Elevated Atmospheric CO2 Concentrations Reduce Tomato Mosaic Virus Severity in Tomato Plants
Abstract
1. Introduction
2. Results
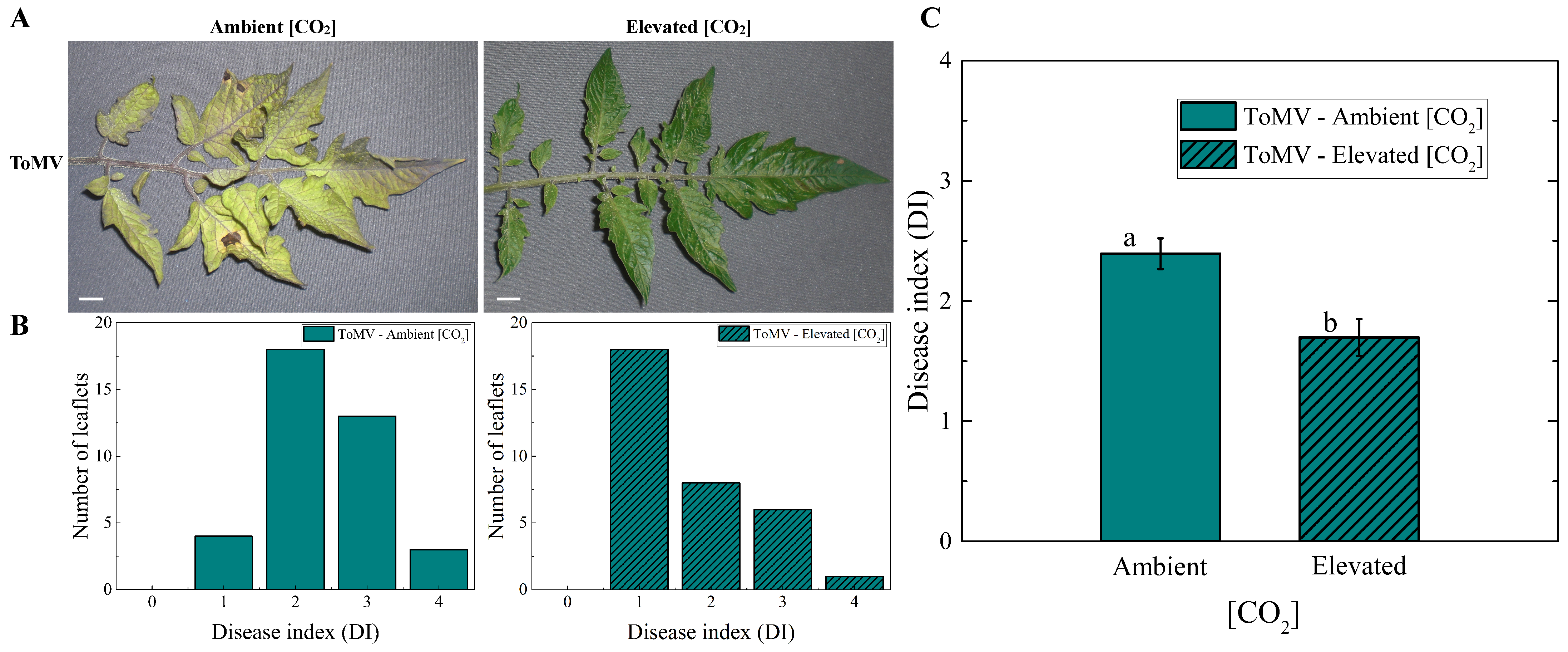
2.1. Plants Grown Under Elevated [CO2] Levels Show Reduced Tomato Mosaic Disease Index
2.2. Elevated [CO2] Enhances Above-Ground Plant Growth in ToMV-Infected Tomato Plants
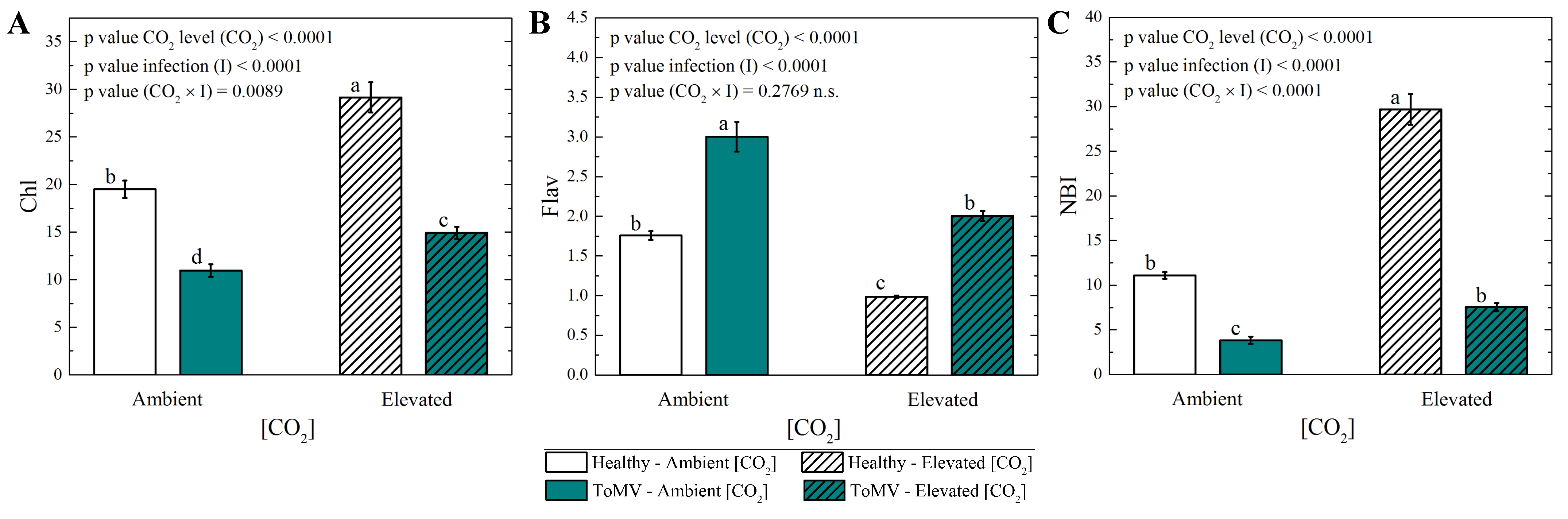
2.3. Effects of Elevated [CO2] on Physiological Parameters in Healthy and ToMV-Infected Tomato Plants
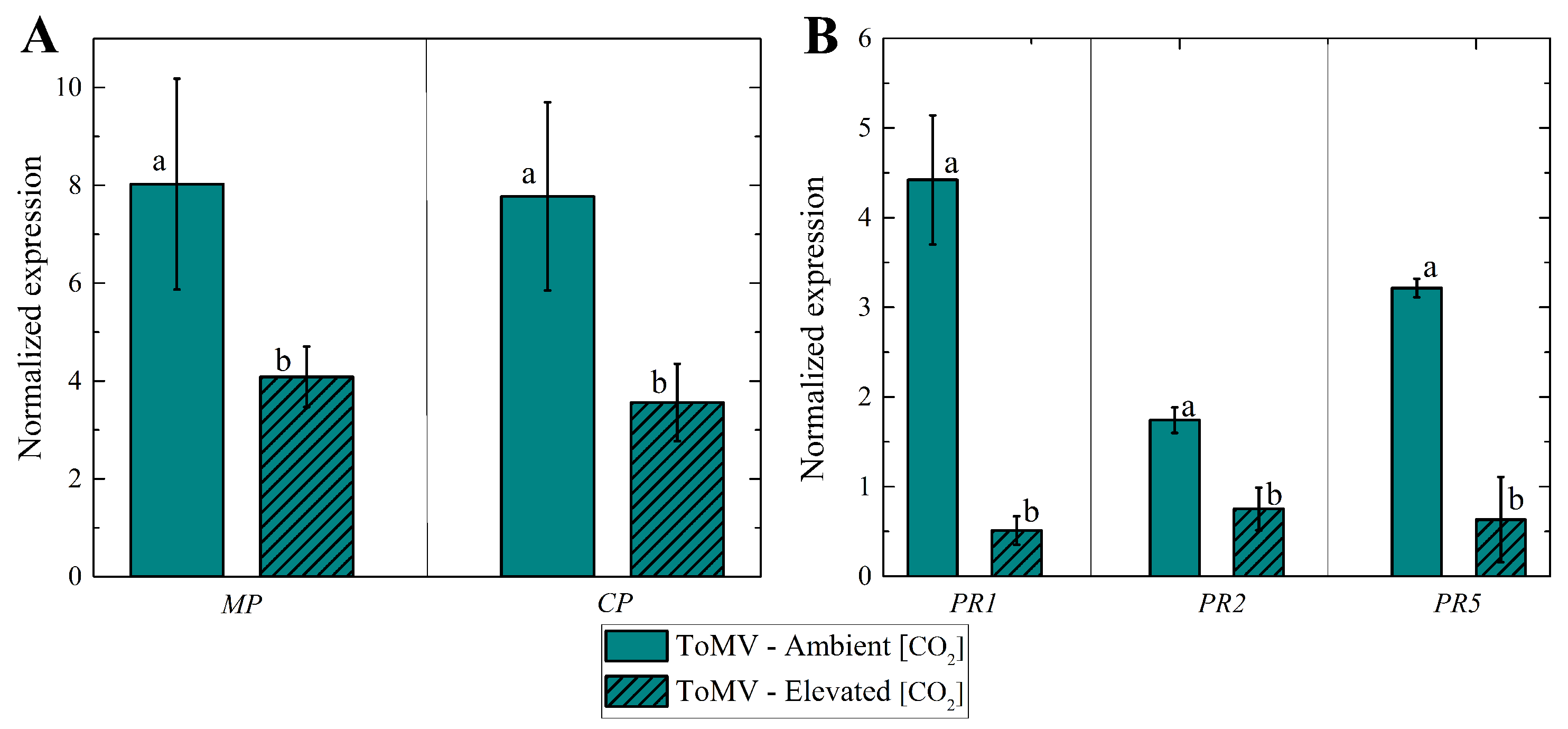
2.4. Effects of Elevated [CO2] on ToMV Expression and Pathogenesis-Related Gene Expression in Infected Tomato Plants
3. Discussion
3.1. Increased [CO2] Reduced the Tomato Mosaic Disease Index
3.2. Elevated [CO2] Promotes Above-Ground Plant Development in ToMV-Infected Tomato Plants
3.3. Impact of Increased [CO2] on Physiological Characteristics in Healthy and ToMV-Infected Tomato Plants
3.4. Impact of Increased [CO2] on ToMV Expression and Pathogenesis-Related Gene Expression in Infected Tomato Plants
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Experimental Site
4.3. Infection Evaluation Through Assessment of Disease Index
4.4. Leaf Area Measurement
4.5. Leaf Gas Exchange and Chlorophyll Fluorescence Measurements
4.6. Measurement of Foliar Chlorophyll, Flavonoids, and Nitrogen
4.7. Total RNA Isolation and Real-Time RT-PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | photosynthesis rate |
| ANOVA | analysis of variance |
| Chl | chlorophyll |
| CMV | cucumber mosaic virus |
| CP | coat protein |
| DI | disease index |
| dpi | days post-inoculation |
| ETR | electron transport rate |
| FACE | free-air CO2 enrichment |
| Flav | flavonoids |
| stomatal conductance | |
| H2S | hydrogen sulfide |
| MP | movement protein |
| NBI | nitrogen balance index |
| OTC | open-top chamber |
| ΦPSII | quantum yield of fluorescence |
| PR | pathogenesis-related |
| PVY | potato virus Y |
| SE | standard error of the mean |
| ToMV | tomato mosaic virus |
| TMV | tobacco mosaic virus |
| TYLCV | tomato yellow leaf curl virus |
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2021—The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar]
- Centritto, M.; Lee, H.S.J.; Jarvis, P.G. Increased growth in elevated [CO2]: An early, short-term response? Glob. Chang. Biol. 1999, 5, 623–633. [Google Scholar] [CrossRef]
- Centritto, M.; Tognetti, R.; Leitgeb, E.; Střelcová, K.; Cohen, S. Above ground processes: Anticipating climate change influences. In Forest Management and the Water Cycle; Ecological studies: Analysis and synthesis; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands, 2010; pp. 31–64. [Google Scholar]
- Poorter, H.; Knopf, O.; Wright, I.J.; Temme, A.A.; Hogewoning, S.W.; Graf, A.; Cernusak, L.A.; Pons, T.L. A meta-analysis of responses of C3 plants to atmospheric CO2: Dose-response curves for 85 traits ranging from the molecular to the whole-plant level. New Phytol. 2022, 233, 1560–1596. [Google Scholar] [CrossRef]
- Bhadra, P.; Maitra, S.; Shankar, T.; Hossain, A.; Praharaj, S. Chapter 1—Climate change impact on plants: Plant responses and adaptations. In Plant Perspectives to Global Climate Changes; Aftab, T., Roychoudhury, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–24. [Google Scholar]
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the Mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Siddell, S.G.; Smith, D.B.; Adriaenssens, E.; Alfenas-Zerbini, P.; Dutilh, B.E.; Garcia, M.L.; Junglen, S.; Krupovic, M.; Kuhn, J.H.; Lambert, A.J.; et al. Virus taxonomy and the role of the International Committee on Taxonomy of Viruses (ICTV). J. Gen. Virol. 2023, 104, 001840. [Google Scholar] [CrossRef] [PubMed]
- Genus: Tobamovirus. Available online: https://ictv.global/report/chapter/virgaviridae/virgaviridae/tobamovirus (accessed on 29 January 2025).
- Boben, J.; Kramberger, P.; Petrovič, N.; Cankar, K.; Peterka, M.; Štrancar, A.; Ravnikar, M. Detection and quantification of Tomato mosaic virus in irrigation waters. Eur. J. Plant Pathol. 2007, 118, 59–71. [Google Scholar] [CrossRef]
- Broadbent, L. The epidemiology of tomato mosaic. Ann. Appl. Biol. 1965, 56, 177–205. [Google Scholar] [CrossRef]
- Miglietta, F.; Raschi, A.; Bettarini, I.; Resti, R.; Selvi, F. Natural CO2 springs in Italy: A resource for examining long-term response of vegetation to rising atmospheric CO2 concentrations. Plant Cell Environ. 1993, 16, 873–878. [Google Scholar] [CrossRef]
- Scholefield, P.A.; Doick, K.J.; Herbert, B.M.J.; Hewitt, C.N.S.; Schnitzler, J.P.; Pinelli, P.; Loreto, F. Impact of rising CO2 on emissions of volatile organic compounds: Isoprene emission from Phragmites australis growing at elevated CO2 in a natural carbon dioxide spring. Plant Cell Environ. 2004, 27, 393–401. [Google Scholar] [CrossRef]
- Matsuura, S. Suppression of Tomato mosaic virus disease in tomato plants by deep ultraviolet irradiation using light-emitting diodes. Lett. Appl. Microbiol. 2014, 59, 457–463. [Google Scholar] [CrossRef]
- Scandolera, T.; Teano, G.; Naderpour, M.; Geffroy, V.; Pflieger, S. Insights into the effects of elevated atmospheric carbon dioxide on plant-virus interactions: A literature review. Environ. Exp. Bot. 2024, 221, 105737. [Google Scholar] [CrossRef]
- Trebicki, P. Climate change and plant virus epidemiology. Virus Res. 2020, 286, 198059. [Google Scholar] [CrossRef] [PubMed]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Alnaggar, A.E.A.M.; Soliman, A.M.; El-Dougdoug, N.K. Ameliorating the adverse effects of tomato mosaic tobamovirus infecting tomato plants in Egypt by boosting immunity in tomato plants using zinc oxide nanoparticles. Molecules 2021, 26, 1337. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Sun, Z.; Shao, S.; Hu, L.; Ye, M.; Zhou, Y.; Xia, X.; Yu, J.; Shi, K. Antagonism between phytohormone signalling underlies the variation in disease susceptibility of tomato plants under elevated CO2. J. Exp. Bot. 2015, 66, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.N.; Tregunna, E.B.; Ragetli, H.W. CO2 effects on local-lesion production by tobacco mosaic virus and turnip mosaic virus. Virology 1975, 65, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Cucu, M.A.; Garibaldi, A.; Gullino, M.L. Combined effect of CO2 and temperature on wheat powdery mildew development. Plant Pathol. J. 2018, 34, 316–326. [Google Scholar] [CrossRef]
- Guo, H.; Huang, L.; Sun, Y.; Guo, H.; Ge, F. The contrasting effects of elevated CO2 on TYLCV infection of tomato genotypes with and without the resistance gene, Mi-1.2. Front. Plant Sci. 2016, 7, 1680. [Google Scholar] [CrossRef]
- Guo, H.; Ge, P.; Tong, J.; Zhang, Y.; Peng, X.; Zhao, Z.; Ge, F.; Sun, Y. Elevated carbon dioxide levels decreases cucumber mosaic virus accumulation in correlation with greater accumulation of rgs-CaM, an inhibitor of a viral suppressor of RNAi. Plants 2020, 10, 59. [Google Scholar] [CrossRef]
- Ye, L.; Fu, X.; Ge, F. Elevated CO2 alleviates damage from Potato virus Y infection in tobacco plants. Plant Sci. 2010, 179, 219–224. [Google Scholar] [CrossRef]
- Thayer, R.H.; Eco Enterprises. Carbon Dioxide Enrichment Methods. 2021. Available online: https://www.hydrofarm.com/carbon-dioxide-enrichment-methods (accessed on 29 January 2025).
- Pugliese, M.; Liu, J.; Titone, P.; Garibaldi, A.; Gullino, M.L. Effects of elevated CO2 and temperature on interactions of zucchini and powdery mildew. Phytopathol. Mediterr. 2012, 51, 480–487. [Google Scholar]
- Fu, X.; Ye, L.; Kang, L.; Ge, F. Elevated CO2 shifts the focus of tobacco plant defences from cucumber mosaic virus to the green peach aphid. Plant Cell Environ. 2010, 33, 2056–2064. [Google Scholar] [CrossRef]
- Khan, M.R.; Rizvi, T.F. Effect of elevated levels of CO2 on powdery mildew development in five cucurbit species. Sci. Rep. 2020, 10, 4986. [Google Scholar] [CrossRef] [PubMed]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.L.; Barbottin, A.; Jeuffroy, M.H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Tenllado, F.; Canto, T. Effects of a changing environment on the defenses of plants to viruses. Curr. Opin. Virol. 2020, 42, 40–46. [Google Scholar] [CrossRef] [PubMed]
- López-Gresa, M.P.; Lisón, P.; Kim, H.K.; Choi, Y.H.; Verpoorte, R.; Rodrigo, I.; Conejero, V.; Bellés, J.M. Metabolic fingerprinting of Tomato Mosaic Virus infected Solanum lycopersicum. J. Plant Physiol. 2012, 169, 1586–1596. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Khalid, M.; Saeed-ur-Rahman; Bilal, M.; Huang, D.F. Role of flavonoids in plant interactions with the environment and against human pathogens—A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Padmanabhan, C.; Ma, Q.; Shekasteband, R.; Stewart, K.S.; Hutton, S.F.; Scott, J.W.; Fei, Z.; Ling, K.S. Comprehensive transcriptome analysis and functional characterization of PR-5 for its involvement in tomato Sw-7 resistance to tomato spotted wilt tospovirus. Sci. Rep. 2019, 9, 7673. [Google Scholar] [CrossRef]
- Pečenková, T.; Pejchar, P.; Moravec, T.; Drs, M.; Haluška, S.; Šantrůček, J.; Potocká, A.; Žárský, V.; Potocký, M. Immunity functions of Arabidopsis pathogenesis-related 1 are coupled but not confined to its C-terminus processing and trafficking. Mol. Plant Pathol. 2022, 23, 664–678. [Google Scholar] [CrossRef]
- D’Errico, C.; Forgia, M.; Pisani, M.; Pavan, S.; Noris, E.; Matić, S. Overexpression of the C4 protein of tomato yellow leaf curl Sardinia virus increases tomato resistance to powdery mildew. Front. Plant Sci. 2023, 14, 1163315. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef]
- Available online: https://sunrisesunset.io/it/piedmont/turin/ (accessed on 20 February 2025).
- Available online: https://world-weather.info/forecast/italy/siena/ (accessed on 20 February 2025).
- Etiope, G.; Guerra, M. Carbon Dioxide and Radon Geohazards over a Gas-bearing Fault in the Siena Graben (Central Italy). Terr. Atmos. Ocean. Sci. 2005, 16, 885–896. [Google Scholar] [CrossRef]
- Agati, G.; Tuccio, L.; Kusznierewicz, B.; Chmiel, T.; Bartoszek, A.; Kowalski, A.; Grzegorzewska, M.; Kosson, R.; Kaniszewski, S. Nondestructive Optical Sensing of Flavonols and Chlorophyll in White Head Cabbage (Brassica oleracea L. var. capitata subvar. alba) Grown under Different Nitrogen Regimens. J. Agric. Food Chem. 2016, 64, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kissoudis, C.; Sunarti, S.; van de Wiel, C.; Visser, R.G.F.; van der Linden, C.G.; Bai, Y. Responses to combined abiotic and biotic stress in tomato are governed by stress intensity and resistance mechanism. J. Exp. Bot. 2016, 67, 5119–5132. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Xu, Z.S.; Wang, F.; Xiong, A.S. Salicylic acid-induced differential resistance to the Tomato yellow leaf curl virus among resistant and susceptible tomato cultivars. BMC Plant Biol. 2019, 19, 173. [Google Scholar] [CrossRef]
- Digilio, M.C.; Corrado, G.; Sasso, R.; Coppola, V.; Iodice, L.; Pasquariello, M.; Bossi, S.; Maffei, M.E.; Coppola, M.; Pennacchio, F.; et al. Molecular and chemical mechanisms involved in aphid resistance in cultivated tomato. New Phytol. 2010, 187, 1089–1101. [Google Scholar] [CrossRef]


| Primer Name | Sequence | Ref. |
|---|---|---|
| SR1a4F | GTGTCCGAGAGGCCAGACTA | [41] |
| SR1a4R | CATTGTTGCAACGAGCCCGA | [41] |
| SlPR2F | TCCAGGTAGAGACAGTGGTAAA | [42] |
| SlPR2R | CCTAAATATGTCGCGGTTGAGA | [42] |
| SlPR5F | GGCCCATGTGGTCCTACAAA | [42] |
| SlPR5R | GGCAACATAGTTTAGCAGACCG | [42] |
| SlEF-fw | CTCCATTGGGTCGTTTTGCT | [43] |
| SlEF5-rv | GGTCACCTTGGCACCAGTTG | [43] |
| ToMVF | TTGCCGTGGTGGTGTGAGT | [9] |
| ToMVR | GACCCCAGTGTGGCTTCGT | [9] |
| ToMVCPf | AAAACCAGCAGAATCCGACAA | [13] |
| ToMVCPr | TGCAACCGTAGCGTCGTCTA | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, G.; Carli, A.; Raschi, A.; Centritto, M.; Noris, E.; D’Errico, C.; Matić, S. Elevated Atmospheric CO2 Concentrations Reduce Tomato Mosaic Virus Severity in Tomato Plants. Plants 2025, 14, 811. https://doi.org/10.3390/plants14050811
Marino G, Carli A, Raschi A, Centritto M, Noris E, D’Errico C, Matić S. Elevated Atmospheric CO2 Concentrations Reduce Tomato Mosaic Virus Severity in Tomato Plants. Plants. 2025; 14(5):811. https://doi.org/10.3390/plants14050811
Chicago/Turabian StyleMarino, Giovanni, Andrea Carli, Antonio Raschi, Mauro Centritto, Emanuela Noris, Chiara D’Errico, and Slavica Matić. 2025. "Elevated Atmospheric CO2 Concentrations Reduce Tomato Mosaic Virus Severity in Tomato Plants" Plants 14, no. 5: 811. https://doi.org/10.3390/plants14050811
APA StyleMarino, G., Carli, A., Raschi, A., Centritto, M., Noris, E., D’Errico, C., & Matić, S. (2025). Elevated Atmospheric CO2 Concentrations Reduce Tomato Mosaic Virus Severity in Tomato Plants. Plants, 14(5), 811. https://doi.org/10.3390/plants14050811









