Effects of Different Postharvest Treatments on Fruit Quality, Sucrose Metabolism, and Antioxidant Capacity of ‘Newhall’ Navel Oranges During Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagent Material
2.2. Experimental Treatment
2.3. Measurement of Flavonoids and Total Phenols
2.4. Measurement of DPPH, ABTS and FRAP Radical Scavenging
2.5. Measurement of Fruit Weight Loss
2.6. Measurement of Total Soluble Solid, Titratable Acidity, and Total Sugar
2.7. Determination of Glucose, Fructose, and Sucrose Contents
2.8. Determination of Enzyme Activities Related to Sucrose Metabolism
2.9. qRT-PCR Analysis
2.10. Data Analysis
3. Results
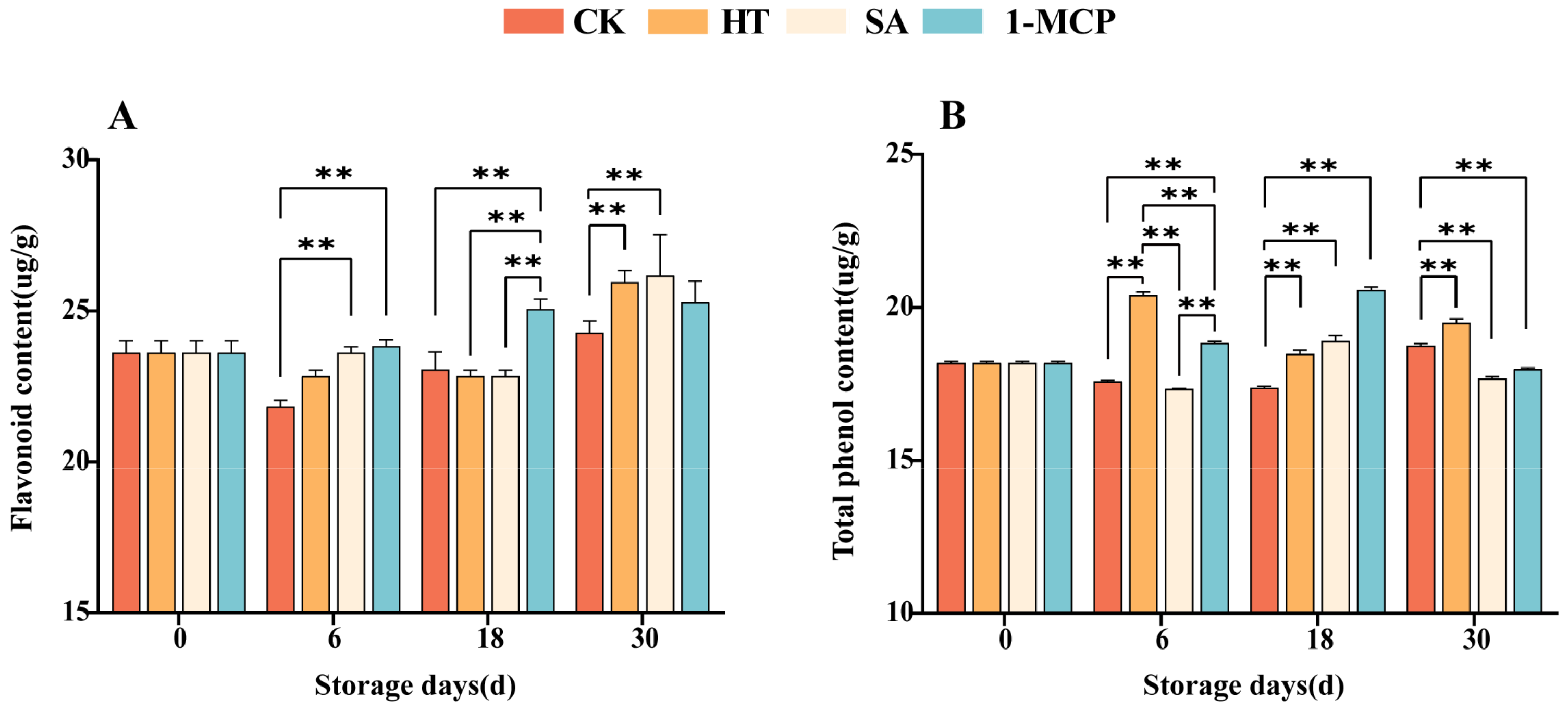
3.1. Changes in Flavonoids and Total Phenolic Content of Fruit Pulp During Storage
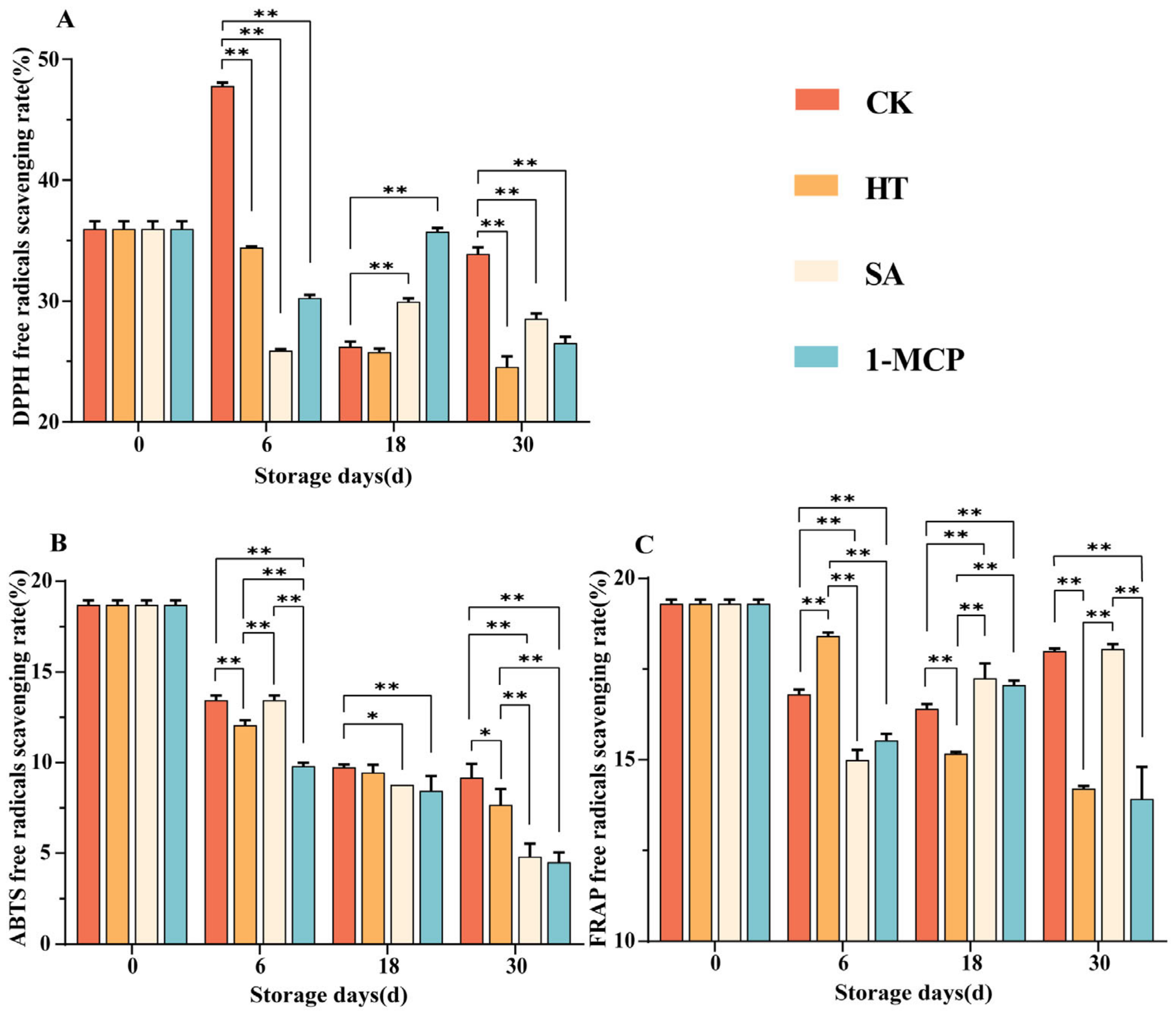
3.2. Changes in DPPH Free Radicals Scavenging, ABTS Free Radicals Scavenging and FRAP Reduction Capacity in Fruit Pulp During Storage
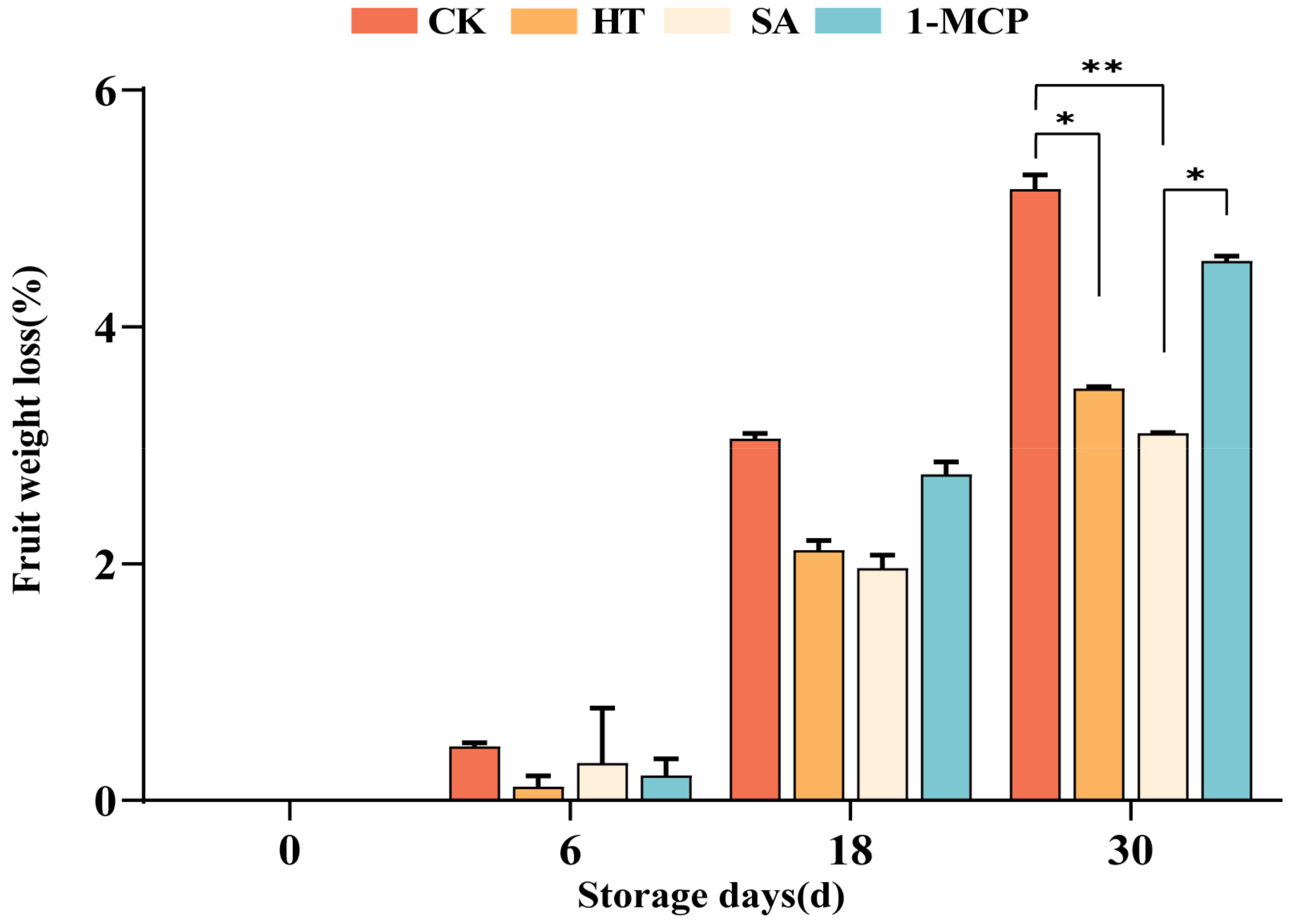
3.3. Effect of Different Treatments on the Rate of Fruit Weight Loss During Storage
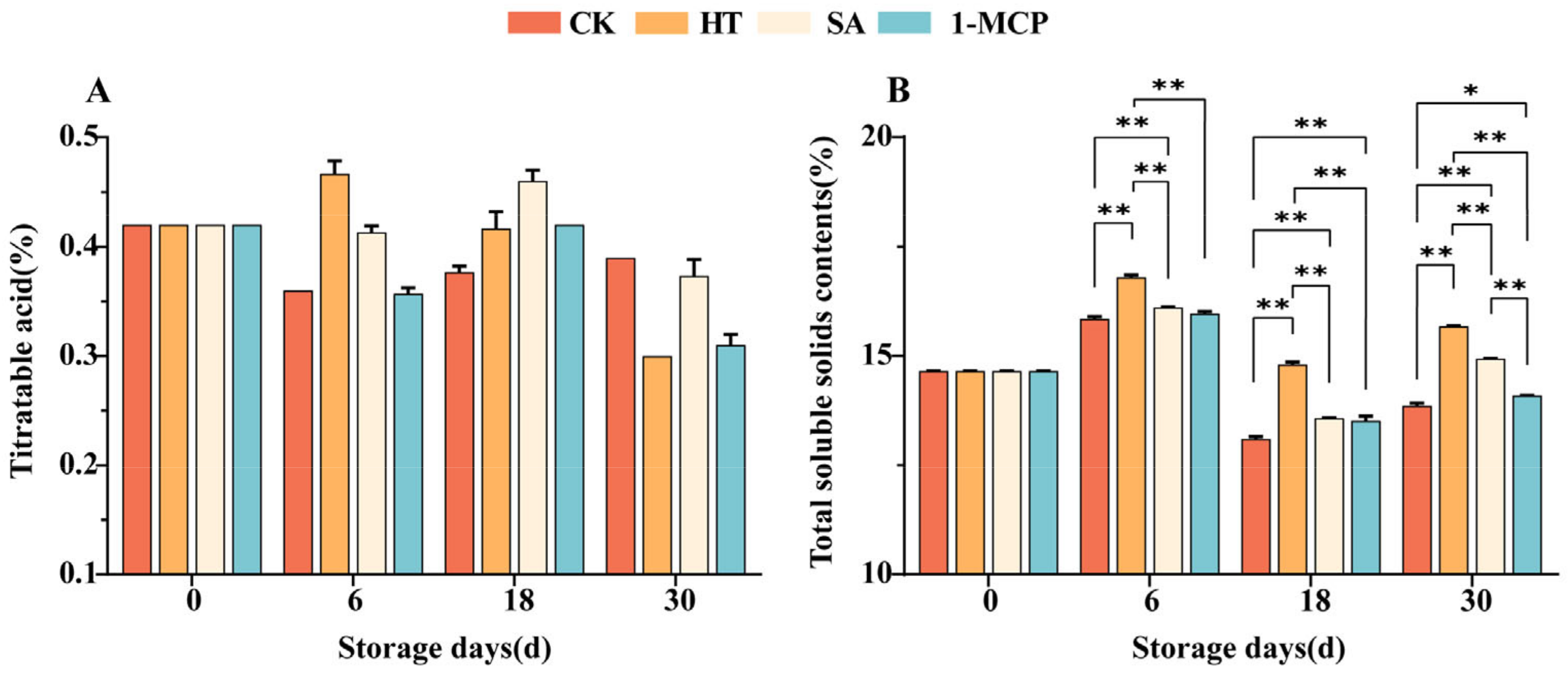
3.4. Changes in Titratable Acid and Total Soluble Solids Content of Fruit Pulp During Storage
3.5. Changes in Total Sugar and Sugar Components During Storage
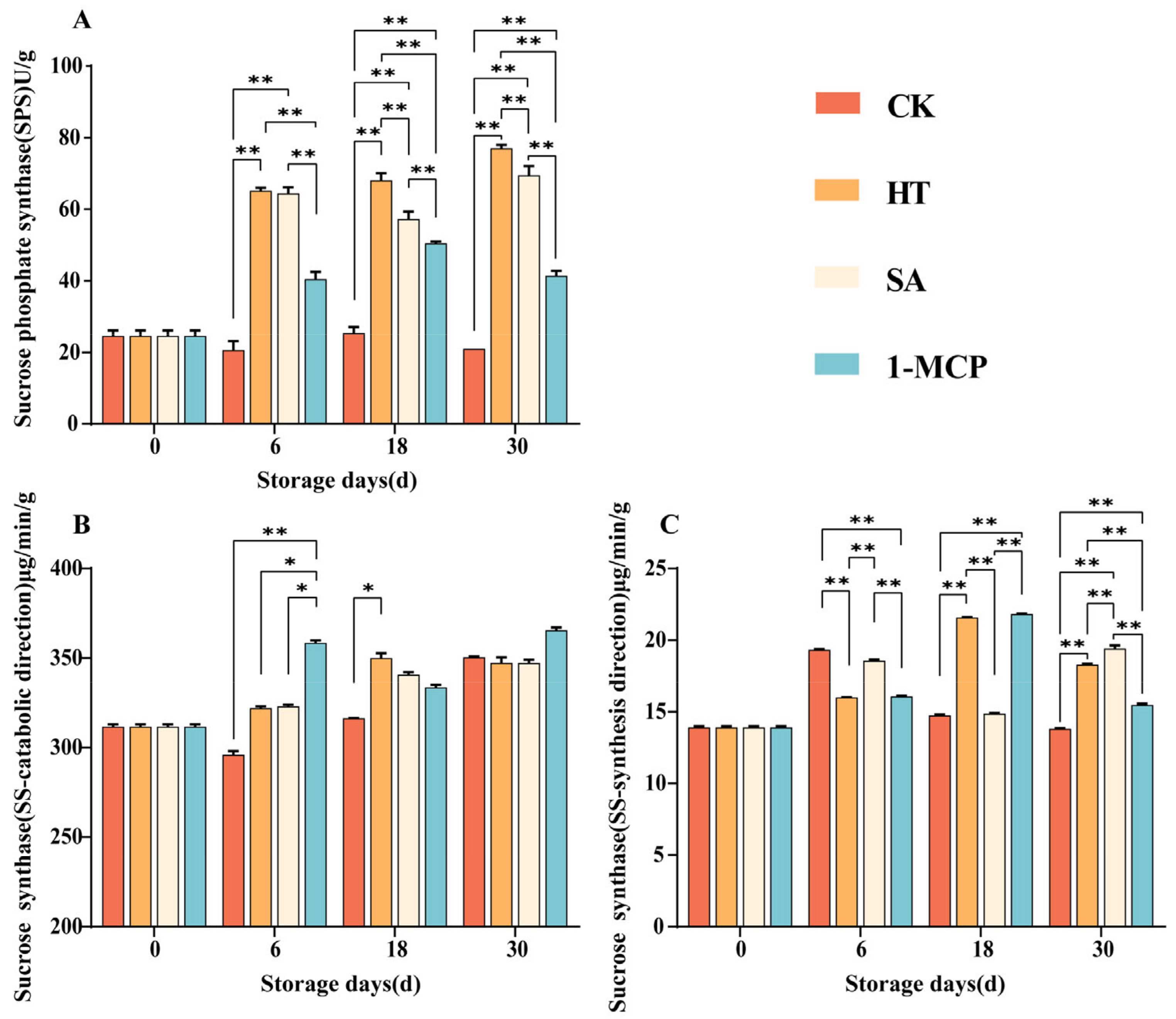
3.6. Changes in the Activities of Enzymes Related to Sucrose Metabolism
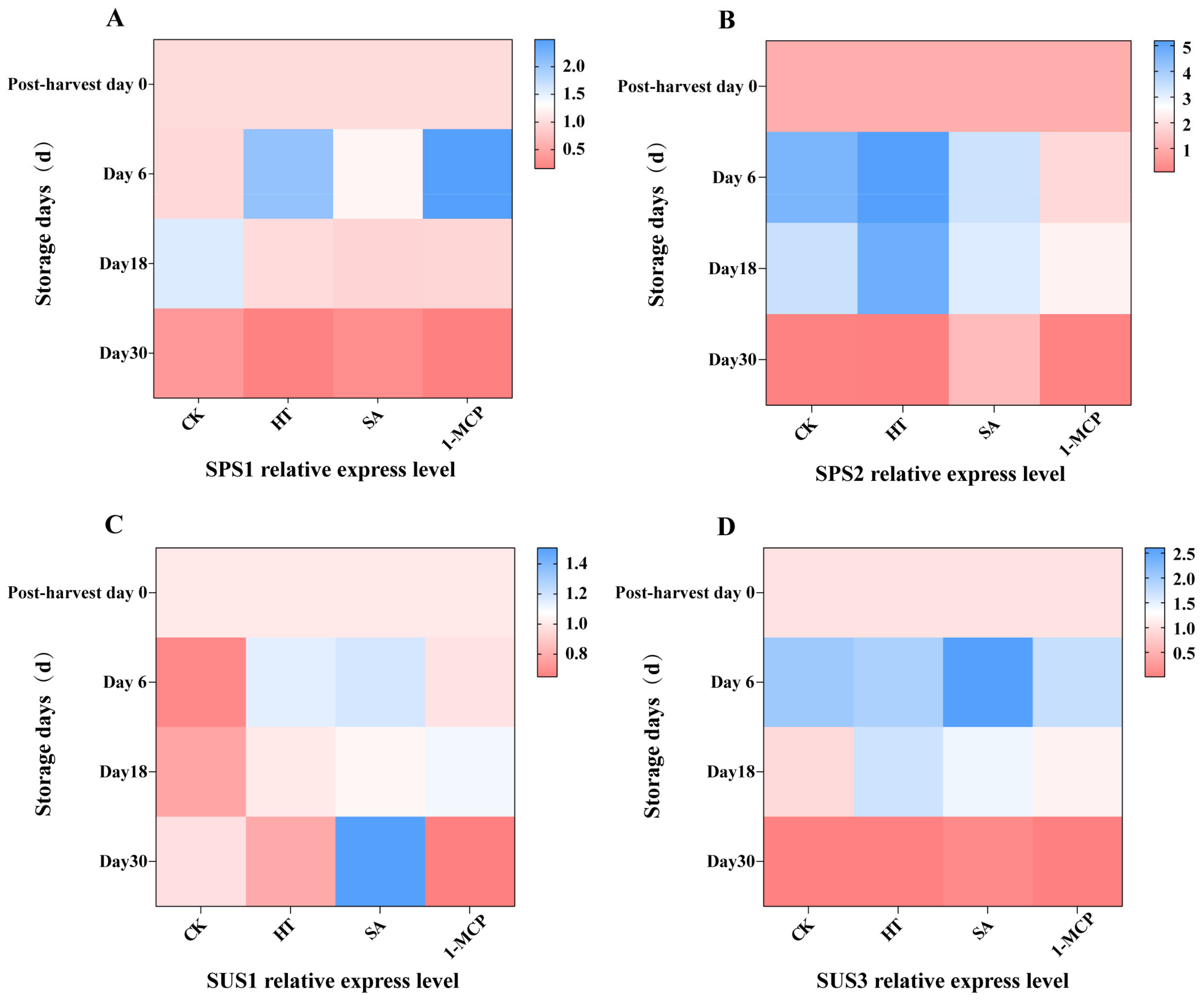
3.7. Expression of Genes Related to Rate-Limiting Enzymes of Sucrose Metabolism
4. Discussion
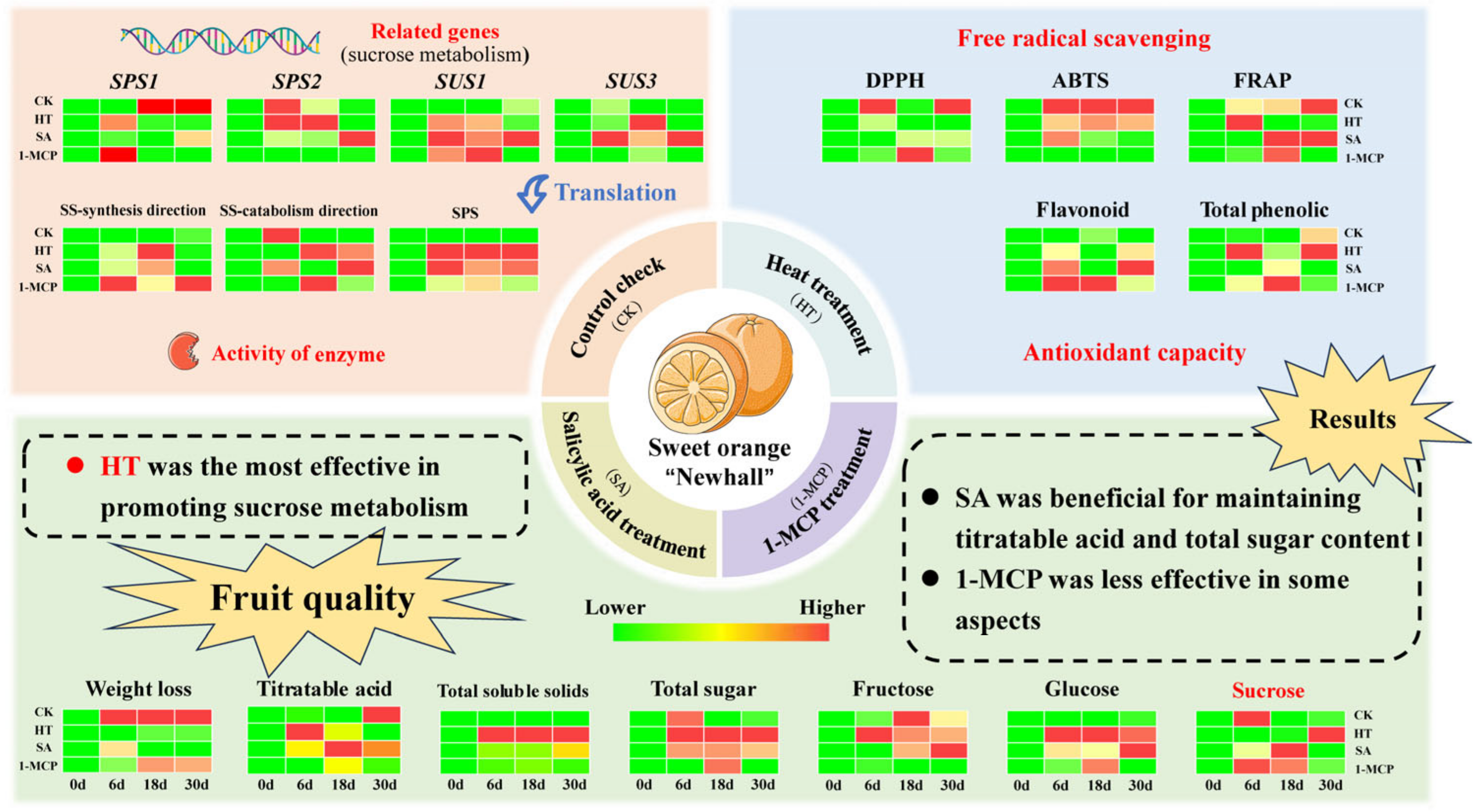
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Liang, Y.; He, L.; Kaliaperumal, K.; Tan, H.; Jiang, Y.; Zhong, B.; Zhang, J. Effects of storage time and temperature on the chemical composition and organoleptic quality of Gannan navel orange (Citrus sinensis Osbeck cv. Newhall). J. Food Meas. Charact. 2021, 16, 935–944. [Google Scholar] [CrossRef]
- Lowell, C.A.; Tomlinson, P.T.; Koch, K.E. Sucrose-Metabolizing Enzymes in Transport Tissues and Adjacent Sink Structures in Developing Citrus Fruit 1. Plant Physiol. 1989, 90, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Legua, P.; Modica, G.; Porras, I.; Conesa, A.; Continella, A. Bioactive compounds, antioxidant activity and fruit quality evaluation of eleven blood orange cultivars. J. Sci. Food Agric. 2022, 102, 2960–2971. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Hao, W.; Liu, K.; Liu, J.; Yin, C.; Su, Y.; Hang, Z.; Peng, B.; Liu, H.; Xiong, B.; et al. Analysis of sugar components and identification of SPS genes in citrus fruit development. Front. Plant Sci. 2024, 15, 1372809. [Google Scholar] [CrossRef]
- Islam, M.Z.; Hu, X.-M.; Jin, L.-F.; Liu, Y.-Z.; Peng, S.-A. Genome-Wide Identification and Expression Profile Analysis of Citrus Sucrose Synthase Genes: Investigation of Possible Roles in the Regulation of Sugar Accumulation. PLoS ONE 2014, 9, e113623. [Google Scholar] [CrossRef]
- Hussain, S.B.; Guo, L.-X.; Shi, C.-Y.; Khan, M.A.; Bai, Y.-X.; Du, W.; Liu, Y.-Z. Assessment of sugar and sugar accumulation-related gene expression profiles reveal new insight into the formation of low sugar accumulation trait in a sweet orange (Citrus sinensis) bud mutant. Mol. Biol. Rep. 2020, 47, 2781–2791. [Google Scholar] [CrossRef]
- Zacarías-García, J.; Rodrigo, M.J.; Rambla, J.L.; Granell, A.; Zacarías, L. Physiological responses, nutritional quality and aroma volatiles of the red-fleshed kirkwood navel and ruby valencia oranges during postharvest cold storage. Postharvest Biol. Technol. 2023, 199, 112303. [Google Scholar] [CrossRef]
- Aravinthkumar, A.; Raj, H.; Kumar, P.; Sharma, P.L.; Verma, S.; Sheela, J.; Parwan, S.; Shankar, S.V.; Ananthakrishnan, S.; Chauhan, A.; et al. Comparative efficacy of GRAS chemicals, botanicals and yeast in controlling green mould and fruit nutritional quality enhancement in Kinnow mandarin (Citrus nobilis Lour x Citrus deliciosa Tenora). Sci. Hortic. 2025, 339, 113869. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Lapuente, M.; Estruch, R.; Shahbaz, M.; Casas, R. Relation of Fruits and Vegetables with Major Cardiometabolic Risk Factors, Markers of Oxidation, and Inflammation. Nutrients 2019, 11, 2381. [Google Scholar] [CrossRef]
- Nakao, K.; Murata, K.; Itoh, K.; Hanamoto, Y.; Masuda, M.; Moriyama, K. Anti-hyperuricemia effects of extracts of immature Citrus unshiu fruit. J. Trad. Med. 2011, 28, 10–15. [Google Scholar] [CrossRef]
- Sarvarian, M.; Jafarpour, A.; Awuchi, C.G.; Adeleye, A.O.; Okpala, C.O.R. Changes in Physicochemical, Free Radical Activity, Total Phenolic and Sensory Properties of Orange (Citrus sinensis L.) Juice Fortified with Different Oleaster (Elaeagnus angustifolia L.) Extracts. Molecules 2022, 27, 1530. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, G.T.; Juneja, V.K.; Pazir, F. Application of ultraviolet-C light on oranges for the inactivation of postharvest wound pathogens. Food Control 2015, 57, 9–13. [Google Scholar] [CrossRef]
- Huan, C.; Han, S.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Ma, R.; Yu, Z. Postharvest hot air and hot water treatments affect the antioxidant system in peach fruit during refrigerated storage. Postharvest Biol. Technol. 2017, 126, 1–14. [Google Scholar] [CrossRef]
- Li, S.; Hu, J.; Ning, S.; Li, W.; Jiang, R.; Huang, J.; Li, Y. Bacillus velezensis HY19 as a sustainable preservative in post-harvest citrus (Citrus reticulata Blanco L.) fruit management. Food Control 2024, 155, 110068. [Google Scholar] [CrossRef]
- Yang, C.; Lin, Z.; Luo, Z.; Wang, Z.; Liu, P.; Xu, R.; Zhu, F.; Cheng, Y. The volatile compound (E)-2-hexenal in wampee (Clausena lansium) represses the development of Penicillium italicum and enhances the disease resistance of postharvest citrus fruit. Postharvest Biol. Technol. 2025, 219, 113241. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Xu, Y.; Sang, Y.; Mei, S.; Xu, C.; Yu, X.; Pan, T.; Cheng, C.; Zhang, J.; et al. The Effects of Storage Temperature, Light Illumination, and Low-Temperature Plasma on Fruit Rot and Change in Quality of Postharvest Gannan Navel Oranges. Foods 2022, 11, 3707. [Google Scholar] [CrossRef]
- Ennab, H.A.; El-Shemy, M.A.; Alam-Eldein, S.M. Salicylic Acid and Putrescine to Reduce Post-Harvest Storage Problems and Maintain Quality of Murcott Mandarin Fruit. Agronomy 2020, 10, 115. [Google Scholar] [CrossRef]
- Maghoumi, M.; Amodio, M.L.; Cisneros-Zevallos, L.; Colelli, G. Prevention of Chilling Injury in Pomegranates Revisited: Pre- and Post-Harvest Factors, Mode of Actions, and Technologies Involved. Foods 2023, 12, 1462. [Google Scholar] [CrossRef]
- Li, J.; Hussain, I.; Azam, M.; Khan, M.A.; Akram, M.T.; Naveed, K.; Asif, M.; Anjum, N.; Zeng, J.; Zhang, J.; et al. Hot Water Treatment Improves Date Drying and Maintains Phytochemicals and Fruit Quality Characteristics of Date Palm (Phoenix dactylifera). Foods 2023, 12, 2405. [Google Scholar] [CrossRef]
- Baswal, A.K.; Dhaliwal, H.S.; Singh, Z.; Mahajan, B.V.C. Post-harvest Application of Methyl Jasmonate, 1-Methylcyclopropene and Salicylic Acid Elevates Health-promoting Compounds in Cold-stored ‘Kinnow’ Mandarin (Citrus nobilis Lour x C. deliciosa Tenora) Fruit. Int. J. Fruit Sci. 2021, 21, 147–157. [Google Scholar] [CrossRef]
- Kaewsuksaeng, S.; Tatmala, N.; Srilaong, V.; Pongprasert, N. Postharvest heat treatment delays chlorophyll degradation and maintains quality in Thai lime (Citrus aurantifolia Swingle cv. Paan) fruit. Postharvest Biol. Technol. 2015, 100, 1–7. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, L.; Chen, J.; Zhang, Y.; Kai, W.; Chen, C. Maintenance of postharvest storability and overall quality of ‘Jinshayou’ pummelo fruit by salicylic acid treatment. Front. Plant Sci. 2023, 13, 1086375. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, Z.; Deng, L.; Li, W.; Yuan, Y.; Zhang, M.; Sun, G.; He, S.; Wang, J.; Wang, Z.; et al. Effect of Natural Variation and Rootstock on Fruit Quality and Volatile Organic Compounds of ‘Kiyomi tangor’ (Citrus reticulata Blanco) Citrus. Int. J. Mol. Sci. 2023, 24, 16810. [Google Scholar] [CrossRef]
- Molan, A.L.; Flanagan, J.; Wei, W.; Moughan, P.J. Selenium-containing green tea has higher antioxidant and prebiotic activities than regular green tea. Food Chem. 2009, 114, 829–835. [Google Scholar] [CrossRef]
- Guo, N.; Yan, Y.; Li, Q.; Yang, Y. Pyruvic acid improves cold-storage quality of plum fruit by stimulating cyanide-resistant respiration and regulating sugar metabolism. Sci. Hortic. 2025, 340, 113926. [Google Scholar] [CrossRef]
- Liu, J.; Yue, R.; Si, M.; Wu, M.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Effects of Exogenous Application of Melatonin on Quality and Sugar Metabolism in ‘Zaosu’ Pear Fruit. J. Plant Growth Regul. 2019, 38, 1161–1169. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, X.; Wei, Y.; Xu, F.; Wang, H. At-harvest fruit maturity affects sucrose metabolism during cold storage and is related to chilling injury in peach. J. Food Sci. Technol. 2020, 57, 2000–2009. [Google Scholar] [CrossRef]
- Cui, M.; Liang, Z.; Liu, Y.; Sun, Q.; Wu, D.; Luo, L.; Hao, Y. Flavonoid profile of Anoectochilus roxburghii (Wall.) Lindl. Under short-term heat stress revealed by integrated metabolome, transcriptome, and biochemical analyses. Plant Physiol. Biochem. 2023, 201, 107896. [Google Scholar] [CrossRef]
- Almeida, J.; Perez-Fons, L.; Fraser, P.D. A transcriptomic, metabolomic and cellular approach to the physiological adaptation of tomato fruit to high temperature. Plant Cell Environ. 2020, 44, 2211–2229. [Google Scholar] [CrossRef]
- Wang, L.; Ma, K.-B.; Lu, Z.-G.; Ren, S.-X.; Jiang, H.-R.; Cui, J.-W.; Chen, G.; Teng, N.-J.; Lam, H.-M.; Jin, B. Differential physiological, transcriptomic and metabolomic responses of Arabidopsis leaves under prolonged warming and heat shock. BMC Plant Biol. 2020, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Balaguera-López, H.E.; Espinal-Ruiz, M.; Rodríguez-Nieto, J.M.; Herrera-Arévalo, A.; Zacarías, L. 1-Methylcyclopropene inhibits ethylene perception and biosynthesis: A theoretical and experimental study on cape gooseberry (Physalis peruviana L.) fruits. Postharvest Biol. Technol. 2021, 174, 111467. [Google Scholar] [CrossRef]
- Mare, R.; Pujia, R.; Maurotti, S.; Greco, S.; Cardamone, A.; Coppoletta, A.R.; Bonacci, S.; Procopio, A.; Pujia, A. Assessment of Mediterranean Citrus Peel Flavonoids and Their Antioxidant Capacity Using an Innovative UV-Vis Spectrophotometric Approach. Plants 2023, 12, 4046. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Liu, C.-C.; Li, Y.-J.; Liao, C.-M.; Vivek, S.; Chuo, G.-L.; Tseng, C.-Y.; Wu, Z.-Q.; Shimada, T.; Suetsugu, N.; et al. Multifaceted roles of Arabidopsis heat shock factor binding protein in plant growth, development, and heat shock response. Environ. Exp. Bot. 2024, 226, 105878. [Google Scholar] [CrossRef]
- Kaya, C.; Akin, S.; Sarioğlu, A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Enhancement of soybean tolerance to water stress through regulation of nitrogen and antioxidant defence mechanisms mediated by the synergistic role of salicylic acid and thiourea. Plant Physiol. Biochem. 2024, 207, 108320. [Google Scholar] [CrossRef]
- Li, Z.; Jin, R.; Yang, Z.; Wang, X.; You, G.; Guo, J.; Zhang, Y.; Liu, F.; Pan, S. Comparative study on physicochemical, nutritional and enzymatic properties of two Satsuma mandarin (Citrus unshiu Marc.) varieties from different regions. J. Food Compos. Anal. 2021, 95, 103614. [Google Scholar] [CrossRef]
- Zhou, H.-j.; Ye, Z.-w.; Su, M.-s.; Du, J.-h.; Li, X.-w. Effect of Heat Treatment on Protein Content and the Quality of ‘Hujingmilu’ Peach [Prunus persica (L.) Batsch]. HortScience Horts 2015, 50, 1531–1536. [Google Scholar] [CrossRef]
- Ancillotti, C.; Bogani, P.; Biricolti, S.; Calistri, E.; Checchini, L.; Ciofi, L.; Gonnelli, C.; Del Bubba, M. Changes in polyphenol and sugar concentrations in wild type and genetically modified Nicotiana langsdorffii Weinmann in response to water and heat stress. Plant Physiol. Biochem. 2015, 97, 52–61. [Google Scholar] [CrossRef]
- Mehdi, F.; Liu, X.; Riaz, Z.; Javed, U.; Aman, A.; Galani, S. Expression of sucrose metabolizing enzymes in different sugarcane varieties under progressive heat stress. Front. Plant Sci. 2023, 14, 1269521. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic acid and salicylic acid induce the accumulation of sucrose and increase resistance to chilling injury in peach fruit. J. Sci. Food Agric. 2021, 101, 4250–4255. [Google Scholar] [CrossRef] [PubMed]
- Falagán, N.; Terry, L.A. 1-Methylcyclopropene maintains postharvest quality in Norwegian apple fruit. Food Sci. Technol. Int. 2020, 26, 420–429. [Google Scholar] [CrossRef] [PubMed]


| Target Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| SPS1 | TGTAACAGTGGCAGTGAT | GTGTGAGTGGTAATAGAAGTC |
| SPS2 | GTTACAACACAAGACACAAT | ATCACCTCAGACCACATT |
| SUS1 | GGCCTTTGGCTTGACTGTTG | GGGATCTGCCTTGCACTTCT |
| SUS3 | ATTCCGAGCATCAGAGAGCG | TCGTCGATCAGTACATGCGG |
| Ingredient | Volume (µL) |
|---|---|
| cDNA | 1.00 |
| PCR Forward Primer (10 µM) | 0.50 |
| PCR Reverse Primer (10 µM) | 0.50 |
| 2× M5SYBR | 6.25 |
| dd H2O | 4.25 |
| Total | 12.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, B.; Han, L.; Ou, Y.; Wu, W.; Wang, J.; Yao, J.; Li, Y.; Chen, S.; Deng, T.; Chen, H.; et al. Effects of Different Postharvest Treatments on Fruit Quality, Sucrose Metabolism, and Antioxidant Capacity of ‘Newhall’ Navel Oranges During Storage. Plants 2025, 14, 802. https://doi.org/10.3390/plants14050802
Xiong B, Han L, Ou Y, Wu W, Wang J, Yao J, Li Y, Chen S, Deng T, Chen H, et al. Effects of Different Postharvest Treatments on Fruit Quality, Sucrose Metabolism, and Antioxidant Capacity of ‘Newhall’ Navel Oranges During Storage. Plants. 2025; 14(5):802. https://doi.org/10.3390/plants14050802
Chicago/Turabian StyleXiong, Bo, Linlv Han, Yinghong Ou, Wenjia Wu, Jialu Wang, Junfei Yao, Yisong Li, Siyu Chen, Taimei Deng, Hongzhen Chen, and et al. 2025. "Effects of Different Postharvest Treatments on Fruit Quality, Sucrose Metabolism, and Antioxidant Capacity of ‘Newhall’ Navel Oranges During Storage" Plants 14, no. 5: 802. https://doi.org/10.3390/plants14050802
APA StyleXiong, B., Han, L., Ou, Y., Wu, W., Wang, J., Yao, J., Li, Y., Chen, S., Deng, T., Chen, H., Wang, C., Ma, Q., Fan, Y., Li, Y., & Wang, Z. (2025). Effects of Different Postharvest Treatments on Fruit Quality, Sucrose Metabolism, and Antioxidant Capacity of ‘Newhall’ Navel Oranges During Storage. Plants, 14(5), 802. https://doi.org/10.3390/plants14050802






