lamiaceae | P | Ge | − | − | + | FN |
| Scutellaria scandens D. Don | Lamiaceae | P | Ge | + | − | − | FON |
| Solanum xanthocarpum Schrad. & Wendl | Solanaceae | P | Ge | − | − | + | FN |
| UNDERSHRUBS | |||||||
| Barleria cristata L. | Acanthaceae | P | Ch | + | − | + | FN |
| Cassia occidentalis L. | Fabaceae | P | Ge | + | + | − | FN |
| Crotolaria alata Buch. Ham. Ex Roxb. | Fabaceae | A/P | Ge | + | + | + | FN |
| Desmodium heterocarpon (L.) DC. | Fabaceae | P | Ge | + | − | + | FON, FN |
| Inula cappa (Buch.-Ham. ex D.Don) DC. | Asteraceae | P | Ph | − | − | + | FON, FN |
| Lepidagathis cuspidata Nees | Acanthaceae | P | Ge | + | + | + | FN |
| Lespedeza eriocarpa DC. | Papilionaceae | A | Ch | − | + | − | FN |
| S. No. | Study Area | Ph | Ch | He | Ge | Th | References |
|---|---|---|---|---|---|---|---|
| 1. | - | 46.00 | 9.00 | 26.00 | 6.00 | 13.00 | Raunkiaer [3] |
| 2. | Varanasi | - | 3.10 | 20.30 | 7.80 | 68.70 | Singh [49] |
| 3. | Varanasi | 40.00 | 6.00 | 1.00 | 10.00 | 43.00 | Rao [50] |
| 4. | Kurukshetra | 2.09 | 10.41 | 18.75 | 6.25 | 62.50 | Singh and Yadava [47] |
| 5. | Varanasi | - | 4.20 | 19.20 | 6.30 | 70.20 | Singh and Ambasht [45] |
| 6. | Berhampur | 5.70 | 25.70 | 14.30 | 5.70 | 48.60 | Misra and Misra [51] |
| 7. | Berhampur | 10.00 | 26.66 | 23.33 | 3.33 | 36.33 | Malana and Misra [52] |
| 8. | Berhampur | 5.40 | 21.60 | 18.90 | 2.70 | 51.30 | Rath and Misra [53] |
| 9. | Western Orissa | 3.00 | 21.20 | 18.20 | 6.00 | 51.50 | Naik [54] |
| 10. | South Orissa | 3.58 | 17.86 | 25.00 | 10.71 | 42.86 | Patnaik [55] |
| 11. | Phulbani | 5.71 | 20.00 | 11.42 | 8.57 | 54.28 | Behera and Misra [56] |
| 12. | Bhubaneswar | 5.88 | 29.42 | 11.76 | 5.88 | 47.05 | Pradhan [57] |
| 13. | Berhampur | - | 25.81 | 12.90 | 9.68 | 51.61 | Barik and Misra [58] |
| 14. | Thoubal, Manipur | - | 4.84 | 11.29 | 14.52 | 69.35 | Devi et al. [59] |
| 15. | Semi-arid grassland, Maharashtra | 21.00 | 17.00 | 16.00 | 14.00 | 32.00 | Jyanthi and Jalal [41] |
| 16. | Bangiriposi, Orissa | - | 46.88 | 12.50 | 6.25 | 34.37 | Rout and Barik [48] |
| 17. | Kumaun Himalaya | 2–4.00 | 20–33.00 | 31–37.00 | 28–30.00 | 5–10.00 | Present study |
| Forest Zone | Altitude (m) | Latitude (N) | Longitude (E) | Slope (°) | Aspect | Shannon-Weiner Index (H′) | Simpson’s Index (Cd) | Dominant Tree Species | Dominant Shrub Species |
|---|---|---|---|---|---|---|---|---|---|
| Oak | 1800–1900 | 29°22′14′′ | 79°28′54′′ | 29.8 | South East | 1.72 | 0.21 | Quercus leucotrichophora | Arudinaria falcata |
| Cypress | 1700–1800 | 29°21′41′′ | 79°27′45′′ | 36.2 | North East | 1.79 | 0.24 | Cupressus torulosa | Berberis asiatica |
| Pine | 1700–1800 | 29°23′03′′ | 79°29′42′′ | 31.5 | South | 1.83 | 0.22 | Pinus roxburghii | Randia tetrasperma |
| Zone | Physical Properties of Soil | Chemical Properties of Soil | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Texture | Gravel (g) | Sand (%) | Silt (%) | Clay (%) | bD (g cm−3) | Porosity (%) | WHC (%) | pH | SOC (%) | TN (%) | P (%) | K (%) | |
| Oak | Sandy-clay-loam | 65.87 | 52.15 | 27.83 | 20.02 | 1.31 | 49.31 | 62.91 | 5.9 | 2.93 | 0.32 | 0.0012 | 0.005 |
| Cypress | Clay-loam | 65.49 | 44.02 | 25.05 | 30.92 | 1.22 | 52.85 | 67.75 | 6.4 | 2.96 | 0.26 | 0.0014 | 0.007 |
| Pine | Sandy-loam | 75.92 | 55.49 | 25.97 | 18.54 | 1.24 | 52.10 | 71.16 | 5.9 | 3.67 | 0.29 | 0.0016 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fartyal, A.; Chaturvedi, R.K.; Bargali, S.S.; Bargali, K. The Relationship Between Phenological Characteristics and Life Forms Within Temperate Semi-Natural Grassland Ecosystems in the Central Himalaya Region of India. Plants 2025, 14, 835. https://doi.org/10.3390/plants14060835
Fartyal A, Chaturvedi RK, Bargali SS, Bargali K. The Relationship Between Phenological Characteristics and Life Forms Within Temperate Semi-Natural Grassland Ecosystems in the Central Himalaya Region of India. Plants. 2025; 14(6):835. https://doi.org/10.3390/plants14060835
Chicago/Turabian StyleFartyal, Archana, Ravi Kant Chaturvedi, Surendra Singh Bargali, and Kiran Bargali. 2025. "The Relationship Between Phenological Characteristics and Life Forms Within Temperate Semi-Natural Grassland Ecosystems in the Central Himalaya Region of India" Plants 14, no. 6: 835. https://doi.org/10.3390/plants14060835
APA StyleFartyal, A., Chaturvedi, R. K., Bargali, S. S., & Bargali, K. (2025). The Relationship Between Phenological Characteristics and Life Forms Within Temperate Semi-Natural Grassland Ecosystems in the Central Himalaya Region of India. Plants, 14(6), 835. https://doi.org/10.3390/plants14060835








