Plant Architecture, Tolerances to NaCl and Heavy Metals May Predispose Tragus racemosus to Growth Around Motorways
Abstract
1. Introduction
2. Results
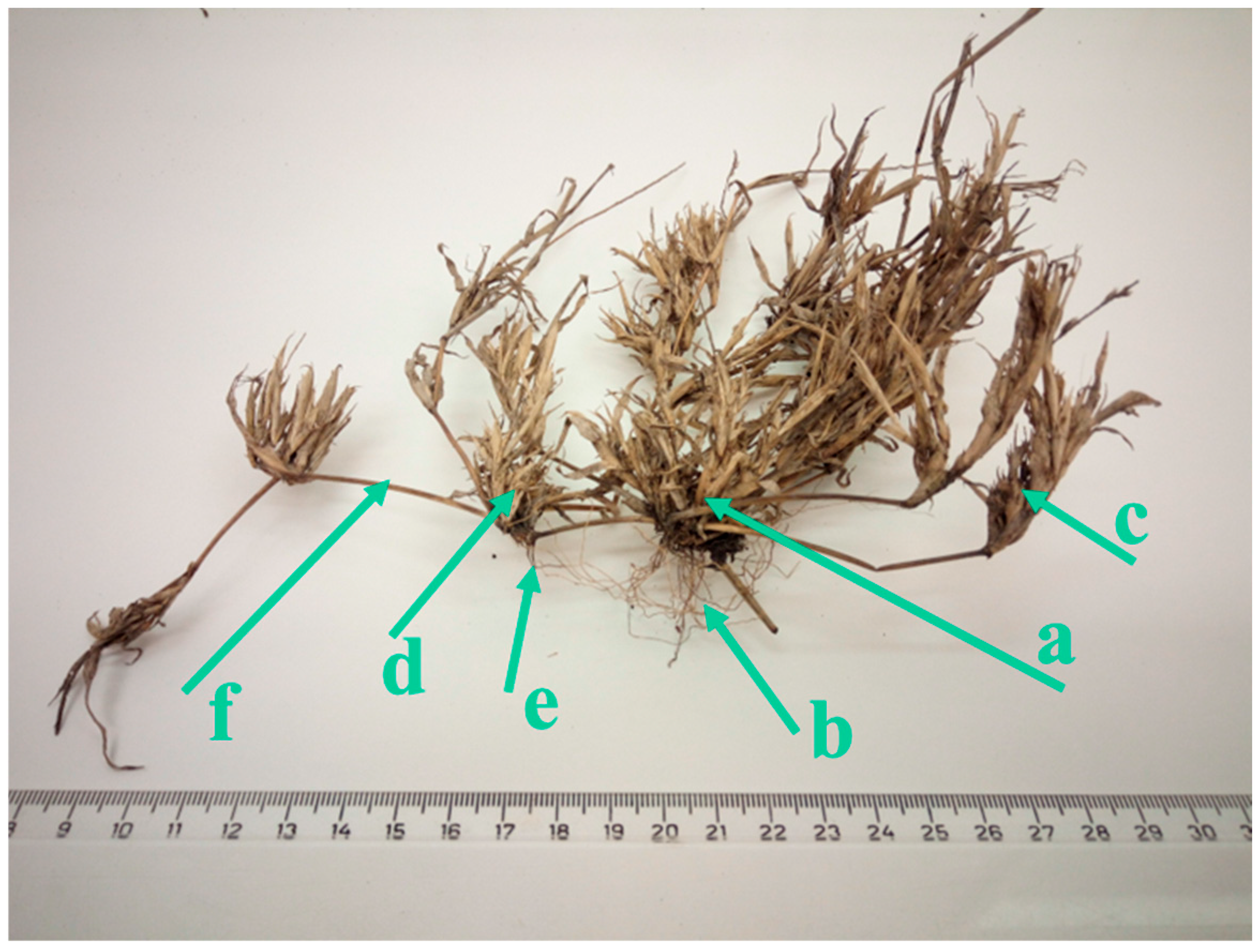
2.1. Plant Morphology and Reproductive Capacity
2.2. Heavy Metals in Plant Biomass and Soil
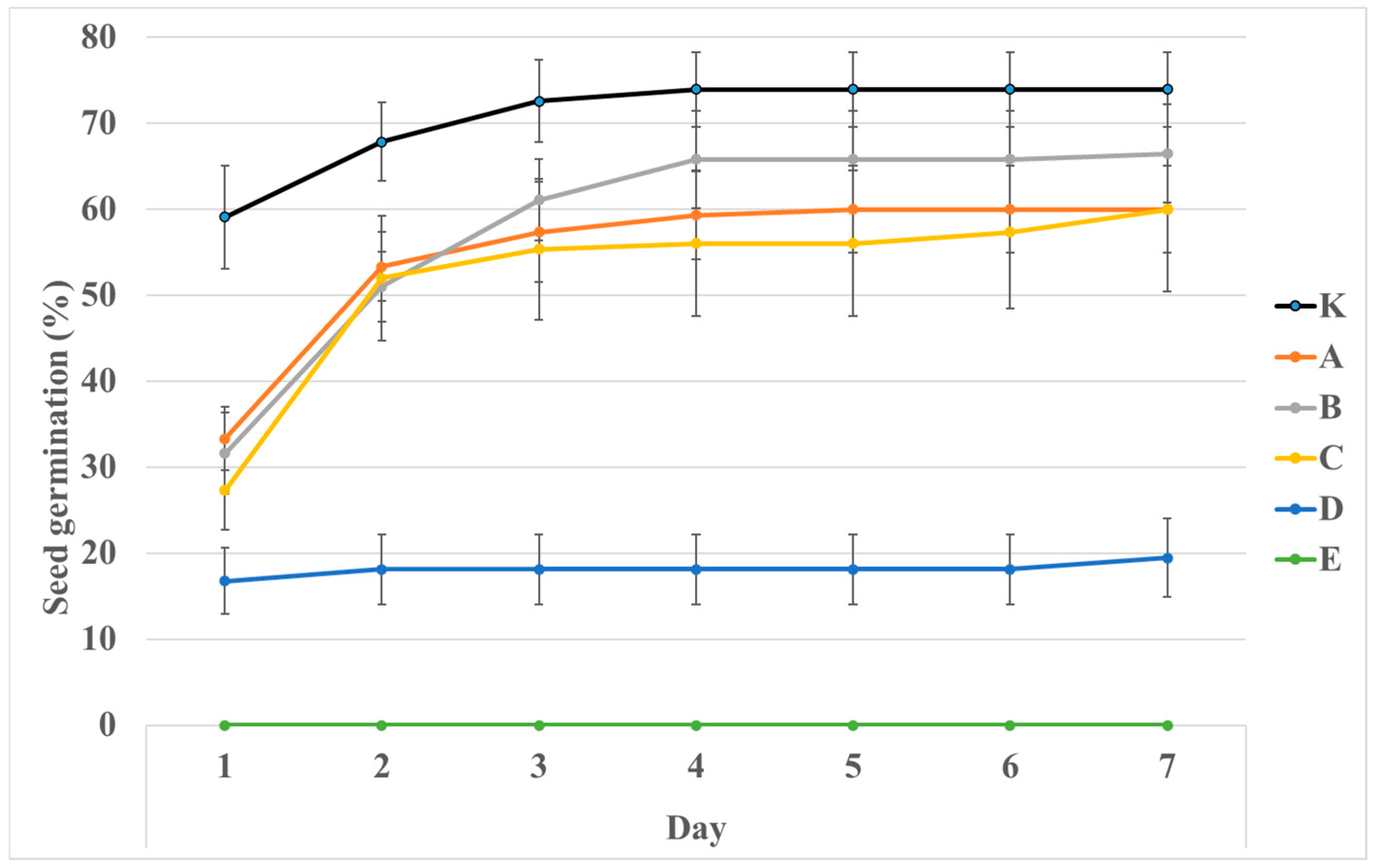
2.3. Salt Stress Test via Seed Germination
3. Discussion
3.1. Plant Architecture and Reproductive Capacity
3.2. Heavy Metal Analyses
3.3. Salt Stress Test
4. Materials and Methods
4.1. Habitat Characteristics
4.2. Plant Architecture and Reproductive Capacity
4.3. Heavy Metal Analyses (in Plant and in Soil)
4.4. Salt Stress Test via Seed Germination
4.5. Data Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eliáš, P.j.; Dítě, D.; Kliment, J.; Hrivnák, R.; Feráková, V. Red list of ferns and flowering plants of Slovakia, 5th ed. Biológia 2015, 70, 218–228. [Google Scholar] [CrossRef]
- Baláž, D. Tragus racemosus (L.) All. na Devínskej Kobyle. Bull. Slov. Bot. Spoločn. 1995, 17, 91–92. [Google Scholar]
- Kocián, P.; Ducháček, M.; Kúr, P. Bodloplev hroznatý (Tragus racemosus) na dálnicích České republiky. Zprávy Čes. Bot. Společ. 2018, 53, 1–9. [Google Scholar]
- Kaplan, Z.; Danihelka, J.; Chrtek, J.j.; Kirschner, J.; Kubát, K.; Štěpánek, J. (Eds.) Klíč ke Květeně České Republiky; Academia: Praha, Czech Republic, 2019; 1168p. [Google Scholar]
- Tutin, I.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1980; 486p. [Google Scholar]
- Schmidt, D.; Fekete, R.; Kis, S. The synanthropic spread of some salt tolerant species along roadsides in the continental part of Croatia. Nat. Croat. 2023, 32, 381–398. [Google Scholar] [CrossRef]
- Anton, A.M. The genus Tragus (Gramineae). Kew Bull. 1981, 36, 55–61. [Google Scholar] [CrossRef]
- Reeder, J.R.; Reeder, C. Tragus racemosus in Arizona. Madroño 1978, 25, 107–108. [Google Scholar]
- Šerá, B.; Žarnovičan, H. Bodnoplev hroznatý podél okraje silnice—Případová studie Selenec. In Proceedings of the Vliv Abiotických a Biotických Stresorů na Vlastnosti Rostlin, Praha, Czech Republic, 3–14 September 2023. [Google Scholar]
- Šerá, B.; Ghulam, M.; Doshi, P.; Žarnovičan, H. Bodloplev hroznatý má specifickou strategii a snáší zasolení půdy—Případová studie Selenec. In Proceedings of the Vliv Abiotických a Biotických Stresorů na Vlastnosti Rostlin, Zvolen, Slovakia, 11–12 September 2024. [Google Scholar]
- Kumar, N.; Haldar, S.; Saikia, R. Root exudation as a strategy for plants to deal with salt stress: An updated review. Environ. Exp. Bot. 2023, 216, 105518. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S.; Mandi, S. Phytosiderophores and absorption of iron and other cations by plants. In Cation Transporters in Plants; Upadhyay, S.K., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2022; pp. 385–399. [Google Scholar] [CrossRef]
- Pouresmaieli, M.; Ataei, M.; Forouzandeh, P.; Azizollahi, P.; Mahmoudifard, M. Recent progress on sustainable phytoremediation of heavy metals from soil. J. Environ. Chem. Eng. 2022, 10, 108482. [Google Scholar] [CrossRef]
- Patra, D.K.; Acharya, S.; Pradhan, C.; Patra, H.K. Poaceae plants as potential phytoremediators of heavy metals and eco-restoration in contaminated mining sites. Environ. Technol. Innov. 2021, 21, 101293. [Google Scholar] [CrossRef]
- Baker, D.V.; Beck, K.G. The weed tunnel: Building an experimental wind tunnel. Weed Technol. 2008, 22, 549–552. [Google Scholar] [CrossRef]
- Banerjee, R.; Goswami, P.; Pathak, K.; Mukherjee, A. Vetiver grass: An environment clean-up tool for heavy metal contaminated iron ore mine-soil. Ecol. Eng. 2016, 90, 25–34. [Google Scholar] [CrossRef]
- Nouri, J.; Khorasani, N.; Lorestani, B.; Karami, M.; Hassani, A.H.; Yousefi, N. Accumulation of heavy metals in soil and uptake by plant species with phytoremediation potential. Environ. Earth Sci. 2009, 56, 315–323. [Google Scholar] [CrossRef]
- Wong, H.M.; Hofmann, R.W.; Reichman, S.M. The interactions of iron nutrition, salinity and ultraviolet-B radiation on the physiological responses of wheat (Triticum aestivum L.). Environ. Exp. Bot. 2023, 207, 105201. [Google Scholar] [CrossRef]
- Nanda, K.; Singh, M.; Yadav, T.; Tiwari, V.K.; Singh, V.; Singh, V.P.; Sawant, S.V.; Singh, S.P. Genome-wide identification and expression analysis of ferric reductase oxidase (FRO) genes in Gossypium spp. Reveal their crucial role in iron homeostasis under abiotic and biotic stress. Plant Physiol. Biochem. 2024, 217, 109281. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.F.R.; Almeida, M.C.; Sousa, E.; Resende, D.I.S.P. Siderophores and metallophores: Metal complexation weapons to fight environmental pollution. Sci. Total Environ. 2024, 932, 173044. [Google Scholar] [CrossRef] [PubMed]
- Nováková, M.; Šerá, B.; Cudlín, P. Roadside habitats: The impact of salinization on the occurrence, growth and reproduction of two weed species Echinochloa crus-galli and Digitaria sanguinalis. Pol. J. Ecol. 2019, 67, 186–193. [Google Scholar] [CrossRef]
- Mazúr, E.; Lukniš, M. Geomorfologické Členenie SSR a ČSSR. Časť Slovensko; Slovenská Kartografia: Bratislava, Slovakia, 1986; pp. 54–55. [Google Scholar]
- Lapin, M.; Faško, P.; Melo, M.; Šťastný, P.; Tomlain, J. Klimatické Oblasti. Atlas Krajiny Slovenskej Republiky; Ministerstvo životného Prostredia; Slovenská Agentúra Životného Prostredia: Banská Bystrica, Slovakia, 2002; p. 95. [Google Scholar]
- Faško, P.; Šťastný, P. Priemerné Ročné Úhrny Zrážok. Atlas Krajiny Slovenskej Republiky; Ministerstvo Životného Prostredia; Slovenská Agentúra Životného Prostredia: Banská Bystrica, Slovakia, 2002; p. 99. [Google Scholar]
- Molnárová, M.; Fargašová, A. Relationship between various physiological and biochemical parameters activated by cadmium in Sinapis alba L. and Hordeum vulgare L. Ecol. Eng. 2012, 49, 65–72. [Google Scholar] [CrossRef]
- Istran. Determination of Zn, Cd, Pb and Cu in Clear as Well as in Turbid Samples; Application list No. 67, manual guide v.2009-10 for EcaFlow 150GLP; Istran: Bratislava, Slovakia, 2009; 2p. [Google Scholar]
- Šerá, B. Methodological contribution on seed germination and seedling initial growth tests in wild plants. Not. Bot. Horti Agrobot. 2023, 51, 13164. [Google Scholar] [CrossRef]
- Dell Inc. Dell Statistica (Data Analysis Software System), version 13.2; Dell Inc.: Round Rock, TX, USA, 2016. Available online: http://www.statsoft.cz/file1/PDF/Dell_Statistica%2013_releasenotes_english.pdf (accessed on 10 September 2024).
| (Semi)Metal | As | Cd | Cr | Cu | Fe | Hg |
|---|---|---|---|---|---|---|
| Soil (mg kg−1 DM) | nd | nd | nd | 6.59 ± 0.43 | 52,783 ± 1416 | nd |
| T. racemosus (mg kg−1 DM) | nd | nd | nd | 1.57 ± 0.09 | 3715 ± 323 | nd |
| BCF | - | - | - | 0.24 | 0.07 | - |
| Concentration range (µg L−1) | 0.5–200 | 0.5–1000 | 2–200 | 0.5–1000 | 10–500 | 0.1–1000 |
| Reproducibility | 4.2% at 50 µg L−1 | 1.5% at 50 µg Pb L−1 * | 1.8% at 100 µg L−1 | 1.5% at 50 µg Pb L−1 * | 1.5% at 200 µg L−1 | 2.0% at 50 µg L−1 |
| (Semi)Metal | Mn | Ni | Pb | Sb | Se | Zn |
| Soil (mg kg−1 DM) | nd | 4.37 ± 0.05 | 20.53 ± 3.26 | nd | nd | nd |
| T. racemosus (mg kg−1 DM) | nd | 15.92 ± 0.36 | 14.30 ± 1.44 | nd | nd | 19.74 ± 1.66 |
| BCF | - | 3.64 | 0.7 | - | - | - |
| Concentration range (µg L−1) | 1–500 | 1–200 | 0.5–1000 | 0.5–500 | 0.5–500 | 0.5–1000 |
| Reproducibility | 1.5% at 50 µg L−1 | 2.5% at 50 µg L−1 | 1.5% at 50 µg Pb L−1 * | 3.2% at 50 µg L−1 | 3.2% at 50 µg L−1 | 1.5% at 50 µg Pb L−1 * |
| NaCl (%) | Seed Germination (%) | Germination Index | |||||
|---|---|---|---|---|---|---|---|
| Mean | SE | HSD | Mean | SE | HSD | ||
| K | 0.00 | 73.931 | 4.820 | a | 51.610 | 3.944 | a |
| A | 0.12 | 60.000 | 5.676 | a | 37.355 | 3.226 | d |
| B | 0.25 | 66.460 | 6.475 | a | 37.982 | 4.081 | d |
| C | 0.50 | 60.000 | 3.801 | a | 34.531 | 2.285 | d |
| D | 0.99 | 19.471 | 1.273 | b | 13.659 | 0.646 | b |
| E | 1.96 | - | - | - | - | - | - |
| NaCl (%) | Seedling Fresh Weight (mg) | Seedling Dry Matter Weight (mg) | Shoot Length (mm) | Root Length (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | ||
| K | 0.00 | 1.637 | 0.116 | a | 0.214 | 0.018 | a | 11.173 | 0.827 | a | 36.073 | 2.657 | a |
| A | 0.12 | 1.395 | 0.109 | a | 0.156 | 0.011 | a | 9.127 | 0.796 | a | 30.733 | 2.423 | a |
| B | 0.25 | 1.556 | 0.117 | a | 0.189 | 0.008 | a | 9.577 | 0.941 | a | 33.749 | 3.430 | a |
| C | 0.50 | 1.332 | 0.078 | a | 0.182 | 0.010 | a | 7.287 | 0.407 | a | 27.713 | 1.539 | a |
| D | 0.99 | - | - | - | - | - | - | - | - | - | - | - | - |
| E | 1.96 | - | - | - | - | - | - | - | - | - | - | - | - |
| NaCl (%) | Seedling Vigor Index I (mm) | Seedling Vigor Index II (mg) | Seedling Vigor Index III (mg) | Root-to-Shoot Length Ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | Mean | SE | HSD | ||
| K | 0.00 | 3557.856 | 490.040 | a | 123.246 | 16.877 | a | 15.942 | 1.983 | a | 3.230 | 0.066 | a |
| A | 0.12 | 2464.033 | 420.070 | a | 86.184 | 14.533 | a | 9.582 | 1.594 | a | 3.378 | 0.079 | ab |
| B | 0.25 | 2990.579 | 531.397 | a | 106.324 | 16.535 | a | 12.695 | 1.677 | a | 3.523 | 0.059 | ac |
| C | 0.50 | 2125.356 | 238.145 | a | 80.891 | 9.131 | a | 11.016 | 1.128 | a | 3.804 | 0.006 | d |
| D | 0.99 | - | - | - | - | - | - | - | - | - | - | - | - |
| E | 1.96 | - | - | - | - | - | - | - | - | - | - | - | - |
| Locality | Nitra Selenec, Feeder to Expressway R1, Direction Towards Trnava, in Operation Since 2011 |
|---|---|
| Coordinates | 48°18′36.33″ N, 18°08′25.49″ E |
| Description of habitat | Left edge of the road under the guard rail |
| Altitude (m a.s.l.) | 161 |
| Exposition | SE-S |
| Slope (°) | 10 |
| Geomorphological unit [22] | Podunajská pahorkatina |
| Climatic region [23] | Warm region, 50 or more summer days annually on average (with daily maximum air temperature ≥ 25 °C) |
| Mean annual precipitation total (mm) [24] | 500–600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šerá, B.; Molnárová, M.; Ghulam, M.; Doshi, P.; Žarnovičan, H. Plant Architecture, Tolerances to NaCl and Heavy Metals May Predispose Tragus racemosus to Growth Around Motorways. Plants 2025, 14, 784. https://doi.org/10.3390/plants14050784
Šerá B, Molnárová M, Ghulam M, Doshi P, Žarnovičan H. Plant Architecture, Tolerances to NaCl and Heavy Metals May Predispose Tragus racemosus to Growth Around Motorways. Plants. 2025; 14(5):784. https://doi.org/10.3390/plants14050784
Chicago/Turabian StyleŠerá, Božena, Marianna Molnárová, Mustafa Ghulam, Pratik Doshi, and Hubert Žarnovičan. 2025. "Plant Architecture, Tolerances to NaCl and Heavy Metals May Predispose Tragus racemosus to Growth Around Motorways" Plants 14, no. 5: 784. https://doi.org/10.3390/plants14050784
APA StyleŠerá, B., Molnárová, M., Ghulam, M., Doshi, P., & Žarnovičan, H. (2025). Plant Architecture, Tolerances to NaCl and Heavy Metals May Predispose Tragus racemosus to Growth Around Motorways. Plants, 14(5), 784. https://doi.org/10.3390/plants14050784







