Abstract
In semi-arid regions, wetlands often face water scarcity, salinity, and alkalinity stresses. Agricultural drainage water has been used to restore degraded wetlands, but it alters water quality and plant growth and resource distribution. Nitrogen (N) and phosphorus (P) stoichiometry reflect plant resource strategies. In China’s Songnen Plain, Bolboschoenus planiculmis, a key plant in soda–alkali wetlands and food for the rare white crane (Grus leucogeranus), is impacted by agricultural water input. However, the N and P stoichiometry in B. planiculmis and the influencing water variables remain unclear. This study analyzed N and P contents in B. planiculmis leaves, stems, tubers, and roots, and water variables. Results showed that leaf N content was highest, while tuber P content exceeded that of other organs. Leaf nitrogen to phosphorus (N:P) ratio was highest, and tuber’s was the lowest. N and P contents in plants were positively correlated, except between roots and stems. Redundancy analysis (RDA) revealed water temperature (T), oxidation-reduction potential (ORP), N contents, and water depth (WD) as key factors influencing N and P stoichiometry. Structural equation models (SEMs) indicated water T negatively affected plant N, while water nitrate nitrogen positively affected it. Water P content directly influenced leaf and stem P, and ammonium nitrogen affected aboveground P accumulation. Water T and WD directly impacted N:P ratios. These findings show that while agricultural drainage water alleviated aridification and salinization in degraded soda–alkali wetlands, exogenous N and P inputs significantly affected vegetation’s N and P utilization strategies.
1. Introduction
Nitrogen (N) and phosphorus (P) are crucial elements in ecosystems, influencing plant growth, energy conversion, metabolism, and stress resistance [1]. N serves as a fundamental constituent of chlorophyll, playing a pivotal role in plant physiology. Elevated N content significantly promotes chlorophyll biosynthesis, consequently enhancing photosynthetic efficiency and facilitating increased energy production for optimal plant growth and development [2]. N deficiency severely impairs plant metabolic processes, primarily by limiting the biosynthesis of essential proteins and chlorophyll pigments. Consequently, leaves turn yellow, and plants become stunted, ultimately resulting in significantly compromised plant vigor and productivity [3]. P significantly enhances root system development by stimulating root growth and branching, thereby improving the plant’s capacity for water and nutrient uptake [4]. Plants with low levels of N and P are more vulnerable to adversity [5]. The nitrogen to phosphorus (N:P) ratio is an important indicator of nutrient limitation in terrestrial ecosystems [6]. It can directly reflect the absorption and utilization of N and P by plants. When the ratio of N:P falls within the optimal range, it signifies balanced nutrient acquisition during plant growth, reflecting favorable nutritional conditions for optimal physiological development [7]. The N:P ratio in plant organs not only reflects the nutrient status of the plant itself but is also closely related to nutrient cycling in the ecosystem. Plants with a high N:P ratio may release more N during decomposition, thus affecting the N:P balance in the soil [8]. Consequently, investigating N and P stoichiometry of wetland plants provides critical insights into nutrient cycling processes and mechanisms within wetland ecosystems under global climate change scenarios.
Hydrological regimes in wetlands fundamentally govern vegetation composition and spatial distribution patterns [9,10]. Hydrological alterations significantly affect nutrient dynamics and vegetation stability in wetland ecosystems [11,12]. In recent years, the hydrology of natural wetlands has been increasingly impacted by drainage, ditching, and reclamation for agricultural development projects [13]. The inflow of agricultural drainage water can meet the water requirements of wetlands but also alters the water quality. Agricultural runoff typically carries a large amount of nutrients such as N and P. The influx of nutrients into wetlands alters aquatic nutrient concentrations and may infiltrate soil systems, modifying soil physicochemical properties and subsequently affecting plant growth dynamics and spatial distribution patterns [14]. Moreover, a large quantity of pesticides and fertilizers frequently used in farmland may be transferred to wetlands and cause pollution to water environments [15,16]. In the face of pollution stress, plants often adapt to the environment by adjusting their nutrient absorption and utilization strategies, ultimately affecting vegetation distribution and community structure, as well as population growth and reproduction [17]. Consequently, investigating N and P stoichiometry in wetland plants is crucial for understanding ecological adaptation mechanisms and optimizing water management under persistent anthropogenic influences, particularly agricultural drainage water input.
The soda–alkali wetland in the Songnen Plain, China, is of great significance for maintaining biodiversity, protecting the habitat of rare waterbirds, conserving water, and preventing desertification [18]. In recent years, the construction of upstream reservoirs along the Nenjiang River has significantly reduced water inflow to downstream wetlands, altering their natural hydrological regime. Anthropogenic activities, including agricultural drainage inputs, road construction, ditch drainage, and cofferdam development, coupled with climate-induced warming and drying trends, have substantially altered wetland hydrological regimes and aquatic environments [10,19]. Receiving agricultural drainage water is regarded as an effective measure to replenish the ecological water demand for vegetation and maintain it in wetlands. However, the content of pollutants and salinity in the water has been increased, and the distribution pattern and growth of typical wetland vegetation are severely threatened [20]. Alterations in wetland aquatic environments accelerate vegetation succession processes while increasing the unpredictability of their developmental trajectories. The current status and change trend in wetland vegetation in the context of hydrological and water environment changes need to be comprehensively evaluated. Although existing studies have paid some attention to the responses of plant communities to hydrological and climatic changes [21,22,23], studies on the effects of water environmental changes on N and P stoichiometry of typical wetland plants are relatively limited.
Bolboschoenus planiculmis is widely distributed in the typical soda–alkali wetland of the semi-arid area of the Songnen Plain and serves as an important food source for the migratory rare water bird, the white crane (Grus leucogeranus). In recent years, anthropogenic disturbances and climate change have drastically transformed the plant community composition and structure of B. planiculmis wetlands, resulting in a substantial decline in suitable white crane habitats [10,18]. Studies on vegetation in the B. planiculmis wetlands mainly focus on the response of plant communities and populations to water level changes and the ecological adaptation of individual plants to salt and alkali conditions [24,25]. However, the adaptive responses of plant N and P stoichiometry to multiple aquatic environmental stressors remain unexplored. The aim of this study is threefold: (1) to investigate the differences in N and P content and their ratios in leaves, stems, tubers, and roots of B. planiculmis; (2) to identify the key water variables governing the N and P stoichiometry of B. planiculmis plants; and (3) to clarify the influence path of water variables on the N and P stoichiometry of the B. planiculmis plants. The results of this study can provide crucial data for predicting the population stability of B. planiculmis under the influence of continuous changes in the water environment caused by agricultural drainage water input in the semi-arid regions.
2. Results
2.1. Variations in N and P Stoichiometry in Organs of B. planiculmis Plants
The results regarding N content in the organs of B. planiculmis indicated that the N content in leaves was the highest at 16.22 g kg−1 (p < 0.05; Table 1). There was no significant difference in N content among other organs (p > 0.05). The tuber had the highest P content at 2.78 g kg−1, followed by the leaf, stem, and roots. In terms of the N:P ratio, the leaf had the highest level of 1.2, followed by stem and roots. The tuber had the lowest level at 2.89. Nevertheless, the leaf N:P ratio was less than 14, suggesting that plant growth was more restricted by N limitation.

Table 1.
Contents of N, P, and their ratios in organs of B. planiculmis plants.
2.2. Relationships of N and P Contents in Organs of B. planiculmis Plants
Pearson’s correlation analyses revealed that there was a significant linear relationship among the N contents in each organ (Figure 1A–D,F; p < 0.05), with the exception of the relationship between stem N and root N contents (Figure 1E, p > 0.05). Similarly, there was a significant positive correlation among the P contents in all plant organs (Figure 2A–D,F; p < 0.05), except for the relationship between stem P and root P contents (Figure 2E, p > 0.05).
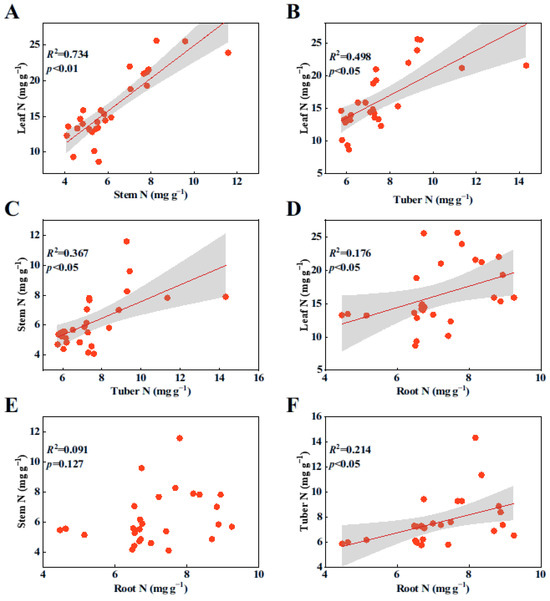
Figure 1.
Relationships among N contents in various organs of B. planiculmis plants. (A), relationship between stem N and leaf N; (B), relationship between tuber N and leaf N; (C), relationship between tuber N and stem N; (D), relationship between root N and leaf N; (E), relationship between root N and stem N; and (F), relationship between root N and tuber N.
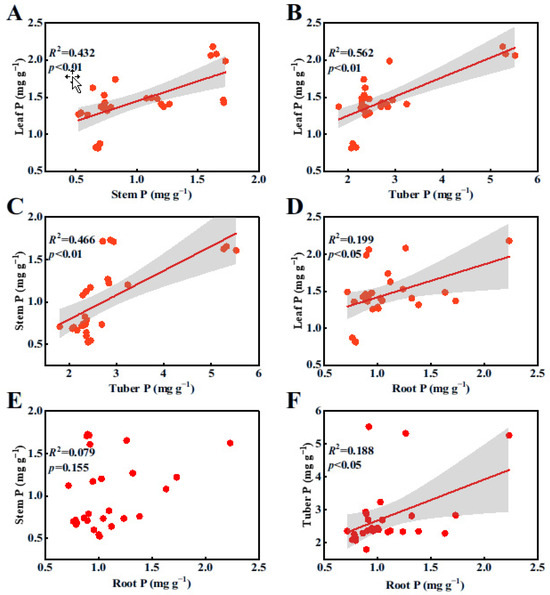
Figure 2.
Relationships among P contents in various organs of B. planiculmis plants. (A), relationship between stem P and leaf P; (B), relationship between tuber P and leaf P; (C), relationship between tuber P and stem P; (D), relationship between root P and leaf P; (E), relationship between root P and stem P; and (F), relationship between root P and tuber P.
2.3. Effects of Water Variables on N, P, and N:P in Organs of B. planiculmis Plants
The relationships in Figure 3 indicate that some water variables had significant effects on N, P, and N:P ratio in organs of the B. planiculmis plant, encompassing both positive and negative correlations. The N content in water, such as TN, NH₄–N, and NO₃–N, had significant positive correlations with the N and P contents in B. planiculmis organs. However, there was no significant positive correlation with N:P ratio (p < 0.05). In fact, there was a negative correlation between NO₃-N and the tuber N:P ratio (p < 0.05). Additionally, N and N:P in organs were negatively correlated with water T (p < 0.05), but there was no significant relationship between the P content in organs and T (p > 0.05). Significant negative correlations were observed between water TP and leaf N and P contents (p < 0.05). Water quality parameters (pH, ORP, DO, WD) exhibited both positive and negative correlations with N, P, and N:P ratios across plant organs.
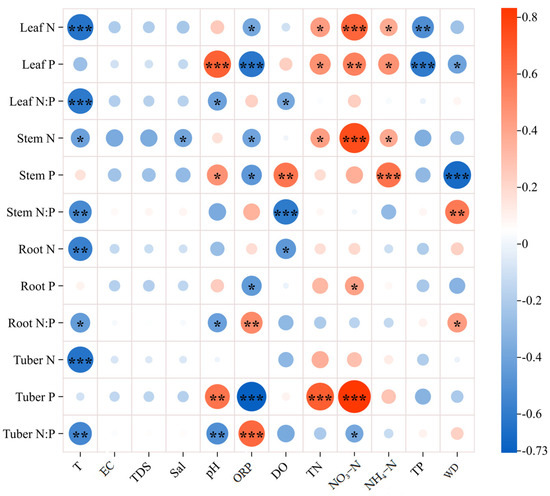
Figure 3.
Correlations of N, P, and N:P ratio of B. planiculmis organs with water variables. T, water temperature; EC, electrical conductivity in water; TDS, total dissolved solids in water; Sal, salinity of water; ORP; oxidation reduction potential of water; DO, dissolved oxygen in water; TN, total nitrogen content in water; NO3–N, nitrate nitrogen content in water; NH4–N, ammonium nitrogen content in water; TP, total phosphorus content in water; WD, water depth. ***, p < 0.001; **, p < 0.01; *, p < 0.05.
RDA was used to analyze the effects of water variables on N, P, and N:P ratios across plant organs (Figure 4). Results revealed that overall water variables explained 61.61% (axis 1, 35.24%; axis 2, 26.37%) of the total variations (F = 3.1; p = 0.002). Conditional effects showed that ORP, NH4–N, NO3–N, WD, and TN could effectively explain N and P stoichiometry (Table 2), accounting for 19.9% (F = 6.2; p = 0.002), 18.6% (F = 7.3; p = 0.002), 11.8% (F = 5.5; p = 0.002), 8.7% (F = 4.7; p = 0.002), 5.0% (F = 2.9; p = 0.022), and 3.5% (F = 2.3; p = 0.036) of the variations, respectively. These results indicate that these water variables, especially T, N, and WD, were the most important factors affecting N and P stoichiometry.
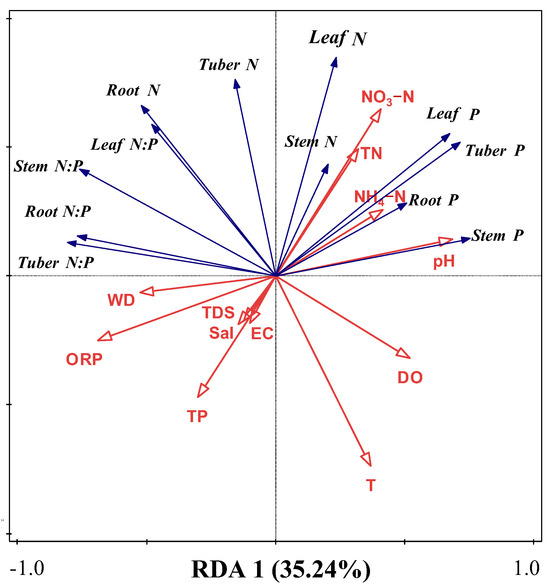
Figure 4.
RDA between water environmental parameters and N, P, and N:P ratio of B. planiculmis. T, water temperature; DO, dissolved oxygen in water; TN, total nitrogen content in water; NH4–N, ammonium nitrogen content in water; NO3–N, nitrate nitrogen content in water; TP, total phosphorus content in water; EC, electrical conductivity in water; TDS, total dissolved solids in water; Sal, salinity of water; ORP; oxidation reduction potential of water; WD, water depth.

Table 2.
Simple and conditional effects of water variables on N, P, and N:P ratio of B. planiculmis.
2.4. Influences of Water Variables on N and P Contents and N:P Ratio in Leaves, Stems, Tubers, and Roots
The results of the SEMs demonstrated that the impacts of water variables on N and P contents in all organs were significant (Figure 5), with nearly all exhibiting significant paths (p > 0.05). Water T had a direct negative effect on leaf, root, and tuber N (Figure 5A,E,G), while it had indirect negative effect on stem N (Figure 5C). Additionally, N in water, especially NO3–N, had a direct or indirect positive effect on N contents in each organ. ORP and TP also directly or indirectly affect N content in various organs. The content of TP in water had a direct negative effect on leaf P and a direct positive effect on stem P. The NH4–N in water directly influenced the accumulation of P in aboveground organs. The effects of various water environmental factors on root P were valid, but no water variables had a direct impact on root P.
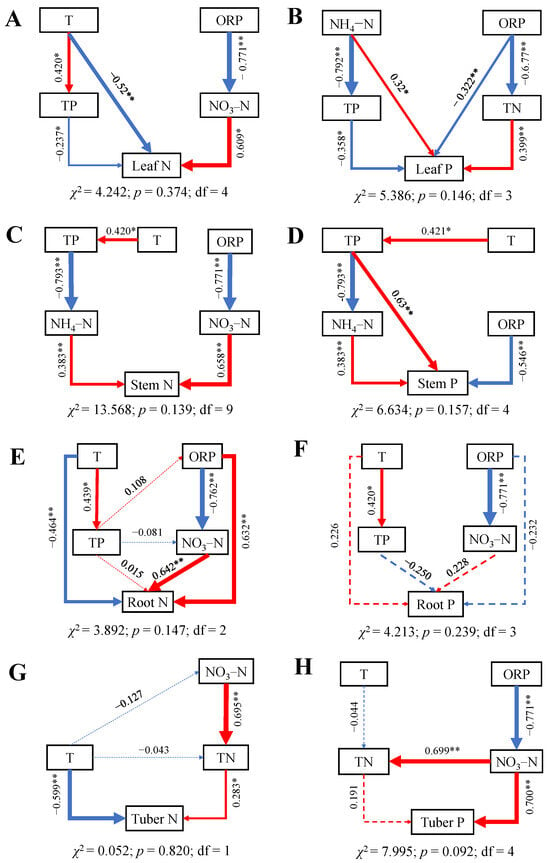
Figure 5.
SEMs of water variables on N and P contents of B. planiculmis organs. (A), SEM for leaf N; (B), SEM for leaf P; (C), SEM for stem N; (D), SEM for stem P; (E), SEM for root N; (F), SEM for root P; (G), SEM for tuber N; and (H), SEM for tuber P. Abbreviations are as follows: T, water temperature; TN, total nitrogen content in water; NH4–N, ammonium nitrogen content in water; NO3–N, nitrate nitrogen content in water; TP, total phosphorus content in water; ORP; oxidation reduction potential of water. **, p < 0.01; *, p < 0.05.
Based on the results of RDA, some significant variables were incorporated into SEM to analyze their effects on N:P ratios (Figure 6). All SEMs were saturated. Water T had a direct negative effect on N:P ratios in each organ, WD had direct positive effect on stem N:P and root N:P, and pH had direct and indirect effect on leaf and tuber N:P ratios. The ORP had a direct positive effect on tuber N:P ratio.
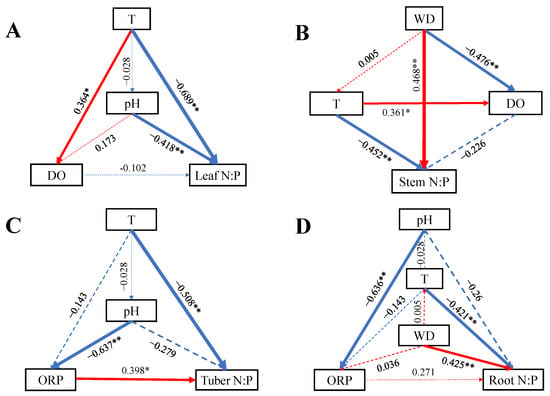
Figure 6.
SEMs of water variables on B. planiculmis organs N:P (saturated models). (A), SEM for leaf N:P; (B), SEM for stem N:P; (C), SEM for tuber N:P; and (D), SEM for root N:P. Abbreviations are as follows: T, water temperature; DO, dissolved oxygen in water; ORP; oxidation reduction potential of water; WD, water depth. **, p < 0.01; *, p < 0.05.
3. Discussion
When the water environment changes, such as with fluctuations in water level and changes in water quality, plants need to adjust their physiological metabolism to adapt to the new environment. The change in the water environment due to agricultural drainage water entering wetlands may impact plants’ absorption and distribution of nutrients [26,27]. This study revealed significant interspecific variation in N content across plant organs, with leaf tissues exhibiting the highest level. This phenomenon primarily stems from aquatic environmental changes exerting survival pressures on plants, with leaf growth and expansion serving as key adaptive strategies to environmental fluctuations. For instance, plants tend to enhance their own adaptability by increasing the N content of leaves [28]. Moreover, due to their location and physiological characteristics, leaves may possess certain advantages in obtaining nutrients such as N. Plants may preferentially allocate N to their leaves to maintain key physiological processes like photosynthesis [29]. Organs like roots and stems are primarily responsible for the absorption and transportation of water and nutrients. Their N requirements are relatively focused on maintaining basic physiological functions and providing structural support [5], consequently exhibiting a lower content than that of leaves. The tubers of B. planiculmis play the role of storing nutrients within its body. Under fluctuating aquatic conditions, nutrient availability may become unstable. B. planiculmis adapts by allocating essential nutrients, particularly P, to tuber storage for rapid remobilization during resource scarcity. In the case of rising water levels that reduce the availability of P in the soil, plants can obtain P from tubers to maintain growth. Compared with other organs, the growth and metabolism of bulbous rhizomes are relatively slow, and the demand for P is relatively stable [30]. Plants tend to prioritize the allocation of limited P resources to rhizomes to ensure their viability under adverse conditions. This also explains why the P content in B. planiculmis tubers was higher than that in leaves, stems, and roots in this study.
The N:P ratio of plant organs can reflect the nutrient restriction status of plants. A high N:P ratio implies that the plant is relatively deficient in P, and its growth is mainly limited by P. Conversely, a lower N:P ratio may indicate that the plant is relatively deficient in N and its growth is restricted by N [31]. In this study, the N:P ratio of B. planiculmis leaves was higher than that of other organs; however, it was still less than 14, indicating that plant growth was more limited by N. Agricultural drainage often brings more N into the water. Nitrogen limitation primarily arises from two interconnected factors. On the one hand, agricultural drainage induces soil waterlogging, which compromises soil aeration, thereby impairing root respiration and N uptake efficiency [32,33]. On the other hand, the N in the agricultural drainage water may predominantly exist in forms that are hard for some plants to use; or the N might have chemical imbalances, which may not be appropriate for the growth requirements of plants [15,34,35]. Therefore, changes in the wetland water environment, such as water level changes, water quality deterioration, and salinity increase, will affect the absorption and utilization of N and P by plants, thus altering the N:P ratio in the organs of B. planiculmis.
Nutrient allocation across plant organs is a highly dynamic physiological process. In the case of a changing water environment, plants may adjust their resource allocation strategies [36]. In this study, there was a significant positive correlation between N and P in the B. planiculmis tubers, stems, and leaves. Plants possess a sophisticated N and P uptake and translocation system that facilitates nutrient transport from roots to aboveground organs [37,38]. When the water environment changes, plants may adjust the distribution and metabolism of N and P according to the growth needs of different organs [39,40], resulting in the N content in each organ maintaining a relatively stable positive correlation. In addition, the relationship between root and stem N and P was not significant. This phenomenon is primarily attributed to the direct yet variable impacts of aquatic environmental changes on root and stem growth and their physiological status. For example, too much water may cause oxygen deprivation to the roots, affecting their N and P uptake and metabolism [41,42]. However, the stem is probably affected by water conduction, gas exchange, and other factors [43]. Other factors in the water environment, such as pH, salinity, and heavy metal content, may also have different effects on roots and stems, and thus affect the relationship between N and P contents [44].
Correlations and RDA results showed that water T, ORP, and WD, as well as N levels— including TN, NH4–N, and NO3–N—had important effects on the distribution pattern of N and P in B. planiculmis plants. Otherwise, RDA indicated that salinity and pH were not the main influencing factors. The main reason is that receiving agricultural drainage water has alleviated drought and saline–alkali conditions to a certain extent [18]. In addition, SEMs also confirmed that these water environment variables also directly and indirectly affected the distribution pattern of N and P in plants. Water T can affect the nutrient absorption capacity of roots, changes the metabolic process in plants, affects plant growth rate and biomass allocation, and then affects the transport and allocation of N and P in roots [45]. Water T levels that are too low or too high may inhibit plant growth, resulting in reduced biomass. In this case, plants may adjust their allocation strategies for N and P. In addition, water T affects the form and availability of N and P in the water body, as well as the solubility and diffusion rate of N and P in the water body, thus affecting the absorption of them by plants. A warmer water T generally increases the rate of respiration, making plants use more energy [45]. This may affect the N and P requirements of plants, which in turn affects the N:P ratio in roots and leaves. This also explains the effect of water T on the N and P contents and their ratios in B. planiculmis organs in the SEM. Consequently, water T significantly shapes plant N and P distribution patterns by regulating physiological processes, growth dynamics, and morphological adaptations in wetland plants, while simultaneously influencing environmental parameters. In addition, water ORP had a significant effect on the distribution pattern of N and P in B. planiculmis plants, and the change in water ORP likely affected the form of N in water. In the high-ORP environment, N mainly exists in the form of NO3–N, which is easily absorbed by plants and has strong mobility in plants [46], and P exists mainly in the form of orthophosphate, which has a high solubility and is easily absorbed by plants [47]. Therefore, the B. planiculmis plants in this situation could take up more N and P and distribute it to areas of strong growth, such as leaves and stems.
This study revealed that agricultural drainage-induced water level fluctuations significantly alter B. planiculmis N:P stoichiometry. Elevated water levels from agricultural drainage cause soil inundation, creating anaerobic conditions that suppress aerobic microbial activity, thereby inhibiting organic matter decomposition and nutrient mineralization processes [48]. For instance, the mineralization of organic N is reduced, resulting in less NH4– and NO3–N that can be taken up and utilized by plants. At the same time, the anaerobic environment will also promote some chemical reactions that are not conducive to plant growth, such as an enhancement of denitrification, so that the nitrate in the soil is converted to lost N, further reducing the N available to plants [49]. Furthermore, under anaerobic conditions, metal ions such as iron and aluminum in soil may combine with P to form insoluble compounds, and then reduce P availability [50]. In addition, water level changes can affect the growth and distribution of wetland plant roots, as well as photosynthesis [51]. Therefore, water level fluctuations directly impact both soil conditions and root development, subsequently influencing nutrient acquisition. Simultaneously, root systems adapt to water level variations through morphological and physiological adjustments, thereby modulating N and P uptake and allocation patterns.
When the contents of N and P in agricultural drainage water are high, wetland plants will absorb a large amount of N and P, resulting in a significant increase in the content of these nutrients in plants [15]. This may disrupt the balance of N and P in plants and affect the normal growth and metabolism of plants [52], as there is a certain correlation between N and P metabolism in plants. A high N content in water may cause plants to distribute more N to the aboveground portion to promote photosynthesis and growth [53]. Simultaneously, a high N content may also inhibit the uptake of P by plants or cause plants to distribute more P to the roots to maintain the normal metabolic function of the roots [54,55]. Therefore, the SEM results of this study indicated that the P content in the tubers, stems, and leaves was also directly affected by the N content in water. In addition, a high level of N tends to affect hormone levels and signaling in plants, further regulating the distribution pattern of N and P in plants [56]. Both the RDA and SEM results of this study identified TN, NH4–N, and NO3–N as key environmental drivers of N and P distribution in B. planiculmis.
4. Materials and Methods
4.1. Study Area
The study was conducted in the Momoge wetlands (45°28′–46°18′ N, 122°47′–124°43′ E), situated on the western bank of the Nenjiang River within China’s Songnen Plain. This area occupies 1440 km2, with a wetland area of 1040 km2. The wetland develops in the lakeside depression, flood plain, and low depression. The water source is supplied by Nenjiang River tributaries, surface water, and atmospheric precipitation. In this area, the climate is a continental monsoon climate. In spring, it is characterized by drought and scant rainfall, accompanied by a rapid rise in temperature. Summer is swelteringly hot, with rainfall concentrated from July to September. Autumn witnesses a sharp drop in temperature and is mostly sunny. Winter is extremely cold with little snowfall. The average annual temperature stands at 4.4 °C. The average annual precipitation is 390 mm, while the average annual evaporation amounts to 1472 mm.
The B. planiculmis communities are mainly distributed in the central and western parts of Momoge wetlands, providing a food source and habitat for rare water birds such as white cranes. The associated species of B. planiculmis communities in the wetlands located in the transition area of water and land are Suaeda salsa, Setaria viridis, Leymus chinensis, Puccinellia tenuiflora, and Poa spp. In flooded areas, there is often a single population, or one associated with Phragmites australis, Scirpus validus, and Typha angustifolia. At present, the wetlands are undergoing an agricultural drainage water input, which has changed the water environment, and also affected the dynamics of the B. planiculmis community.
4.2. Field Survey and Sampling
In this study, we selected 10 B. planiculmis wetland sites in the middle of the Momoge wetlands as the research area (Figure 7). These sites were flooded and mainly dominated by B. planiculmis populations. The method of combining a transect and quadrat was adopted, and 3 transects (50 m length × 10 m width) at a distance of >20 m were set at each wetland site, and five 1 m × 1 m quadrats were randomly set along the transects. Starting from 10 May 2021, the water depth was recorded in each quadrat every two weeks, and a portable multi-parameter water quality meter (YSI ProQuatro, Columbus, OH, USA) was used to measure the water temperature (T, °C); pH; dissolved oxygen (DO, mg⋅L−1); electrical conductivity (EC, ms cm−1); total dissolved solids including inorganic salts, organics, and ions (TDS, mg⋅L−1); salinity (Sal, ppt); and oxidation reduction potential (ORP, mv). At the same time, wetland water samples were collected and brought back to the laboratory for the determination of total nitrogen content (TN, mg⋅L−1), ammonium nitrogen content (NH4–N, mg⋅L−1), nitrate nitrogen content (NO3–N, mg⋅L−1), and total P content (TP, mg⋅L−1). TN in water was determined by ultraviolet spectrophotometry with potassium persulfate digestion. NH4–N was determined by spectrophotometry with Nesser’s reagent. NO3–N was determined by sulfamic acid ultraviolet spectrophotometry. TP in water was determined by ammonium molybdate spectrophotometry. The water quality was measured until the end of August, and then averaged to represent the various water conditions.
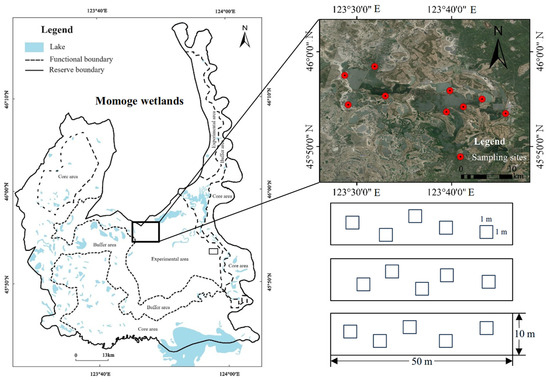
Figure 7.
Schematic diagram of study area, sampling sites, and experimental design.
At the end of August, according to the above transects and quadrats, the aboveground part of B. planiculmis was collected, and the stems and leaves were separated. Root samples within 0–30 cm soil depth were collected and washed with clean water. Afterward, the root tubers were separated and counted. The stem, leaf, root, and tuber samples were placed in a drying oven at 65 °C to reach a constant weight. The contents of N and P were determined after crushing. Plant N was determined using the micro-Kjeldahl method. Plant P was determined using the Vanadate–Molybdate–Yellow colorimetry method. Nitrogen to phosphorus (N:P) ratio was obtained by dividing N content by P content.
4.3. Data Analysis
Data were analyzed by SPSS 16.0, Canoco 5.0, and R v3.4.2. One-way analyses of variance (ANOVAs) were employed to explore the differences in N and P contents among plant leaves, stems, tubers, and roots at a significance level of p ≤ 0.05. Pearson’s correlation analyses were used to explore correlations between N and P contents among plant organs and water variables at p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001 levels. A redundancy analysis (RDA) was employed to identify the main water variables that affect the N and P stoichiometry in plants. Structural equation models (SEMs) were used to evaluate the influencing pathways of water variables on N and P stoichiometry in plants. Prior to the SEM procedure, we reduced the number of water variables based on the results of RDA while considering the collinearity among variables. The chi-squared test (χ2) and p-value were used to evaluate the model fitness.
5. Conclusions
The wetlands in semi-arid regions currently face the dual stressors of drought and saline alkalization. While agricultural drainage water supplementation partially mitigates water scarcity, it significantly alters plant N and P stoichiometry. Firstly, there were significant differences in N, P, and the N:P ratio among B. planiculmis organs. Secondly, there were significant positive correlations between the N:P contents in all organs, except for the relationship between roots and stems. Thirdly, RDA indicated that water T, ORP, N contents, and WD are the main factors affecting N and P stoichiometry. SEMs further demonstrated the direct and indirect effects of these water variables. This study demonstrated that agricultural drainage water inputs mitigated wetland water scarcity and saline–alkali constraints on plant nutrient dynamics, while simultaneously amplifying the influence of key water variables (T, WD, N, and ORP) on plant N and P stoichiometry. Therefore, wetland vegetation and white crane habitat restoration in arid/semi-arid regions requires integrated consideration of hydrological regimes, water quality parameters, and water source characteristics.
Author Contributions
Y.A.: conceptualization, original draft, writing—review and editing, L.W.: review and software. B.L.: investigation, writing, review, and correspondence with the journal. H.W.: data curation, writing, review, and editing. S.T.: investigation, writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (41771108; 42471066), the National Key Research and Development Program of China (2022YFF1300900; 2024YFF1306400), the Key Research and Development Program of Ningxia Hui Autonomous Region, China (2024BEG02001), the Science and Technology Development Program of Jilin Province of China (20230203003SF; 20240602026RC), and the Major Scientific and Technological Project of Jilin Province of China (20230303005SF).
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Li, L.; Chen, D.; Huang, X.; Liu, Q.; Liang, J.; Hu, J.; Liu, Q. Variations of nitrogen and phosphorus between leaf, stem and root in shrubland biomes and responses to climate and soil factors across the Hengduan Mountains, China. Catena 2024, 241, 108008. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Bioch. 2021, 158, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Xiao, W.; Mu, Q.; Li, D.; Chen, X.; Wu, H.; Li, L.; Peng, F. How does nitrate regulate plant senescence? Plant Physiol. Bioch. 2020, 157, 60–69. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Peng, Q.; Li, K.; Gong, Y.; Liu, Y.; Han, W. Patterns of nitrogen and phosphorus stoichiometry among leaf, stem and root of desert plants and responses to climate and soil factors in Xinjiang, China. Catena 2021, 199, 105100. [Google Scholar] [CrossRef]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 2014, 95, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S.; Freeman, C. Nutrient limitation and enzyme activities during litter decomposition of nine wetland species in relation to litter N:P ratios. Funct. Ecol. 2005, 19, 582–593. [Google Scholar] [CrossRef]
- Hong, M.G.; Kim, J.G. Effects of initial density, nutrient, and water level regime on the seedling survival and growth of Typha orientalis Presl. J. Plant Biol. 2016, 59, 369–376. [Google Scholar] [CrossRef]
- An, Y.; Song, T.; Zhang, Y.; Tong, S.; Liu, B. Optimum water depth for restoration of Bolboschoenus planiculmis in wetlands in semi-arid regions. Hydrobiologia 2022, 849, 13–28. [Google Scholar] [CrossRef]
- Magee, T.K.; Kentula, M.E. Response of wetland plant species to hydrologic conditions. Wetl. Ecol. Manag. 2005, 13, 163–181. [Google Scholar] [CrossRef]
- Li, B.; Yang, G.; Wan, R.; Lai, X.; Wagner, P.D. Impacts of hydrological alteration on ecosystem services changes of a large river-connected lake (Poyang Lake), China. J. Environ. Manag. 2022, 310, 114750. [Google Scholar] [CrossRef] [PubMed]
- Mitsch, W.J.; Gosselink, J.G. Wetlands; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- O’geen, A.T.; Budd, R.; Gan, J.; Maynard, J.J.; Parikh, S.J.; Dahlgren, R.A. Mitigating nonpoint source pollution in agriculture with constructed and restored wetlands. Adv. Agron. 2010, 108, 1–76. [Google Scholar]
- Blann, K.L.; Anderson, J.L.; Sands, G.R.; Vondracek, B. Effects of agricultural drainage on aquatic ecosystems: A review. Crit. Rev. Env. Sic. Technol. 2009, 39, 909–1001. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, L.; Xue, Z.; E, M.; Jiang, M.; Lu, X.; Yang, S.; Shen, X.; Liu, Z.; Sun, G.; et al. Impacts of agricultural and reclamation practices on wetlands in the Amur River Basin, Northeastern China. Wetlands 2018, 38, 383–389. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiao, L.; Qin, H.; Li, F. Effect of environmental stress on the nutrient stoichiometry of the clonal plant Phragmites australis in inland riparian wetlands of Northwest China. Front. Plant Sci. 2021, 12, 705319. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Gao, Y.; Tong, S. Emergence and growth performance of Bolboschoenus planiculmis varied in response to water level and soil planting depth: Implications for wetland restoration using tuber transplantation. Aquat. Bot. 2018, 148, 10–14. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, S.; Yu, X.; Fu, G.; Lu, X. Isotopic evidence for soil water sources and reciprocal movement in a semi-arid degraded wetland: Implications for wetland restoration. Fund. Res. 2023, 3, 861–867. [Google Scholar] [CrossRef]
- An, Y.; Gao, Y.; Zhang, Y.; Tong, S.; Liu, X. Early establishment of Suaeda salsa population as affected by soil moisture and salinity: Implications for pioneer species introduction in saline-sodic wetlands in Songnen Plain, China. Ecol. Indic. 2019, 107, 105654. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Jiang, M.; Lu, X. Vegetation change and its response to climate change between 2000 and 2016 in marshes of the Songnen Plain, Northeast China. Sustainability 2020, 12, 3569. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Jiang, M.; Tong, S.; Lu, X. Daytime and nighttime temperatures exert different effects on vegetation net primary productivity of marshes in the western Songnen Plain. Ecol. Indic. 2022, 137, 108789. [Google Scholar] [CrossRef]
- Song, T.; An, Y.; Tong, S.; Zhang, W.; Wang, X.; Wang, L.; Jiang, L. Soil water conditions together with plant nitrogen acquisition strategies control vegetation dynamics in semi-arid wetlands undergoing land management changes. Catena 2023, 227, 107115. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, M.; Tong, S.; Zhang, W.; Zou, C.; Wang, B.; Lu, X. Effects of burial depth and water depth on seedling emergence and early growth of Scirpus planiculmis Fr. Schmidt. Ecol. Eng. 2016, 87, 30–33. [Google Scholar] [CrossRef]
- An, Y.; Gao, Y.; Tong, S.; Liu, B. Morphological and physiological traits related to the response and adaption of Bolboschoenus planiculmis seedlings grown under salt-alkaline stress conditions. Front. Plant Sci. 2021, 12, 567782. [Google Scholar] [CrossRef] [PubMed]
- Moloney, T.; Fenton, O.; Daly, K. Ranking connectivity risk for phosphorus loss along agricultural drainage ditches. Sci. Total Environ. 2020, 703, 134556. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Salinization and drainage problems of agricultural land. Irrig. Drain. 2020, 69, 844–853. [Google Scholar] [CrossRef]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Chen, Z.; Li, Y.; Wang, R.; Yu, G.; Sun, W.; Xiao, C.; et al. Variation and evolution of C:N ratio among different organs enable plants to adapt to N-limited environments. Glob. Change Biol. 2020, 26, 2534–2543. [Google Scholar] [CrossRef]
- Zhong, C.; Jian, S.; Huang, J.; Jin, Q.; Cao, X. Trade-off of within-leaf nitrogen allocation between photosynthetic nitrogen-use efficiency and water deficit stress acclimation in rice (Oryza sativa L.). Plant Physiol. Bioch. 2019, 135, 41–50. [Google Scholar] [CrossRef]
- Li, K.; Ren, H.; Zhao, W.; Zhao, X.; Gan, C. Factors affecting bulblet multiplication in bulbous plants. Sci. Hortic. 2023, 312, 111837. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y. Plant mixture balances terrestrial ecosystem C:N:P stoichiometry. Nat. Commun. 2021, 12, 4562. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312. [Google Scholar] [CrossRef]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Env. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous fertilizers: Impact on environment sustainability, mitigation strategies, and challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–11672. [Google Scholar] [CrossRef]
- Yang, Z.; Midmore, D.J. Modelling plant resource allocation and growth partitioning in response to environmental heterogeneity. Ecol. Model. 2005, 181, 59–77. [Google Scholar] [CrossRef]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root development and nutrient uptake. Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Nacry, P.; Bouguyon, E.; Gojon, A. Nitrogen acquisition by roots: Physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil 2013, 370, 1–29. [Google Scholar] [CrossRef]
- Xing, W.; Han, Y.; Guo, Z.; Zhou, Y. Quantitative study on redistribution of nitrogen and phosphorus by wetland plants under different water quality conditions. Environ. Pollut. 2020, 261, 114086. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Shabala, L.; Barcelo, J.; Poschenrieder, C. Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ. 2014, 37, 2216–2233. [Google Scholar] [CrossRef] [PubMed]
- Daniel, K.; Hartman, S. How plant roots respond to waterlogging. J. Exp. Bot. 2024, 75, 511–525. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, Z.; Shen, X.; Liu, D.; Pedersen, O. Flooding-adaptive root and shoot traits in rice. Funct. Plant Biol. 2024, 51, FP23226. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jiang, D.; Chen, Y.; Wei, J.; Xia, F.; Xie, W.; Zhou, Y.; Li, X.; Deng, S. Spatial-temporal distribution, morphological transformation, and potential risk of dissolved inorganic nitrogen in the contaminated unconfined aquifer from a retired nitrogenous fertilizer plant. J. Environ. Res. Public Health 2022, 19, 8022. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, Y.; Dong, Y. A review of methods, influencing factors and mechanisms for phosphorus recovery from sewage and sludge from municipal wastewater treatment plants. J. Environ. Chem. Eng. 2023, 12, 111657. [Google Scholar] [CrossRef]
- Ou, Y.; Rousseau, A.N.; Wang, L.; Yan, B.; Gumiere, T.; Zhu, H. Identification of the alteration of riparian wetland on soil properties, enzyme activities and microbial communities following extreme flooding. Geoderma 2019, 337, 825–833. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef]
- Wang, Y.; Lambers, H. Root-released organic anions in response to low phosphorus availability: Recent progress, challenges and future perspectives. Plant Soil 2020, 447, 135–156. [Google Scholar] [CrossRef]
- Lai, W.L.; Wang, S.Q.; Peng, C.L.; Chen, Z.H. Root features related to plant growth and nutrient removal of 35 wetland plants. Water Res. 2011, 45, 3941–3950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, C.; Li, H.; Liu, J.; Jiang, T.; Yan, D.; Tong, J.; Dong, L. Review on ecological response of aquatic plants to balanced harvesting. Sustainability 2022, 14, 12451. [Google Scholar] [CrossRef]
- Reich, P.B.; Hobbie, S.E.; Lee, T.D. Plant growth enhancement by elevated CO2 eliminated by joint water and nitrogen limitation. Nat. Geosci. 2014, 7, 920–924. [Google Scholar] [CrossRef]
- Touchette, B.W.; Burkholder, J.M. Review of nitrogen and phosphorus metabolism in seagrasses. J. Exp. Mar. Biol. Ecol. 2000, 250, 133–167. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Wu, H.H.; Smith, M.D.; La Pierre, K.J.; Lü, X.T.; Zhang, H.Y.; Han, X.G.; Yu, Q. Nitrogen deposition promotes phosphorus uptake of plants in a semi-arid temperate grassland. Plant Soil 2016, 408, 475–484. [Google Scholar] [CrossRef]
- Guan, P. Dancing with hormones: A current perspective of nitrate signaling and regulation in Arabidopsis. Front. Plant Sci. 2017, 8, 1697. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).