Ubiquitin Ligase Gene OsPUB57 Negatively Regulates Rice Blast Resistance
Abstract
1. Introduction
2. Results
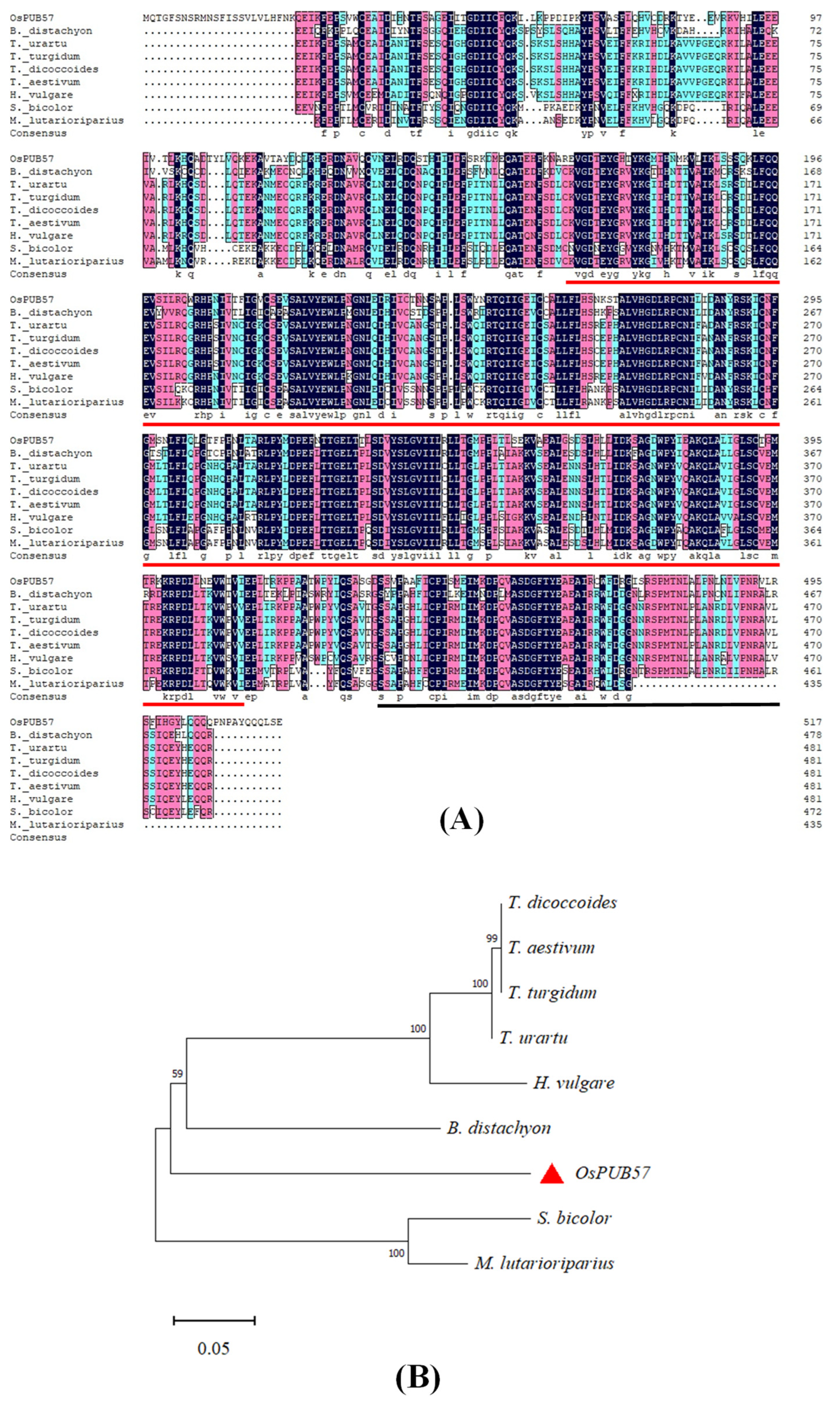
2.1. Molecular Characteristics of OsPUB57 and Its Promoter
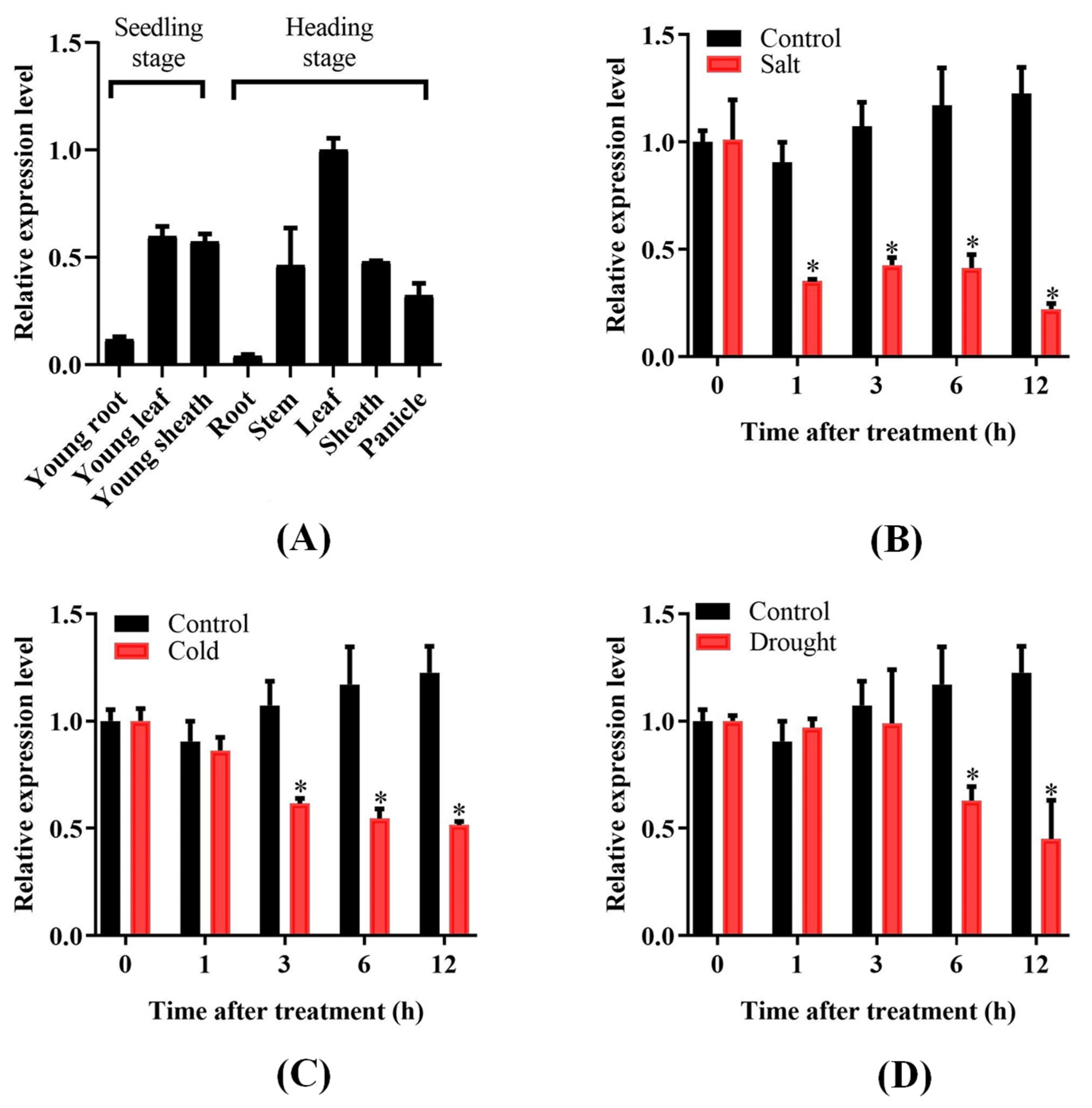
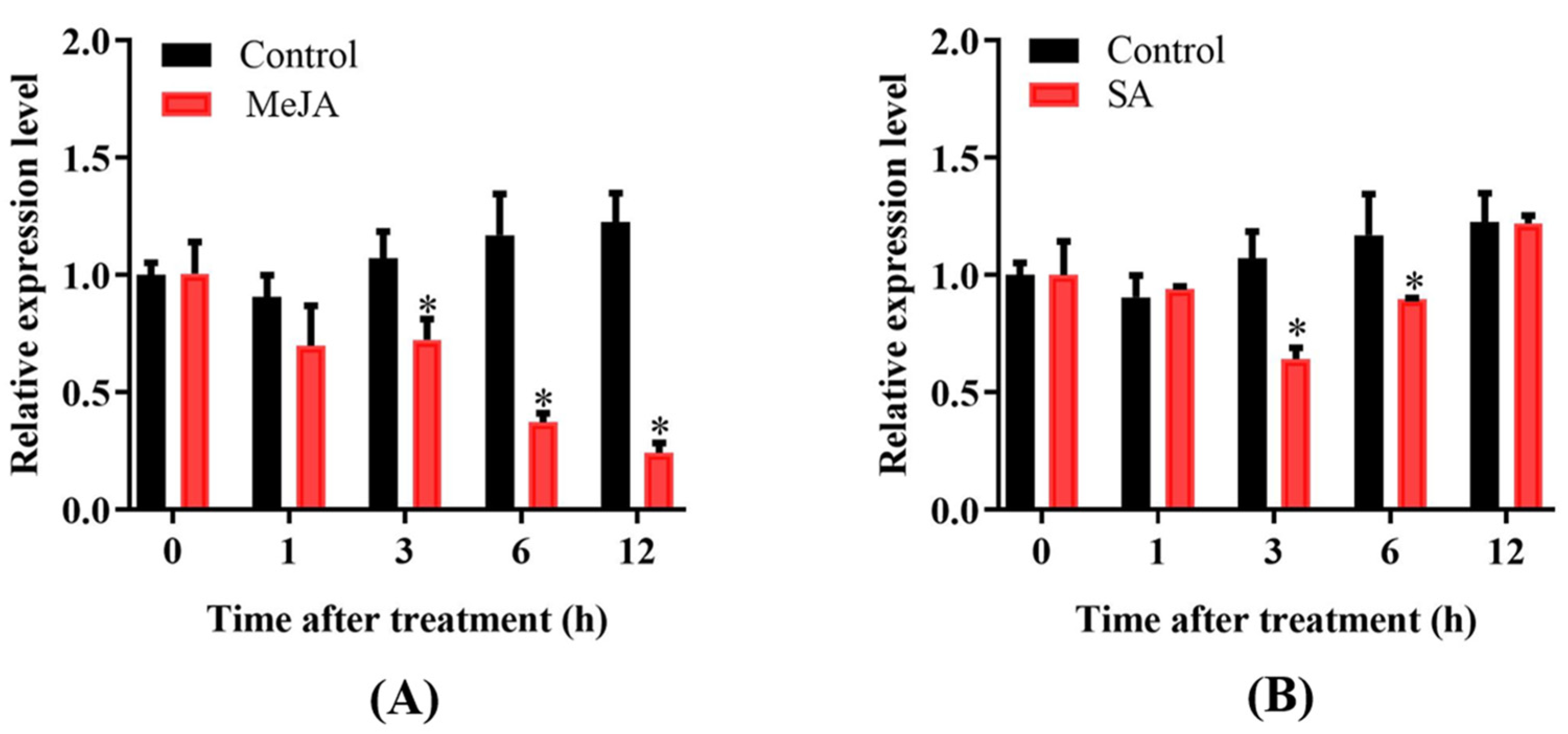
2.2. Expression Profile of OsPUB57
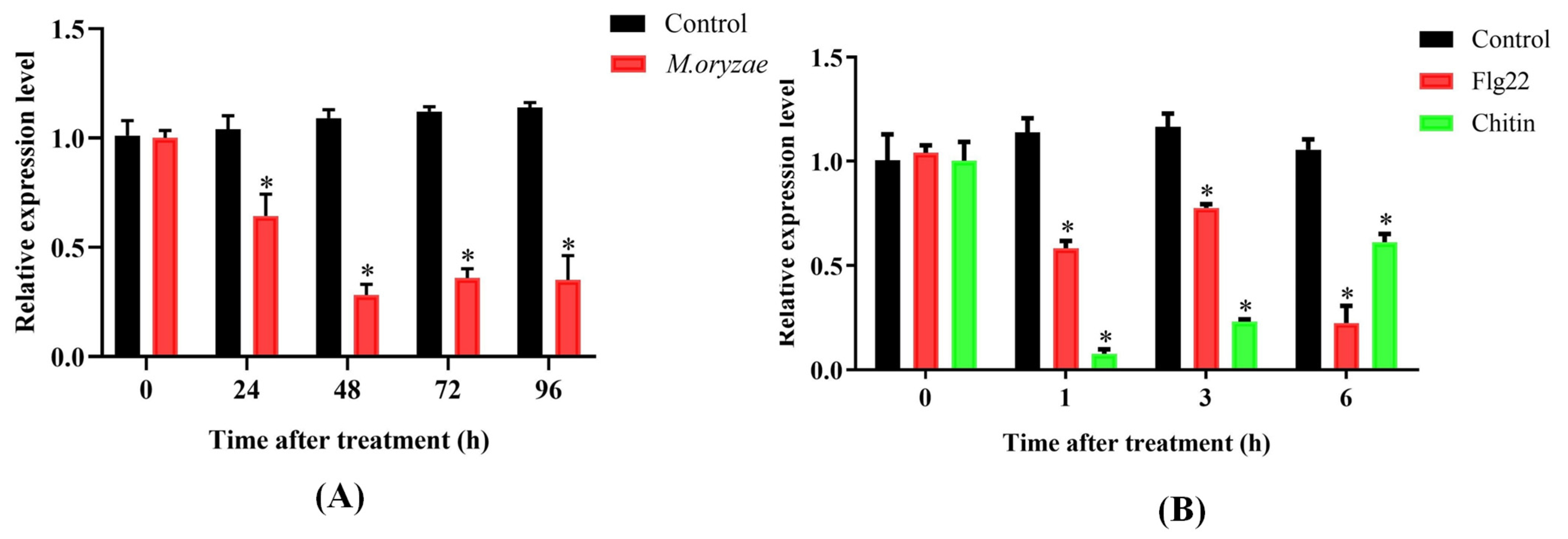
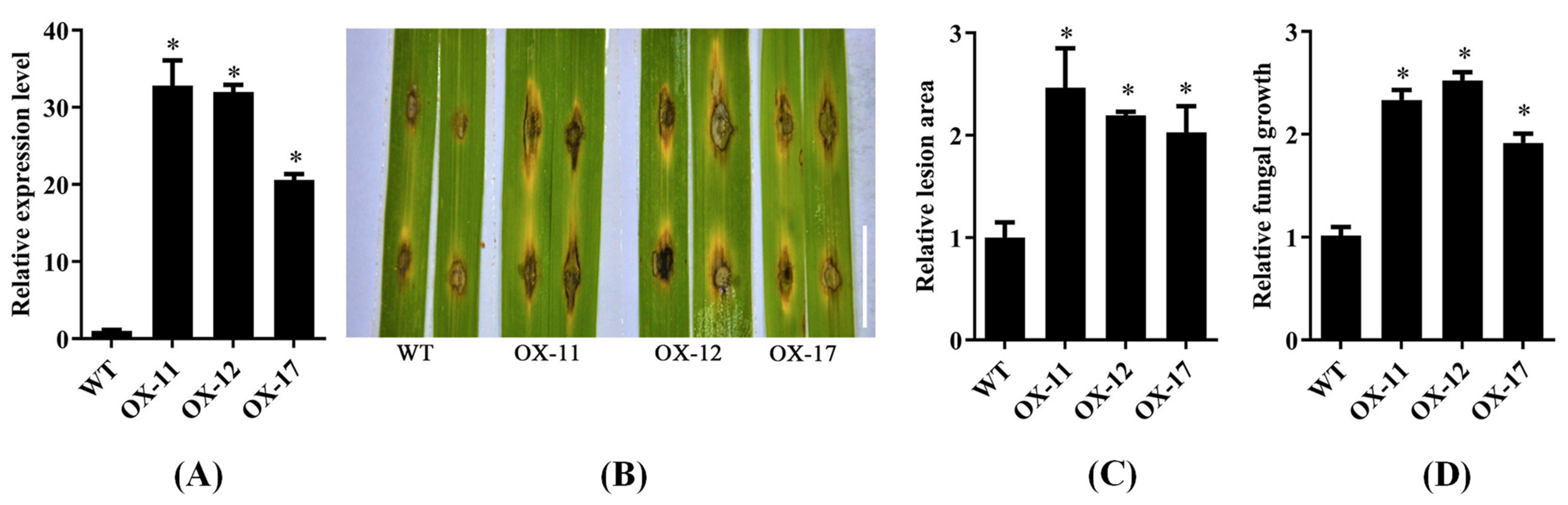
2.3. OsPUB57-Overexpressing Plants Showed Decreased Rice Blast Resistance
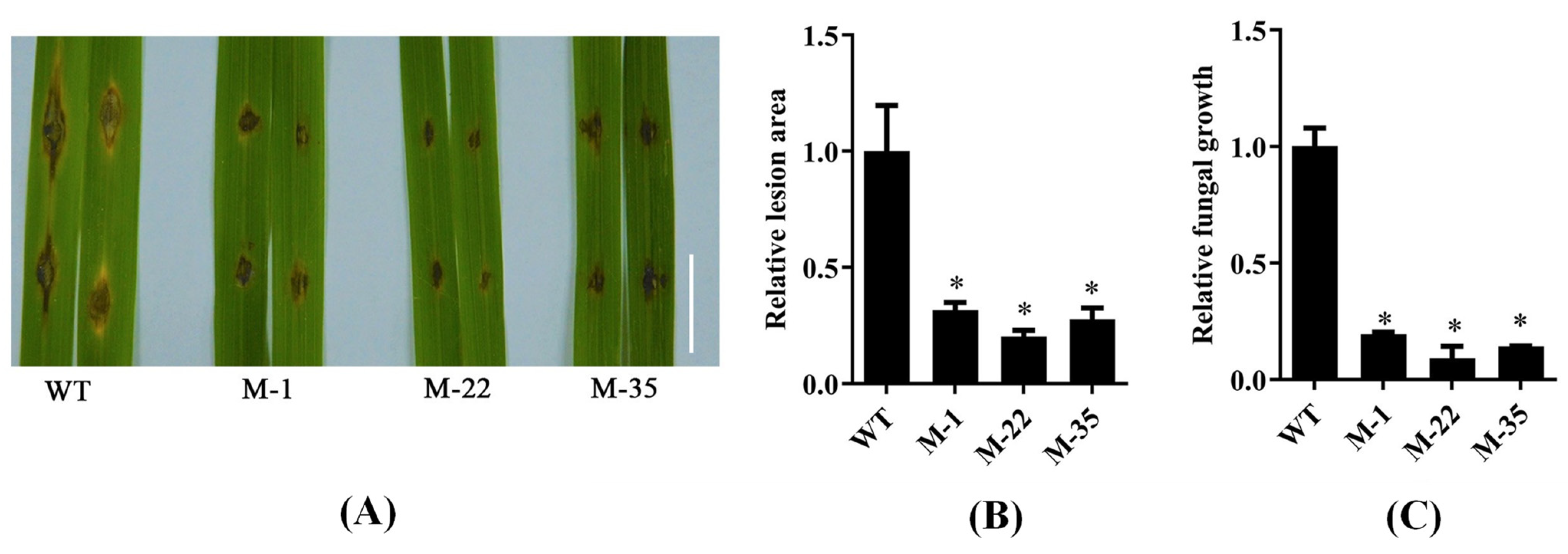
2.4. The Mutant Plants of OsPUB57 Displayed Improved Rice Blast Resistance
2.5. OsPUB57 May Influence the Expression of Defense-Related Genes to Regulate Rice Blast Resistance
3. Discussion
4. Materials and Methods
4.1. Materials and Growth Conditions
4.2. RNA Extraction and qRT-PCR Amplification
4.3. Vector Construction and Genetic Transformation of Rice
4.4. Cultivation of Rice Blast Fungus
4.5. Inoculation of Rice Blast Fungus
4.6. Various Treatments and Samplings
4.7. Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mukhopadhyay, D.; Riezman, H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 2007, 315, 201–205. [Google Scholar] [CrossRef]
- Wang, J.; Qu, B.; Dou, S.; Li, L.; Yin, D.; Pang, Z.; Zhou, Z.; Tian, M.; Liu, G.; Xie, Q.; et al. The E3 ligase OsPUB15 interacts with the receptor-like kinase PID2 and regulates plant cell death and innate immunity. BMC Plant Biol. 2015, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Callis, J. The ubiquitination machinery of the ubiquitin system. Arab. Book 2014, 12, e0174. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Ryu, M.Y.; Kim, J.H.; Hong, J.S.; Oh, T.R.; Kim, W.T.; Yang, S.W. RING E3 ligases: Key regulatory elements are involved in abiotic stress responses in plants. BMB Rep. 2017, 50, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Berndsen, C.E.; Wolberger, C. New insights into ubiquitin E3 ligase mechanism. Nat. Struct. Mol. Biol. 2014, 21, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Yamaguchi, K.; Sakamoto, K.; Yoshimura, S.; Inoue, K.; Tsuge, S.; Kojima, C.; Kawasaki, T. Bacterial effector modulation of host E3 ligase activity suppresses PAMP-triggered immunity in rice. Nat. Commun. 2014, 5, 5430. [Google Scholar] [CrossRef] [PubMed]
- Aravind, L.; Koonin, E.V. The U-box is a modified RING finger–A common domain in ubiquitination. Curr. Biol. 2000, 10, R132–R134. [Google Scholar] [CrossRef] [PubMed]
- Borden, K.L.; Freemont, P.S. The RING finger domain: A recent example of a sequence-structure family. Curr. Opin. Struct. Biol. 1996, 6, 395–401. [Google Scholar] [CrossRef]
- Koegl, M.; Hoppe, T.; Schlenker, S.; Ulrich, H.D.; Mayer, T.U.; Jentsch, S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell 1999, 96, 635–644. [Google Scholar] [CrossRef]
- Ohi, M.D.; Vander Kooi, C.W.; Rosenberg, J.A.; Chazin, W.J.; Gould, K.L. Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat. Struct. Biol. 2003, 10, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, Y.; Shiu, S.H.; Stone, S.L.; Salt, J.N.; Goring, D.R. A large complement of the predicted arabidopsis ARM repeat proteins are members of the U-box E3 ubiquitin ligase family. Plant Physiol. 2004, 134, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.R.; Park, C.H.; Venu, R.C.; Gough, J.; Wang, G.L. Classification, expression pattern, and E3 ligase activity assay of rice U-box containing proteins. Mol. Plant 2008, 1, 800–815. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, Y.P.; Li, J.; Yu, Z.Y.; Guo, Q.R. Genome-wide identification and expression analysis of the plant U-box protein gene family in Phyllostachys edulis. Front. Genet. 2021, 12, 710113. [Google Scholar] [CrossRef] [PubMed]
- Byun, M.Y.; Cui, L.H.; Oh, T.K.; Jung, Y.J.; Lee, A.; Park, K.Y.; Kang, B.G.; Kim, W.T. Homologous U-box E3 Ubiquitin Ligases OsPUB2 and OsPUB3 Are Involved in the Positive Regulation of Low Temperature Stress Response in Rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.H.; Jiang, X.; Jung, K.H. An abiotic stress responsive U-box E3 ubiquitin ligase is involved in OsGI-mediating diurnal rhythm regulating Mechanism. Plants 2020, 9, 1071. [Google Scholar] [CrossRef]
- Kim, M.S.; Le, V.T.; Jung, Y.J.; Kang, K.K.; Cho, Y.G. OsPUB9 Gene Edited by CRISPR/Cas9 Enhanced Resistance to Bacterial Leaf Blight in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2024, 25, 7145. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Park, C.H.; He, F.; Nagano, M.; Wang, M.; Bellizzi, M.; Zhang, K.; Zeng, X.; Liu, W.; Ning, Y.; et al. The RhoGAP SPIN6 associates with SPL11 and OsRac1 and negatively regulates programmed cell death and innate immunity in rice. PLoS Pathog. 2015, 11, e1004629. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, X.; Yu, C.; Shi, X.; Lan, W.; Gao, C.; Yang, J.; Dai, H.; Zhang, X.; Zhang, H.; et al. Release of a ubiquitin brake activates OsCERK1-triggered immunity in rice. Nature 2024, 629, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Yi, J.; Yoon, J.; Cho, L.H.; Ping, J.; Jeong, H.J.; Cho, S.K.; Kim, W.T.; An, G. OsPUB15, an E3 ubiquitin ligase, functions to reduce cellular oxidative stress during seedling establishment. Plant J. 2011, 65, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Qiao, W.; Zhu, Z.; Wang, Z.; Tian, Y.; Liu, S.; Wan, J.; Liu, L. Identification of a novel locus qGW12/OsPUB23 regulating grain shape and weight in rice (Oryza sativa L.). Theor. Appl. Genet. 2024, 137, 267. [Google Scholar] [CrossRef]
- Min, H.J.; Cui, L.H.; Oh, T.R.; Kim, J.H.; Kim, T.W.; Kim, W.T. OsBZR1 turnover mediated by OsSK22-regulated U-box E3 ligase OsPUB24 in rice BR response. Plant J. 2019, 99, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sun, Y.; Zhan, C.; Qu, C.; Jin, N.; Gu, X.; Huang, J. The E3 ligase OsPUB33 controls rice grain size and weight by regulating the OsNAC120-BG1 module. Plant Cell 2024, 5, koae297. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Lee, A.; Yu, S.G.; Cui, L.H.; Min, H.J.; Lee, S.E.; Cho, N.H.; Kim, S.; Bae, H.; Kim, W.T. OsPUB41, a U-box E3 ubiquitin ligase, acts as a negative regulator of drought stress response in rice (Oryza sativa L.). Plant Mol. Biol. 2021, 106, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ko, S.R.; Jung, Y.J.; Kang, K.K.; Lee, Y.J.; Cho, Y.G. Knockout Mutants of OsPUB7 Generated Using CRISPR/Cas9 Revealed Abiotic Stress Tolerance in Rice. Int. J. Mol. Sci. 2023, 24, 5338. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wang, Y.; Huang, L.; Du, F.; Zhao, X.; Li, Z.; Wang, W.; Fu, B. A U-box E3 ubiquitin ligase OsPUB67 is positively involved in drought tolerance in rice. Plant Mol. Biol. 2020, 102, 89–107. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, Y.; Huang, J. CRISPR-Cas9 mediated mutation in OsPUB43 improves grain length and weight in rice by promoting cell proliferation in spikelet hull. Int. J. Mol. Sci. 2022, 23, 2347. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, R.; Liu, G.; Jin, J.; Wu, J.; Liu, X. Cytological and transcriptome analyses reveal OsPUB73 defect affects the gene expression associated with tapetum or pollen exine abnormality in rice. BMC Plant Biol. 2019, 19, 546. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Tian, J.; Fang, H.; Fang, L.; Xu, X.; He, F.; Li, S.; Xie, W.; Du, Q.; You, X.; et al. A VQ-motif-containing protein fine-tunes rice immunity and growth by a hierarchical regulatory mechanism. Cell Rep. 2022, 40. [Google Scholar] [CrossRef]
- Hu, X.; Qian, Q.; Xu, T.; Zhang, Y.; Dong, G.; Gao, T.; Xie, Q.; Xue, Y. The U-box E3 ubiquitin ligase TUD1 functions with a heterotrimeric G α subunit to regulate Brassinosteroid-mediated growth in rice. PLoS Genet. 2013, 9, e1003391. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, X.; Qu, C.; Jin, N.; Qin, T.; Jin, L.; Huang, J. OsPUB75-OsHDA716 mediates deactivation and degradation of OsbZIP46 to negatively regulate drought tolerance in rice. Plant Physiol. 2024, 15, kiae545. [Google Scholar] [CrossRef]
- Mei, C.; Qi, M.; Sheng, G.; Yang, Y. Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol. Plant Microbe Interact. 2006, 19, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Morel, J.B.; Riemann, M.; Nick, P. Jasmonic acid contributes to rice resistance against Magnaporthe oryzae. BMC Plant Biol. 2022, 22, 601. [Google Scholar] [CrossRef]
- Kumari, D.; Prasad, B.D.; Dwivedi, P.; Sahni, S.; Kumar, M.; Alamri, S.; Adil, M.F.; Alakeel, K.A. Comprehensive analysis of transcription factor binding sites and expression profiling of rice pathogenesis related genes (OsPR1). Front. Plant Sci. 2024, 15, 1463147. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell. 2022, 34, 1447–1478. [Google Scholar] [CrossRef]
- Yuan, M.H.; Ngou, B.P.M.; Ding, P.T.; Xin, X.F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Ahammed, G.J.; Wu, C.J.; Fan, S.Y.; Zhou, Y.H. Crosstalk among jasmonate, salicylate and ethylene signaling pathways in plant disease and immune responses. Curr. Protein Pept. Sci. 2015, 16, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Zhang, Y.L. Plant immunity: Danger perception and signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef] [PubMed]
- La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O. Trichoderma Counteractsthe Challenge of Phytophthora nicotianae Infections on Tomato by Modulating Plant Defense Mechanisms and the Expression of Crinkler, Necrosis-Inducing Phytophthora Protein 1, and Cellulose-Binding Elicitor Lectin Pathogenic Effectors. Front. Plant Sci. 2020, 11, 583539. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef]
- Ken, H.; Moritoshi, I. Phytochrome-Mediated Transcriptional Up-regulation of ALLENE OXIDE SYNTHASE in Rice Seedlings. Plant Cell Physiol. 2004, 45, 119–128. [Google Scholar] [CrossRef]
- Takken, F.L.; Albrecht, M.; Tameling, W.I. Resistance proteins: Molecular switches of plant defence. Curr. Opin. Plant Biol. 2006, 9, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Teng, Z.; Li, H.; Wang, W.; Xu, F.; Sun, K.; Chu, J.; Qian, Y.; Loake, G.J.; Chu, C.; et al. An activated form of NB-ARC protein RLS1 functions with cysteine-rich receptor-like protein RMC to trigger cell death in rice. Plant Commun. 2023, 4, 100459. [Google Scholar] [CrossRef] [PubMed]
- Bundó, M.; Coca, M. Enhancing blast disease resistance by overexpression of the calcium-dependent protein kinase OsCPK4 in rice. Plant Biotechnol. J. 2016, 14, 1357–1367. [Google Scholar] [CrossRef]
- Lin, Q.J.; Kumar, V.; Chu, J.; Li, Z.M.; Wu, X.X.; Dong, H.; Sun, Q.; Xuan, Y.H. CBL-interacting protein kinase 31 regulates rice resistance to blast disease by modulating cellular potassium levels. Biochem. Biophys. Res. Commun. 2021, 563, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Miyao, A.; Takahashi, A.; Hirochika, H. Pdk1 kinase regulates basal disease resistance through the OsOxi1-OsPti1a phosphorylation cascade in rice. Plant Cell Physiol. 2010, 51, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Zhong, H.; Deng, X.; Gautam, M.; Gong, Z.; Zhang, Y.; Zhao, G.; Liu, C.; Li, Y. Evolutionary Analysis of GH3 Genes in Six Oryza Species/Subspecies and Their Expression under Salinity Stress in Oryza sativa ssp. japonica. Plants 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wan, H.; Zhang, S.; Yu, J. γ-MYN: A new algorithm for estimating Ka and Ks with consideration of variable substitution rates. Biol. Direct. 2009, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Boubakri, H.; Chihaoui, S.; Najjar, E.; Gargouri, M.; Barhoumi, F.; Jebara, M. Genome-wide analysis and expression profiling of H-type Trx family in Phaseolus vulgaris revealed distinctive isoforms associated with symbiotic N2-fixing performance and abiotic stress response. Plant Physiol. 2021, 260, 153410. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Songkumarn, P.; Liu, J.; Wang, G.L. A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol. 2009, 150, 1111–1121. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A robust CRISPR/Cas9 system for convenient high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chen, S.; Shirsekar, G.; Zhou, B.; Khang, C.H.; Songkumarn, P.; Afzal, A.J.; Ning, Y.; Wang, R.; Bellizzi, M.; et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen associated molecular pattern-triggered immunity in rice. Plant Cell 2012, 24, 4748–4762. [Google Scholar] [CrossRef] [PubMed]
- Chourey, K.; Ramani, S.; Apte, S.K. Accumulation of LEA proteins in salt (NaCl) stressed young seedlings of rice (Oryza sativa L.) cultivar Bura Rata and their degradation during recovery from salinity stress. Plant Physiol. 2003, 160, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Chen, Y.; Pan, X.; Chen, Y.; Zhou, J.; Jiang, X.; Zhang, Z.; Xiao, G.; Zhang, H. OsEIN2-OsEIL1/2 pathway negatively regulates chilling tolerance by attenuating OsICE1 function in rice. Plant Cell Environ. 2024, 47, 2559–2575. [Google Scholar] [CrossRef]
- Ning, Y.; Jantasuriyarat, C.; Zhao, Q.; Zhang, H.; Chen, S.; Liu, J.; Liu, L.; Tang, S.; Park, C.H.; Wang, X.; et al. The SINA E3 ligase OsDIS1 negatively regulates drought response in rice. Plant Physiol. 2011, 157, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Kumar, G.; Arya, P.; Jaswal, R.; Jain, P.; Singh, K.; Sharma, T.R. Genome-Wide Analysis and Expression Profiling of Rice Hybrid Proline-Rich Proteins in Response to Biotic and Abiotic Stresses, and Hormone Treatment. Plants 2019, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Kano, A.; Tamaoki, D.; Miyamoto, A.; Shishido, H.; Miyoshi, S.; Taniguchi, S.; Akimitsu, K.; Gomi, K. Involvement of OsJAZ8 in jasmonate-induced resistance to bacterial blight in rice. Plant Cell Physiol. 2012, 53, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Li, K.; Wu, Y.; Wang, D.; Zhou, J.; Liu, X.; Li, Y.; Jin, C.; Liu, X.; Alejandro, J.L.M.; et al. OsTSD2-mediated cell wall modification affects ion homeostasis and salt tolerance. Plant 2019, 42, 1503–1512. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought- high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef]
- Xie, J.; Yang, F.; Xu, X.; Peng, Y.; Ji, H. Salicylic Acid, Jasmonate, and Ethylene Contribute to Rice Defense Against White Tip Nematodes Aphelenchoides besseyi. Front. Plant Sci. 2022, 12, 755802. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.; Zhang, D.; Li, S.; Gao, J.; Han, L.; Qiu, J. Simple Bioassay for PAMP-Triggered Immunity in Rice Seedlings Based on Lateral Root Growth Inhibition. Rice Sci. 2022, 29, 67–75. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, Q.; Zhao, X.; Yang, C.; Liu, R.; Pang, J.; Zhao, W.; Wang, Q.; Liu, M.; Zhang, Z.; et al. A rice protein modulates endoplasmic reticulum homeostasis and coordinates with a transcription factor to initiate blast disease resistance. Cell Rep. 2022, 39, 110941. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Du, Q.; Wu, Y.; Shen, M.; Gao, F.; Wang, Z.; Xiao, X.; Tang, W.; Chen, Q. Ubiquitin Ligase Gene OsPUB57 Negatively Regulates Rice Blast Resistance. Plants 2025, 14, 758. https://doi.org/10.3390/plants14050758
Zhang J, Du Q, Wu Y, Shen M, Gao F, Wang Z, Xiao X, Tang W, Chen Q. Ubiquitin Ligase Gene OsPUB57 Negatively Regulates Rice Blast Resistance. Plants. 2025; 14(5):758. https://doi.org/10.3390/plants14050758
Chicago/Turabian StyleZhang, Jian, Qiang Du, Yugui Wu, Mengyu Shen, Furong Gao, Zhilong Wang, Xiuwen Xiao, Wenbang Tang, and Qiuhong Chen. 2025. "Ubiquitin Ligase Gene OsPUB57 Negatively Regulates Rice Blast Resistance" Plants 14, no. 5: 758. https://doi.org/10.3390/plants14050758
APA StyleZhang, J., Du, Q., Wu, Y., Shen, M., Gao, F., Wang, Z., Xiao, X., Tang, W., & Chen, Q. (2025). Ubiquitin Ligase Gene OsPUB57 Negatively Regulates Rice Blast Resistance. Plants, 14(5), 758. https://doi.org/10.3390/plants14050758




