Identification of Phenotypic Diversity and DArTseq Loci Associated with Vitamin A Contents in Turkish Common Bean Germplasm Through GWAS
Abstract
1. Introduction
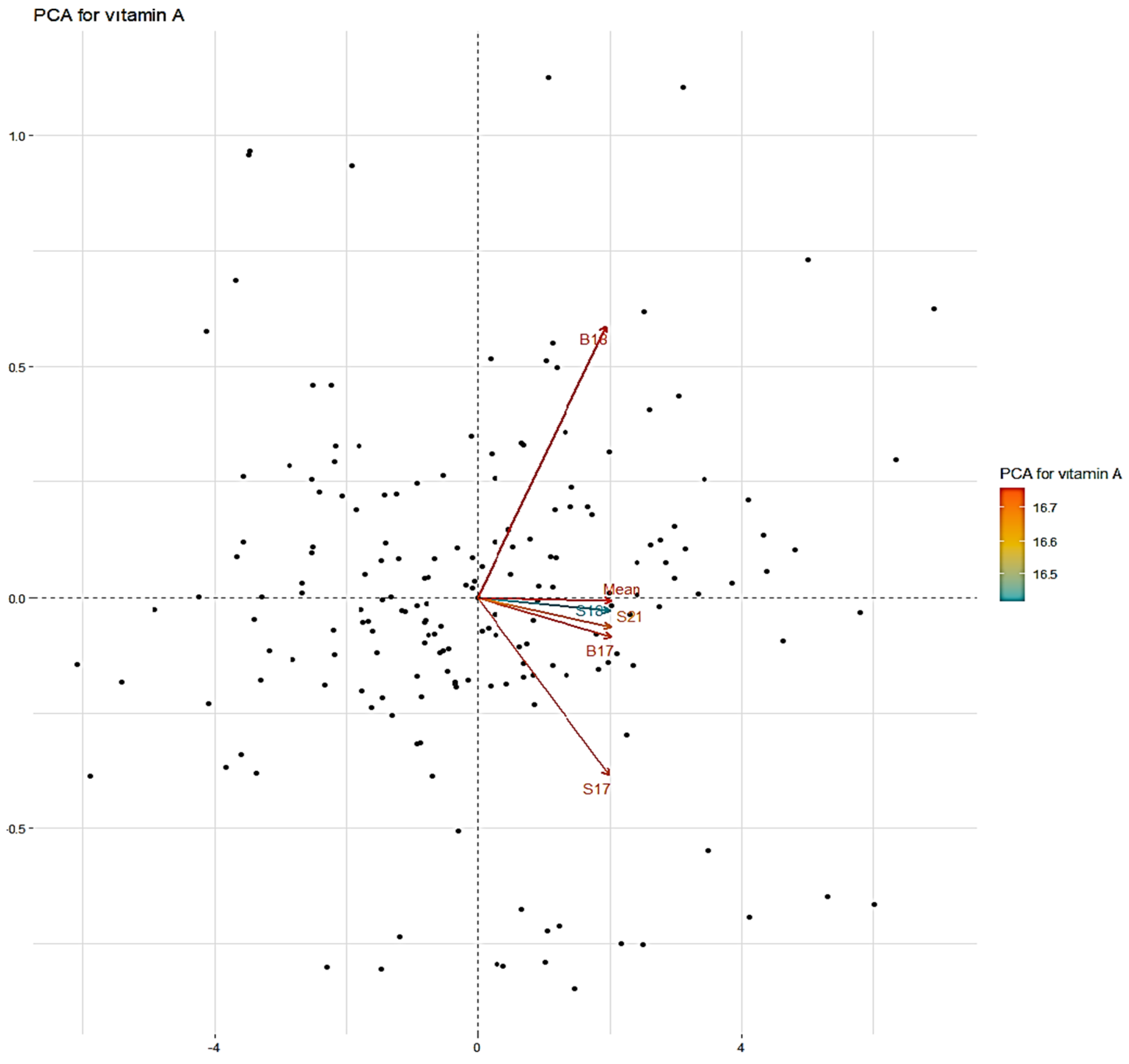
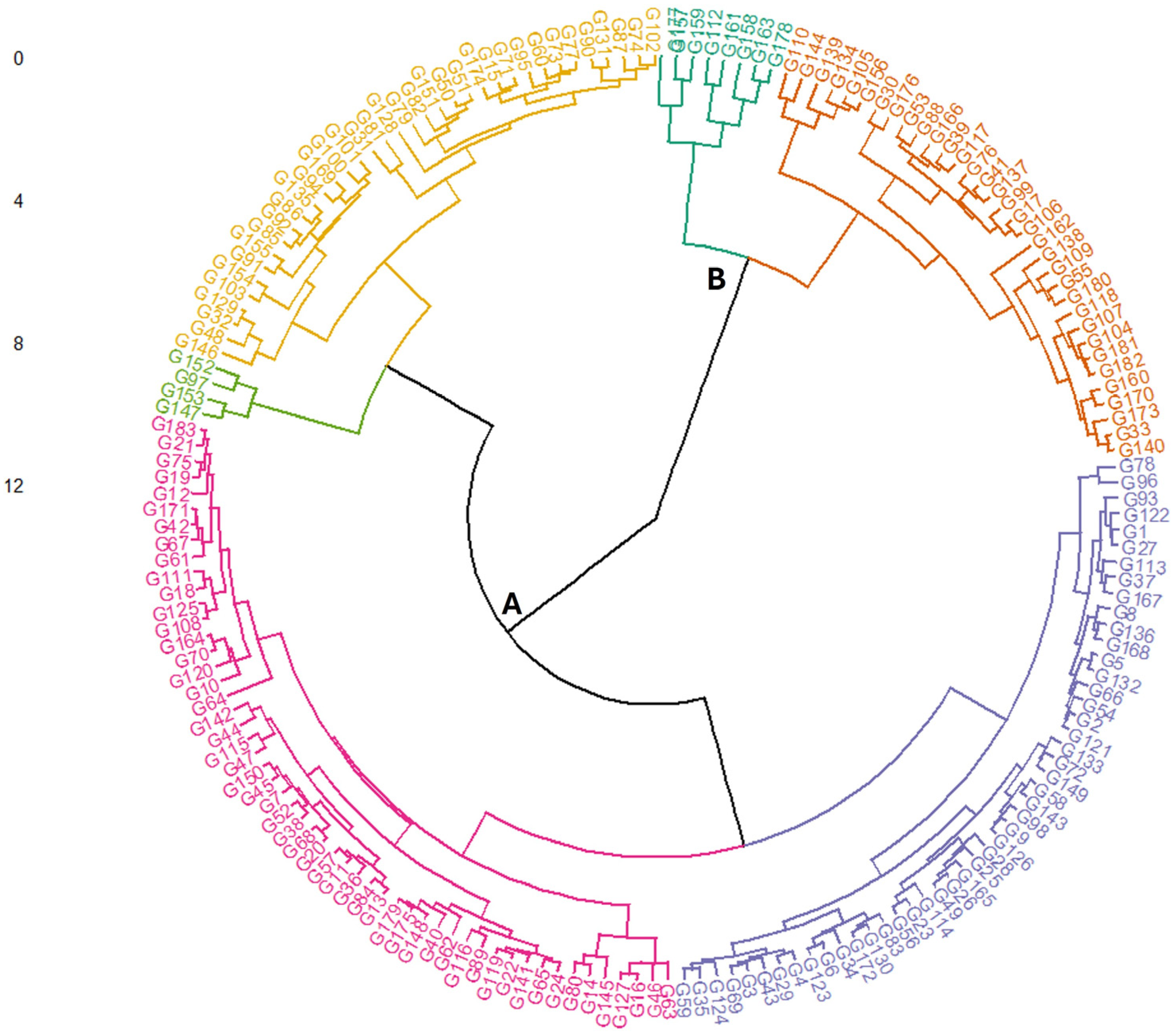
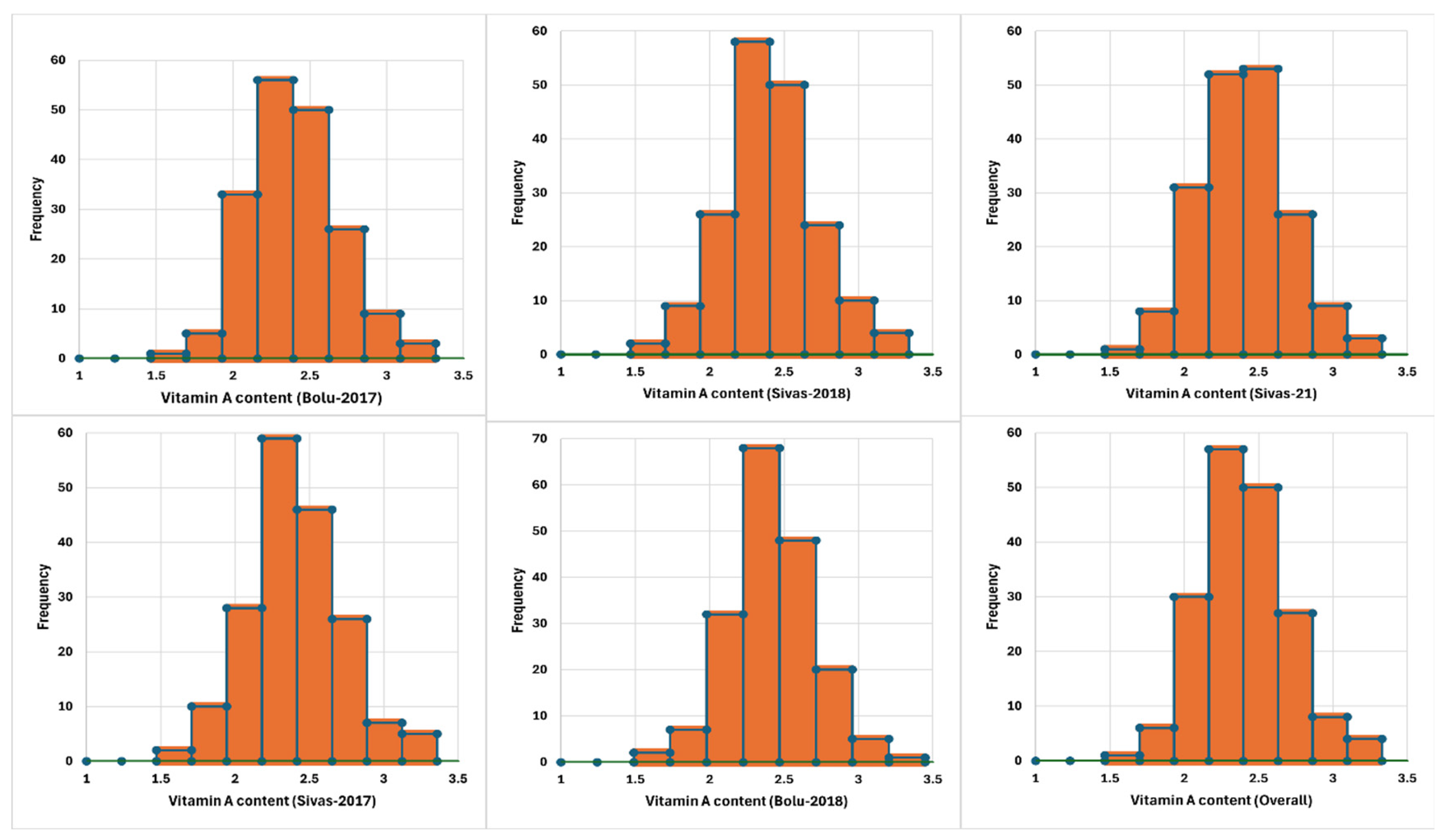
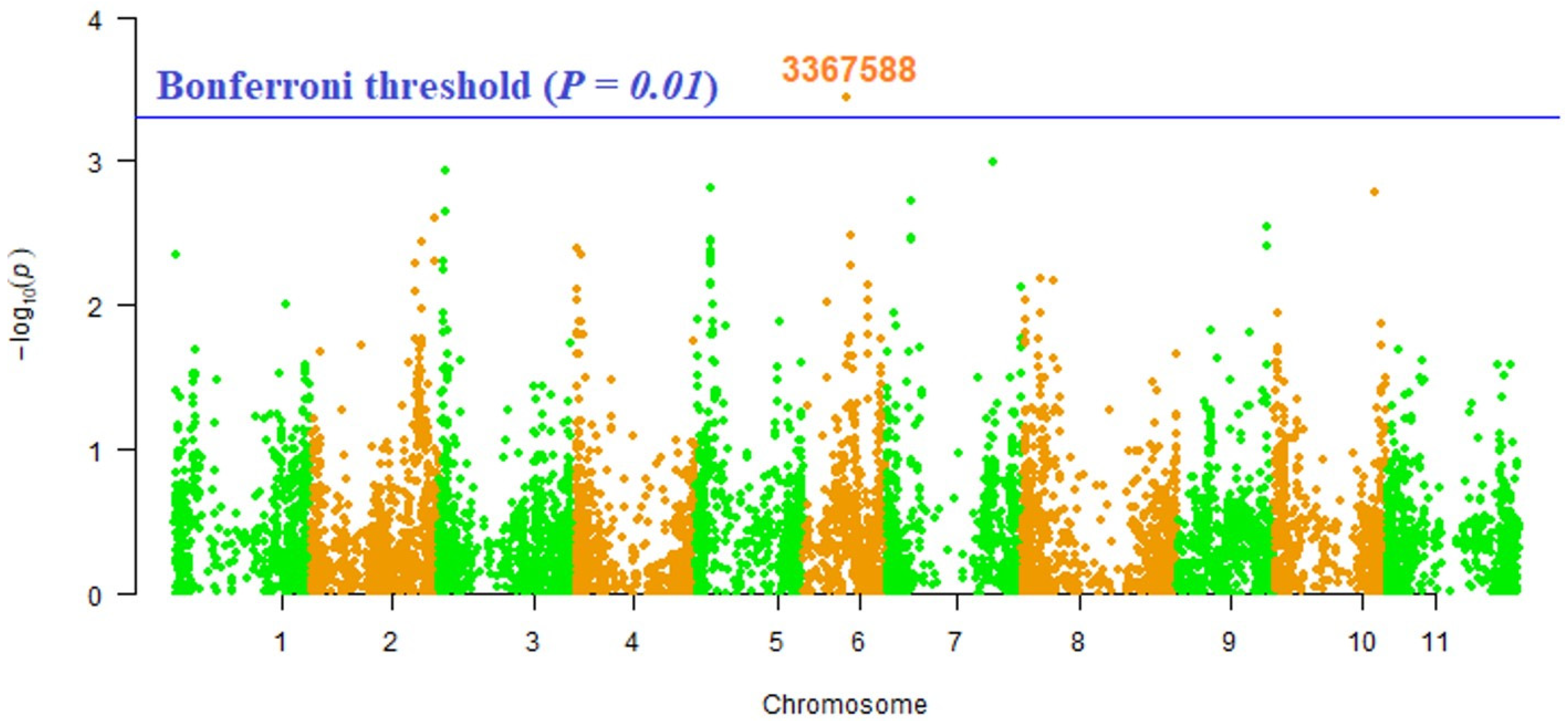
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Field Experimentation
4.3. Seed Vitamin A Contents Profiling
4.4. Molecular Analysis
4.5. Statistical Analysis
4.5.1. Phenotypic Data Analysis
4.5.2. Marker–Trait Association Analysis for Vitamin A Contents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liaqat, W.; Altaf, M.T.; Barutçular, C.; Nawaz, H.; Ullah, I.; Basit, A.; Mohamed, H.I. Ultraviolet-B radiation in relation to agriculture in the context of climate change: A review. Cereal Res. Commun. 2024, 52, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The threat of the combined effect of biotic and abiotic stress factors in forestry under a changing climate. Front. Plant Sci. 2020, 11, 1874. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Bryksa, B.C.; Yada, R.Y. Feeding the world into the future–food and nutrition security: The role of food science and technology. Front. Life Sci. 2016, 9, 155–166. [Google Scholar] [CrossRef]
- ACTION, H. Hundreds of millions of people face hunger as historic food crisis looms. Foods 2022, 11, 166. [Google Scholar]
- Vuppalapati, C. Future. In Artificial Intelligence and Heuristics for Enhanced Food Security; Springer International Publisher: Cham, Switzerland, 2022; p. 823. [Google Scholar]
- Ali, A.; Altaf, M.T.; Nadeem, M.A.; Karaköy, T.; Shah, A.N.; Azeem, H.; Baloch, F.S.; Baran, N.; Hussain, T.; Chung, Y.S.; et al. Recent advancement in OMICS approaches to enhance abiotic stress tolerance in legumes. Front. Plant Sci. 2022, 13, 952759. [Google Scholar] [CrossRef]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef]
- Rautiainen, S.; Manson, J.E.; Lichtenstein, A.H.; Sesso, H.D. Dietary supplements and disease prevention: A global overview. Nat. Rev. Endocrinol. 2016, 12, 407–420. [Google Scholar] [CrossRef]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; De Benoist, B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Aslam, M.; Akbar, W.; Iqbal, Z. Biological importance of vitamins for human health: A review. J. Agric. Basic Sci. 2017, 2, 50–58. [Google Scholar]
- Fitzpatrick, T.B.; Basset, G.J.; Borel, P.; Carrari, F.; DellaPenna, D.; Fraser, P.D.; Hellmann, H.; Osorio, S.; Rothan, C.; Valpuesta, V.; et al. Vitamin deficiencies in humans: Can plant science help? Plant Cell 2012, 24, 395–414. [Google Scholar] [CrossRef]
- Shaik, P.S.; Pachava, S. The role of vitamins and trace elements on oral health: A systematic review. Int. J. Med. Rev. 2017, 4, 22–31. [Google Scholar] [CrossRef]
- West, K.P., Jr.; Sommer, A.; Palmer, A.; Schultink, W.; Habicht, J.P. Commentary: Vitamin A policies need rethinking. Int. J. Epidemiol. 2015, 44, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Lykstad, J.; Sharma, S. Biochemistry, Water Soluble Vitamins; StatPearls Publishing: St. Petersburg, FL, USA, 2019. [Google Scholar] [PubMed]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The nutritional content of common bean (Phaseolus vulgaris L.) landraces in comparison to modern varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Akande, T.; Onyezili, F.N.; Ikwebe, J. Biochemical characterization of soybean germplasms with respect to bioavailable iron and zinc, vitamin A and crude protein. Afr. J. Biochem. Res. 2018, 12, 40–44. [Google Scholar]
- Sommer, A. Vitamin A Deficiency and Its Consequences: A Field Guide to Detection and Control; WHO: Geneva, Switzerland, 1995; Volume 138, pp. 1835–1839. [Google Scholar]
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health 2015, 3, e528–e536. [Google Scholar] [CrossRef]
- Myhre, A.M.; Carlsen, M.H.; Bøhn, S.K.; Wold, H.L.; Laake, P.; Blomhoff, R. Water-miscible, emulsified, and solid forms of retinol supplements are more toxic than oil-based preparations. Am. J. Clin. Nutr. 2003, 78, 1152–1159. [Google Scholar] [CrossRef]
- Frossard, E.; Bucher, M.; Mächler, F.; Mozafar, A.; Hurrell, R. Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. J. Sci. Food Agric. 2000, 80, 861–879. [Google Scholar] [CrossRef]
- Baloch, F.S.; Nadeem, M.A. Unlocking the genomic regions associated with seed protein contents in Turkish common bean germplasm through genome-wide association study. Turk. J. Agric. For. 2022, 46, 113–128. [Google Scholar]
- Bitocchi, E.; Nanni, L.; Bellucci, E.; Rossi, M.; Giardini, A.; Zeuli, P.S.; Logozzo, G.; Stougaard, J.; McClean, P.; Attene, G.; et al. Mesoamerican origin of the common bean (Phaseolus vulgaris L.) is revealed by sequence data. Proc. Natl. Acad. Sci. USA 2012, 109, e788–e796. [Google Scholar] [CrossRef]
- FAOSTAT. 2020. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 2 February 2020).
- Yeken, M.Z.; Nadeem, M.A.; Karaköy, T.; Baloch, F.S.; Çiftçi, V. Determination of Turkish common bean germplasm for morpho-agronomic and mineral variations for breeding perspectives in Türkiye. KSU J. Nat. Sci. 2019, 22, 38–50. [Google Scholar]
- Nadeem, M.A.; Karaköy, T.; Yeken, M.Z.; Habyarimana, E.; Hatipoğlu, R.; Çiftçi, V.; Nawaz, M.A.; Sönmez, F.; Shahid, M.Q.; Yang, S.H.; et al. Phenotypic characterization of 183 Turkish common bean accessions for agronomic, trading, and consumer-preferred plant characteristics for breeding purposes. J. Agron. 2020, 10, 272. [Google Scholar] [CrossRef]
- Köse, M.A.; Ekbiç, E.; Arici, Y.K. Determination of protein, vitamins, amino acids, and mineral element content of Yenice and Pınarlı bean (Phaseolus vulgaris L.) genotypes. Turk. J. Food Agric. Sci. 2019, 1, 6–11. [Google Scholar]
- Altaf, M.T.; Tatar, M.; Ali, A.; Liaqat, W.; Mortazvi, P.; Kayihan, C.; Ölmez, F.; Nadeem, M.A.; Javed, J.; Gou, J.Y.; et al. Advancements in QTL Mapping and GWAS Application in Plant Improvement. Turk. J. Bot. 2024, 48, 376–426. [Google Scholar] [CrossRef]
- Purkaystha, S.; Das, P.; Rashmi, K.; Rout, S.; Nanda, S. Advances in Genetic Mapping of Loci Governing Disease Resistance in Plants. In Biotechnological Advances for Disease Tolerance in Plants; Springer Nature Singapore: Singapore, 2024; pp. 1–27. [Google Scholar]
- Adedze, Y.M.N.; Lu, X.; Fan, W.; Zhang, W.; Yang, X.; Deng, Z.; Alam, A.; Xu, G.; Zhang, L.; Li, W. Development of PCR-based markers associated with powdery mildew resistance using bulked segregant analysis (BSA-seq) in melon. Czech J. Genet. Plant Breed. 2024, 60, 25–33. [Google Scholar] [CrossRef]
- Feng, S.; Wu, J.; Chen, K.; Chen, M.; Zhu, Z.; Wang, J.; Chen, G.; Cao, B.; Lei, J.; Chen, C. Identification and characterization analysis of candidate genes controlling mushroom leaf development in Chinese kale by BSA-seq. Mol. Breed 2023, 43, 17. [Google Scholar] [CrossRef]
- Mengesha, W.; Menkir, A.; Meseka, S.; Bossey, B.; Afolabi, A.; Burgueno, J.; Crossa, J. Factor analysis to investigate genotype and genotype × environment interaction effects on pro-vitamin A content and yield in maize synthetics. Euphytica 2019, 215, 1–5. [Google Scholar] [CrossRef]
- Sarkiyayi, S.; Hamman, B.M. Nutritional evaluation of some legumes and vegetables cultivated and consumed in Yola, Adamawa State, Nigeria. Adv. J. Food Sci. Technol. 2015, 7, 658–667. [Google Scholar] [CrossRef]
- Arslan, M. Diversity for vitamin and amino acid content in grass pea (Lathyrus sativus L.). Legume Res. 2017, 40, 803–810. [Google Scholar] [CrossRef][Green Version]
- Shukla, G.K. Some statistical aspects of partitioning genotype-environmental components of variability. Crop Sci. 1972, 29, 237–245. [Google Scholar] [CrossRef]
- Santos, C.; Simon, P. QTL analyses reveal clustered loci for accumulation of major provitamin A carotenes and lycopene in carrot roots. Mol. Genet. Genom. 2002, 268, 122–129. [Google Scholar] [CrossRef]
- Ross, J.; Li, Y.; Lim, E.K.; Bowles, D.J. Higher plant glycosyltransferases. Genome Biol. 2001, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Khaidizar, M.İ.; Haliloğlu, K.; Elkoca, E.; Aydın, M.; Kantar, F. Genetic diversity of common bean (Phaseolus vulgaris L.) landraces grown in northeast Anatolia of Turkey assessed with simple sequence repeat markers. Turk. J. Field Crops 2012, 17, 145–150. [Google Scholar]
- Ceylan, A.; Öcal, N.; Akbulut, M. Genetic diversity among the Turkish common bean cultivars (Phaseolus vulgaris L.) as assessed by SRAP, POGP and cpSSR markers. Biochem. Syst. Ecol. 2014, 54, 219–229. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Nadeem, M.A.; Habyarimana, E.; Çiftçi, V.; Nawaz, M.A.; Karaköy, T.; Comertpay, G.; Shahid, M.Q.; Hatipoğlu, R.; Yeken, M.Z.; Ali, F.; et al. Characterization of genetic diversity in Turkish common bean gene pool using phenotypic and whole-genome DArTseq-generated silicoDArT marker information. PLoS ONE 2018, 13, e0205363. [Google Scholar] [CrossRef]
- Rathore, A.; Parsad, R.; Gupta, V.K. Computer-aided construction and analysis of augmented designs. J. Indian Soc. Agric. Stat. 2004, 57, 320–344. [Google Scholar]
- Pour-Aboughadareh, A.M.; Yousefian, H.; Moradkhani, P.; Poczai, K.H.M.S.; Siddique, K.H.M. STABILITYSOFT: A new online program to calculate parametric and non-parametric stability statistics for crop traits. Appl. Plant Sci. 2019, 7, e1211. [Google Scholar] [CrossRef]
- Wricke, G. Uber eine methode zur erfassung der okologischen streubreite in feldversuchen. Z. Pflanzenzuchtg. 1962, 47, 92–96. [Google Scholar]
- Eberhart, S.T.; Russell, W.A. Stability parameters for comparing varieties. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Habyarimana, E.; Karaköy, T.; Baloch, F.S. Genetic dissection of days to flowering via genome-wide association studies in Turkish common bean germplasm. Physiol. Mol. Biol. Plants 2021, 27, 1609–1622. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.D. qqman: An R package for visualizing GWAS results using QQ and manhattan plots. Biorxiv 2014, 005165. [Google Scholar] [CrossRef]

| Source | Df | Sum Sq | Mean Sq | F Value | Pr (>F) |
|---|---|---|---|---|---|
| ENV | 4 | 0.310747 | 0.077687 | 23.15593 | 2.95 × 10−18 |
| REP (ENV) | 5 | 0.067811 | 0.013562 | 4.042485 | 0.001236 |
| GEN | 182 | 155.7559 | 0.8465 | 252.3146 | 0 |
| GEN:ENV | 736 | 7.026215 | 0.009546 | 2.845504 | 1.53 × 10−50 |
| Residuals | 920 | 3.086542 | 0.003355 |
| Years | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|
| Bolu17 | 1.670 | 3.220 | 2.395 | 0.293 |
| Sivas17 | 1.660 | 3.260 | 2.395 | 0.310 |
| Bolu18 | 1.660 | 3.350 | 2.412 | 0.284 |
| Sivas18 | 1.630 | 3.240 | 2.404 | 0.298 |
| Sivas21 | 1.650 | 3.230 | 2.397 | 0.294 |
| Mean | 1.670 | 3.230 | 2.400 | 0.292 |
| PCA1 | PCA2 | PCA3 | PCA4 | PCA5 | |
|---|---|---|---|---|---|
| BOLU 2017 | 0.993 | −0.061 | −0.045 | −0.072 | −0.047 |
| SİVAS 2017 | 0.992 | −0.050 | −0.058 | 0.096 | −0.002 |
| BOLU 2018 | 0.996 | −0.046 | 0.011 | −0.037 | 0.072 |
| SİVAS 2018 | 0.987 | 0.158 | −0.031 | −0.008 | 0.002 |
| SİVAS 2021 | 0.992 | −0.001 | 0.123 | 0.020 | −0.024 |
| Eigenvalue | 4.921 | 0.033 | 0.021 | 0.016 | 0.008 |
| Variability (%) | 98.419 | 0.668 | 0.429 | 0.323 | 0.160 |
| Cumulative (%) | 98.419 | 99.087 | 99.517 | 99.840 | 100.000 |
| Genotype | Vitamin A Content | Wi2 | σ2i | s2di |
|---|---|---|---|---|
| Niğde-Dermasyon | 2.46 | 0.00013 | 1.08 × 10−05 | 9.53 × 10−06 |
| Balıkesir-3 | 2.92 | 0.000228 | 3.05 × 10−05 | 2.78 × 10−05 |
| Önceler | 2.74 | 0.00046 | 7.74 × 10−05 | 4 × 10−05 |
| Muş-34 | 2.3 | 0.000584 | 0.000103 | 4.75 × 10−05 |
| Akdağ | 2.54 | 0.000609 | 0.000108 | 5.61 × 10−05 |
| Bitlis-5 | 2.51 | 0.00067 | 0.00012 | 9.57 × 10−05 |
| Bilecik-6 | 1.67 | 0.000677 | 0.000121 | 4.46 × 10−05 |
| Akman | 2.98 | 0.000829 | 0.000152 | 3.64 × 10−05 |
| Göksun | 2.76 | 0.000829 | 0.000152 | 3.64 × 10−05 |
| Göynük | 2.46 | 0.00085 | 0.000156 | 7.21 × 10−06 |
| Trait | Marker | Chromosome | Position | p-Value | MarkerR2 |
|---|---|---|---|---|---|
| Vitamin A | 3367588 | 6 | 16519249 | 3.57 × 10−04 | 0.07977 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çilesiz, Y.; Altaf, M.T.; Nadeem, M.A.; Ali, A.; Sesiz, U.; Alsaleh, A.; İlçim, A.; Özer, M.S.; Erdem, T.; Aziz, I.; et al. Identification of Phenotypic Diversity and DArTseq Loci Associated with Vitamin A Contents in Turkish Common Bean Germplasm Through GWAS. Plants 2025, 14, 776. https://doi.org/10.3390/plants14050776
Çilesiz Y, Altaf MT, Nadeem MA, Ali A, Sesiz U, Alsaleh A, İlçim A, Özer MS, Erdem T, Aziz I, et al. Identification of Phenotypic Diversity and DArTseq Loci Associated with Vitamin A Contents in Turkish Common Bean Germplasm Through GWAS. Plants. 2025; 14(5):776. https://doi.org/10.3390/plants14050776
Chicago/Turabian StyleÇilesiz, Yeter, Muhammad Tanveer Altaf, Muhammad Azhar Nadeem, Amjad Ali, Uğur Sesiz, Ahmad Alsaleh, Ahmet İlçim, Mehmet Sertaç Özer, Tunahan Erdem, Israr Aziz, and et al. 2025. "Identification of Phenotypic Diversity and DArTseq Loci Associated with Vitamin A Contents in Turkish Common Bean Germplasm Through GWAS" Plants 14, no. 5: 776. https://doi.org/10.3390/plants14050776
APA StyleÇilesiz, Y., Altaf, M. T., Nadeem, M. A., Ali, A., Sesiz, U., Alsaleh, A., İlçim, A., Özer, M. S., Erdem, T., Aziz, I., Mansoor, S., Karaköy, T., & Baloch, F. S. (2025). Identification of Phenotypic Diversity and DArTseq Loci Associated with Vitamin A Contents in Turkish Common Bean Germplasm Through GWAS. Plants, 14(5), 776. https://doi.org/10.3390/plants14050776











