InDel Markers for Identifying Interspecific Hybrid Offspring of Apple and Pear
Abstract
1. Introduction
2. Results
2.1. Construction of a Distant Hybridization Population Between Apple and Pear
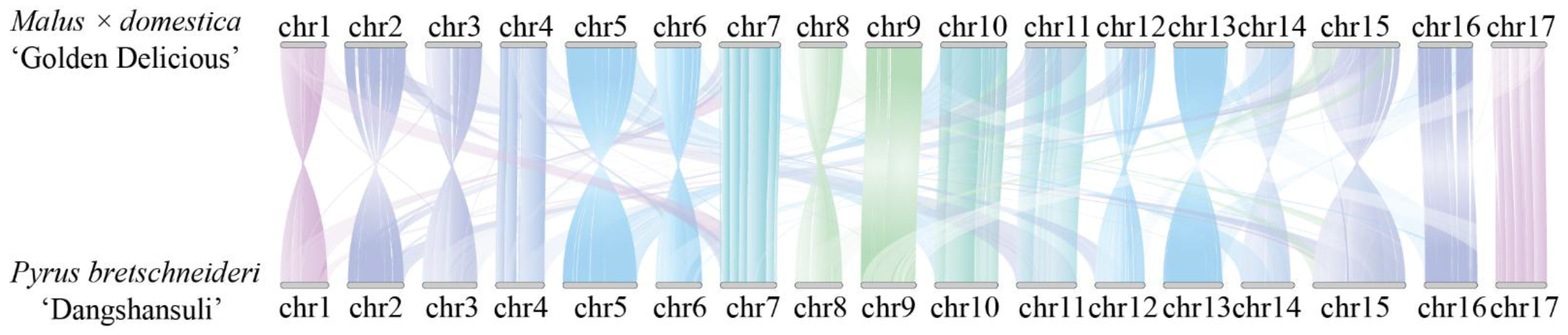
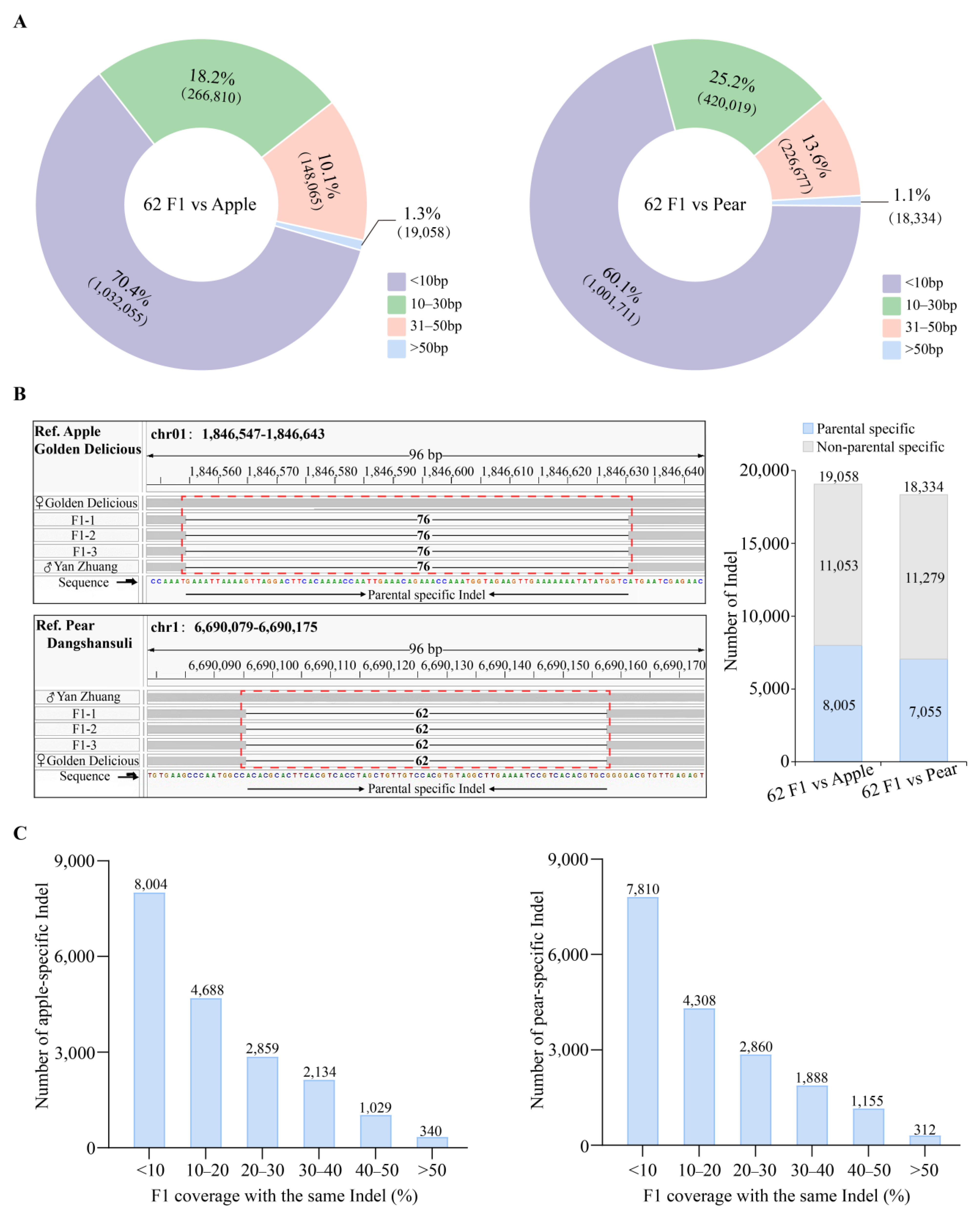
2.2. Resequencing of Distant Hybridization Progeny and Genome-Wide Genetic Variations
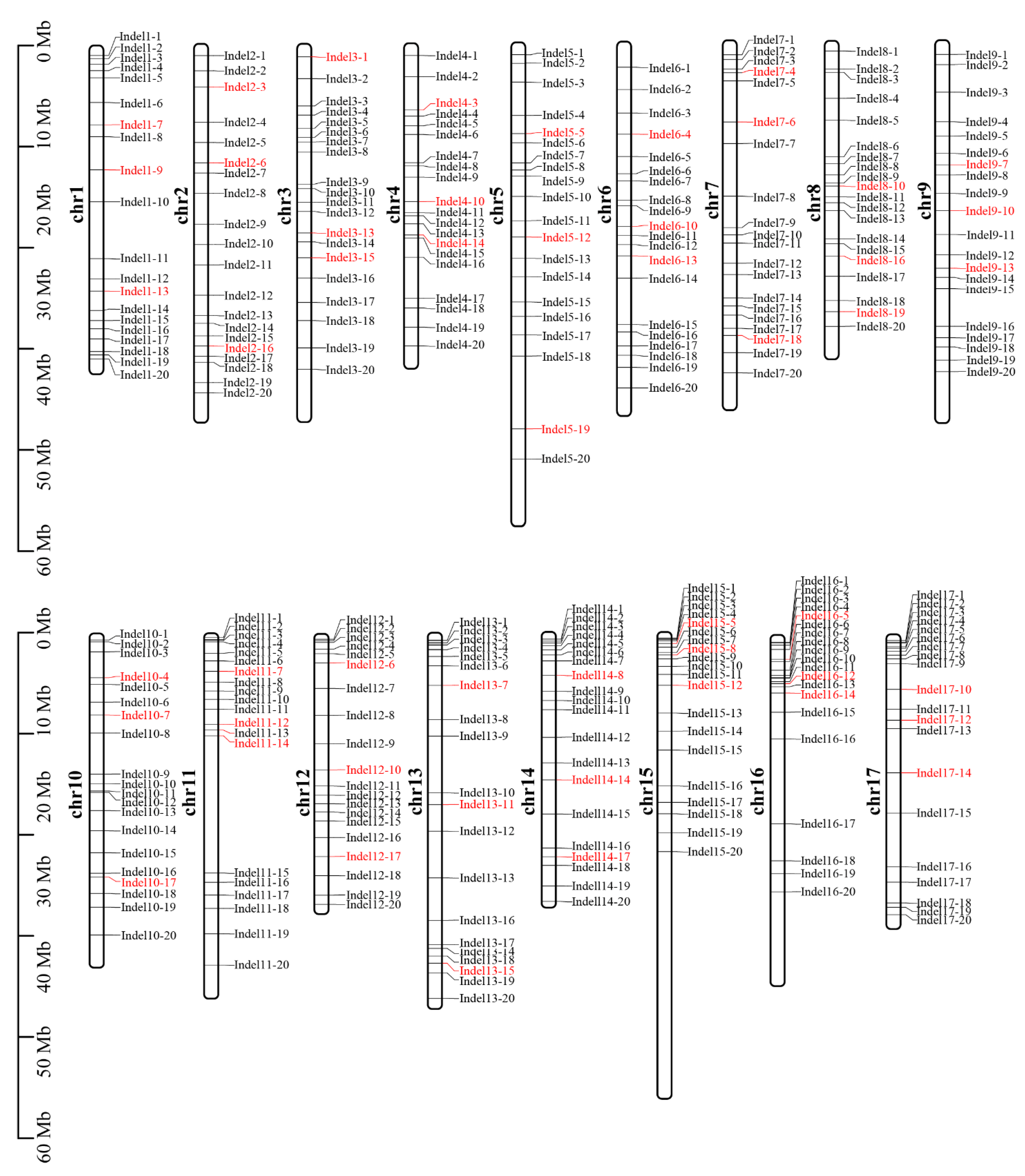
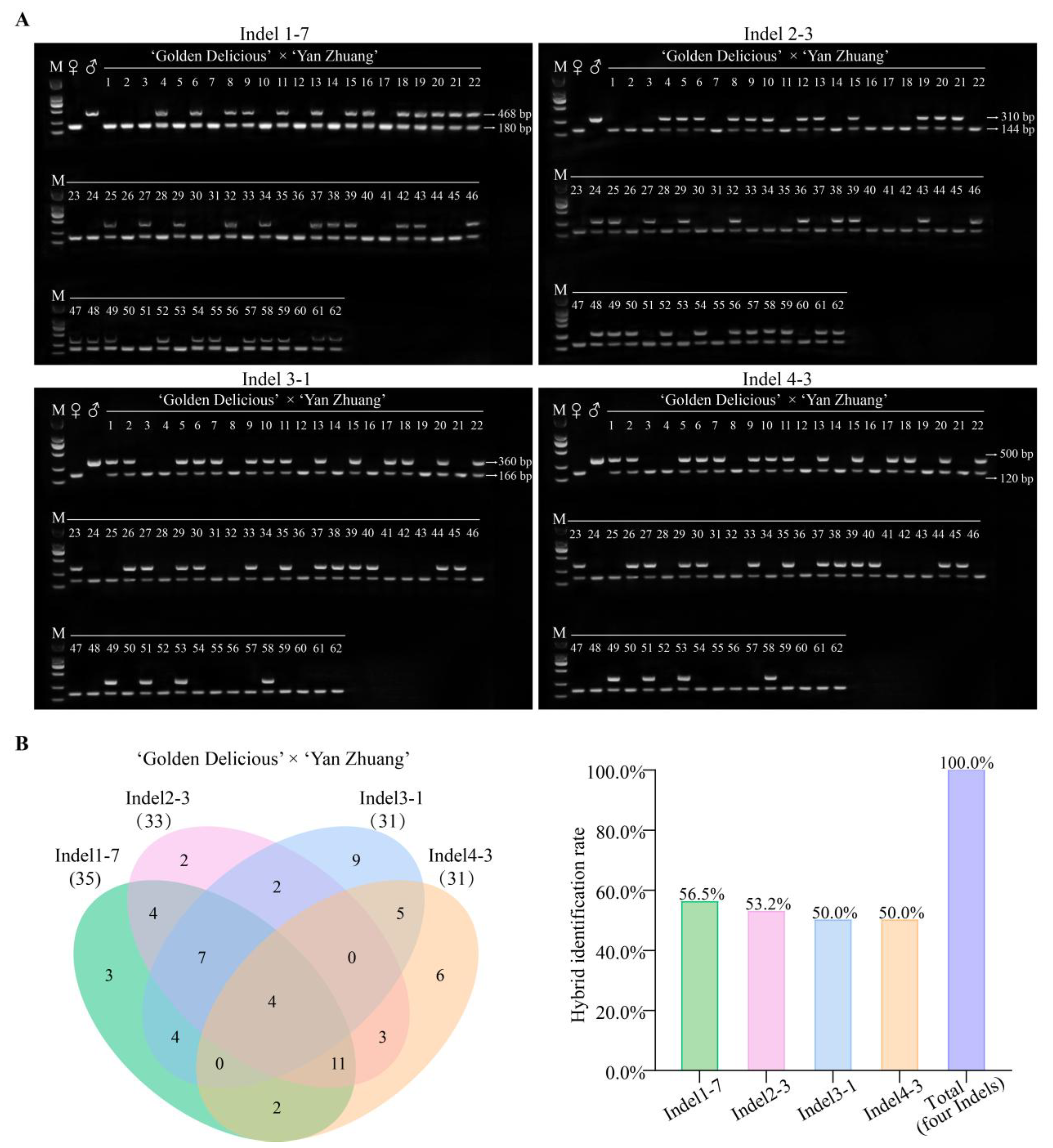
2.3. Development of Identification Markers for Distant Hybridization Progeny
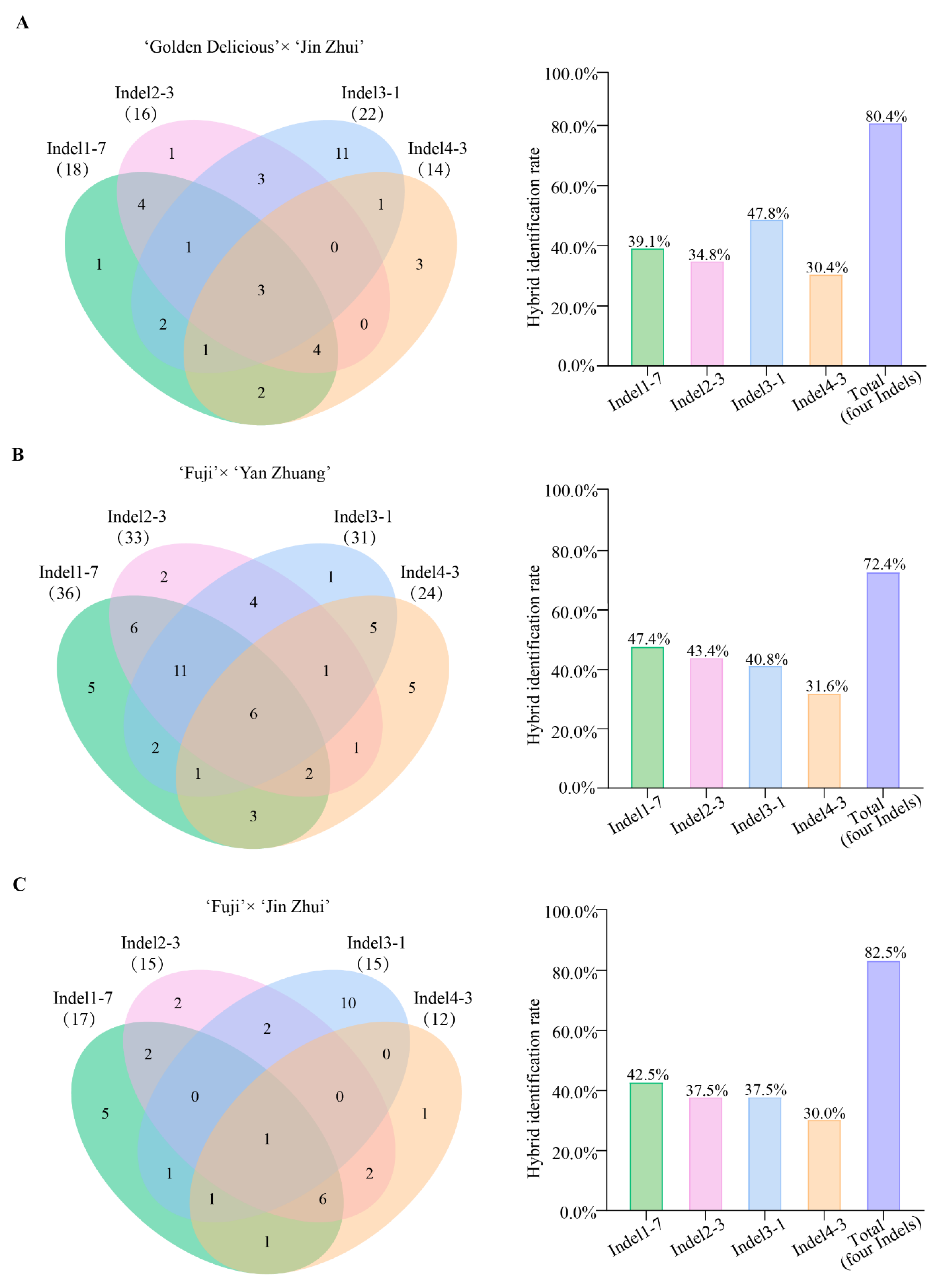
2.4. Universality of Markers
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Genomic DNA Library Preparation and Sequencing
4.3. Bioinformatics Analysis
4.4. Data Filtering, Sequence Alignment and InDel Calling
4.5. Experimental Validation of InDel for Polymorphism
4.6. InDel Marker Transferability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, H.C.; Gill, B.S. Current Status of Wide Hybridization in Wheat. Euphytica 1983, 32, 17–31. [Google Scholar] [CrossRef]
- Grant, P.R.; Grant, B.R.; Petren, K. Hybridization in the Recent Past. Am. Nat. 2005, 166, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Mallet, J. Hybridization as an invasion of the genome. Trends Ecol. Evol. 2005, 20, 229–237. [Google Scholar] [CrossRef]
- Mallet, J. Hybrid speciation. Nature 2007, 446, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhu, Z.; Shen, X.; Zhou, J.; He, J. Essences and Approaches of Distant Hybridization in Crops Breeding. Genom. Appl. Biol. 2010, 29, 144–149. [Google Scholar]
- Yao, Q.; Zhu, H.; Ren, Q.; Yang, J.; Gu, Y. ‘Hongyun’, a new intergeneric hybrid between Sinoc-alycanthus chinensis and Calycanthus floridus. Acta Hortic. Sin. 2014, 41, 1755–1756. [Google Scholar] [CrossRef]
- Xiumei, Z.; Hongxu, L.; Falin, W.; Ruxuan, N.; Chenbing, W.; Kuanying, L. A new very early- ripening of distant hybridization between plum and apricot cultivar ‘Longyuanhong’. J. Fruit. Sci. 2021, 38, 4. [Google Scholar]
- Kui, D.; Yongqing, W. Studies on hybridization compatibility between Loquat and Pear. In Proceedings of the the Fifth National Loquat Academic Symposium, Sichuan, China, 1 May 2011. [Google Scholar]
- Fengwang, M.; Junsheng, K. Test of Peach and Apricot Hybridization Compatibility. J. Fruit Sci. 1996, 13, 251–252. [Google Scholar]
- Crane, M.B.; Marks, E. Pear-Apple Hybrids. Nature 1952, 170, 1017. [Google Scholar] [CrossRef]
- Shimura, I.; Seike, K.; Shishikura, T. Intergeneic htbridization between Japanese pear, pyrus serotina Rehd. Var. culta Rehd, and apple, Malus pumila Mill. var. domestica Schneid. Jpn. J. Breed. 1980, 30, 170–180. [Google Scholar] [CrossRef]
- Banno, K.; Kumashiro, K.; Tabira, H. Growth characters of intergeneric hybrids between apple and Japanese pear. In Proceedings of the XXIV International Horticulture Congress, Kyoto, Japan, 21–27 August 1994; p. 34. [Google Scholar]
- Sakurai, K.; Shimura, I. Apple–pear hybrid. In Proceedings of the Abstract of XXIV International Horticulture Congress, Kyoto, Japan, 21–27 August 1994; p. 230. [Google Scholar]
- Sakuma, F.; Inoue, E.; Kasumi, M. Intergeneric crossing between Japanese pear (Pyrus pyrifolia Nakai) and apple (Malus pumila Mill.). In Bulletin of the Plant Biotechnology Institute Ibaraki Agricultural Center (Japan); FAO: Rome, Italy, 2000. [Google Scholar]
- Fischer, T.C.; Malnoy, M.; Hofmann, T.; Schwab, W.; Palmieri, L.; Wehrens, R.; Schuch, L.A.; Müller, M.; Schimmelpfeng, H.; Velasco, R.; et al. F-1 hybrid of cultivated apple (Malus x domestica) and European pear (Pyrus communis) with fertile F-2 offspring. Mol. Breed. 2014, 34, 817–828. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, P.; Liu, C. Phylogenetic relationship among nine Prunus species based on random amplified polymorphic DNA. Acta Hortic. Sin. 2002, 29, 218–223. [Google Scholar]
- Andersen, J.R.; Lübberstedt, T. Functional markers in plants. Trends Plant Sci. 2003, 8, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, Z.; Appels, R.; Xia, X. Functional markers in wheat: Current status and future prospects. Theor. Appl. Genet. 2012, 125, 1–10. [Google Scholar] [CrossRef]
- Li, S.; Zhang, M.; Li, X.; Qi, K.; Zhang, S.; Xue, L.; Li, J.; WU, J. Identification of progenies of distant hybrids of apple (Malus) and pear (Pyrus). J. Agric. Biotechnol. 2021, 29, 393–401. [Google Scholar]
- Peng, F.; Zhang, Y.; Chen, K.; Dai, Y.; Li, Y.; Cai, X. Research progress of DNA molecular markers in spinach genetic breeding. J. Shanghai Norm. Univ. (Nat. Sci.) 2021, 50, 237–242. [Google Scholar] [CrossRef]
- Pu, T.; Jin, J.; He, L.; Qu, W.; Liao, C.; Yuan, J.; Luo, H.; Zhao, Q. Population and genetic analysis of Phyllanthus emblica by SNP and InDel markers. J. Fruit. Sci. 2023, 40, 875–883. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, H.; Jin, J.; Zhang, Z.; Xi, B.; He, Y.; Wang, G.; Wang, C.; Qian, L.; Li, X.; et al. Development of Genome-Wide DNA Polymorphism Database for Map-Based Cloning of Rice Genes. Plant Physiol. 2004, 135, 1198–1205. [Google Scholar] [CrossRef]
- Hulse-Kemp, A.M.; Ashrafi, H.; Plieske, J.; Lemm, J.; Stoffel, K.; Hill, T.; Luerssen, H.; Pethiyagoda, C.L.; Lawley, C.T.; Ganal, M.W.; et al. A HapMap leads to a Capsicum annuum SNP infinium array: A new tool for pepper breeding. Hortic. Res. 2016, 3, 16036. [Google Scholar] [CrossRef]
- Liu, J.; Qu, J.; Yang, C.; Tang, D.; Li, J.; Lan, H.; Rong, T. Development of genome-wide insertion and deletion markers for maize, based on next-generation sequencing data. BMC Genom. 2015, 16, 601. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Zhai, W.; Deng, J.; Wang, H.; Cui, Y.; Cheng, F.; Wang, X.; Wu, J. Development of InDel markers for Brassica rapa based on whole-genome re-sequencing. Theor. Appl. Genet. 2013, 126, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cheng, J.; Wu, Z.; Qin, C.; Tan, S.; Tang, X.; Cui, J.; Zhang, L.; Hu, K. An InDel-based linkage map of hot pepper (Capsicum annuum). Mol. Breed. 2015, 35, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lan, J.; Bai, S.; Sun, X.; Liu, C.; Zhang, Y.; Dai, H. Screening of SNP and InDel markers glomerella leaf spot resistance gene locus in apple using HRM technology. Acta Hortic. Sin. 2017, 42, 215–222. [Google Scholar] [CrossRef]
- Fernandez i Marti, A.; Saski, C.A.; Manganaris, G.A.; Gasic, K.; Crisosto, C.H. Genomic Sequencing of Japanese Plum (Prunus salicina Lindl.) Mutants Provides a New Model for Rosaceae Fruit Ripening Studies. Front. Plant Sci. 2018, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Zamir, D. Improving plant breeding with exotic genetic libraries. Nat. Rev. Genet. 2001, 2, 983–989. [Google Scholar] [CrossRef]
- Uwimana, B.; Smulders, M.J.M.; Hooftman, D.A.P.; Hartman, Y.; van Tienderen, P.H.; Jansen, J.; McHale, L.K.; Michelmore, R.W.; van de Wiel, C.C.M.; Visser, R.G.F. Hybridization between crops and wild relatives: The contribution of cultivated lettuce to the vigour of crop–wild hybrids under drought, salinity and nutrient deficiency conditions. Theor. Appl. Genet. 2012, 125, 1097–1111. [Google Scholar] [CrossRef][Green Version]
- Newaskar, G.S.; Chimote, V.P.; Mehetre, S.S.; Jadhav, A.S.; Jenkins, J. Interspecific hybridization in Gossypium L.: Characterization of progenies with different ploidy-confirmed multigenomic backgrounds. Plant Breed. 2013, 132, 211–216. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Kell, S.; Maxted, N.; Ford-Lloyd, B.V.; Wei, W.; Kang, D.; Ma, K. Crop wild relatives and their conservation strategies in China. Biodivers. Sci. 2013, 21, 750–757. [Google Scholar]
- Zhang, X.; Wang, A.; Du, C.; Song, Z.; Wang, W.; He, Y.; Cai, D. An efficient method of developing synthetic allopolyploid rice (Oryza spp.). Genet. Resour. Crop Evol. 2014, 61, 809–816. [Google Scholar] [CrossRef]
- Sun, W.; Dong, T.; Wang, P.; Ma, M. Evaluation on fruit quality characteristics and identification at molecular level of distant hybrids Ganjin and Ganhong. J. Fruit. Sci. 2023, 40, 2505–2512. [Google Scholar] [CrossRef]
- Toxopeus, H. Notes on the genetics of a few leaf characters in the genus citrus. Euphytica 1962, 11, 19–25. [Google Scholar] [CrossRef]
- Ailing, Y.; Guojun, Z.; Haiying, X. Embryo rescue and identification of hybrids between diploid Grape and Tetraploid Grape. Acta Agric. Boreali-Occident. Sin. 2008, 17, 4. [Google Scholar]
- Xiuren, Z.; Juli, Z.; Shiming, Z. Breeding of apple pear generic hybrid varity ‘Gan Jin’. J. Fruit. Sci. 1991, 8, 65–70. [Google Scholar] [CrossRef]
- Varshney, R.K.; Nayak, S.N.; May, G.D.; Jackson, S.A. Next-generation sequencing technologies and their implications for crop genetics and breeding—ScienceDirect. Trends Biotechnol. 2009, 27, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Osorio, C.E.; Udall, J.A.; Salvo-Garrido, H.; Maureira-Butler, I.J. Development and characterization of InDel markers for Lupinus luteus L. (Fabaceae) and cross-species amplification in other Lupin species. Electron. J. Biotechnol. 2018, 31, 44–47. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, G.; Pan, B.; Diao, W.; Liu, J.; Ge, W.; Gao, C.; Zhang, Y.; Jiang, C.; Wang, S. Development and Application of InDel Markers for Capsicum spp. Based on Whole-Genome Re-Sequencing. Sci. Rep. 2019, 9, 3691. [Google Scholar] [CrossRef]
- Noda, T.; Daiou, K.; Mihara, T.; Nagano, Y. Development of Indel markers for the selection of Satsuma mandarin (Citrus unshiu Marc.) hybrids that can be used for low-cost genotyping with agarose gels. Euphytica 2020, 216, 115. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, L.; Yu, H.; Huang, Y.; Jiang, X.; Deng, X.; Xu, Q. Development of Species-Specific InDel Markers in Citrus. Plant Mol. Biol. Report. 2018, 36, 653–662. [Google Scholar] [CrossRef]
- Shihang, S.; Miaomiao, L.; Xiujuan, Q.; Leiming, S.; Yunpeng, Z.; Jinbao, F. Application of InDel markers on progeny identification in Actinidia arguta. J. Fruit. Sci. 2018, 35, 6. [Google Scholar]
- Jian, H.; Wenwen, X.; Guibing, Y.; Xuzhao, L.; Songliang, J.; Xianxin, L.; Ziniu, D.; Xianfeng, M. Establishment of Shatian pomelo × P. trifoliata hybrid population and InDel marker identification. J. Fruit. Sci. 2023, 40, 223–229. [Google Scholar]
- Noda, T.; Daiou, K.; Mihara, T.; Nagano, Y. Potential application of simple easy-to-use insertion-deletion (InDel) markers in citrus cultivar identification. Breed. Sci. 2021, 71, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Natali, L.; Giordani, T.; Polizzi, E.; Pugliesi, C.; Fambrini, M.; Cavallini, A. Genomic alterations in the interspecific hybrid Helianthus annuus × Helianthus tuberosus. Theor. Appl. Genet. 1998, 97, 1240–1247. [Google Scholar] [CrossRef]
- Binsfeld, P.C.; Wingender, R.; Schnabl, H. Cytogenetic analysis of interspecific sunflower hybrids and molecular evaluation of their progeny. Theor. Appl. Genet. 2001, 102, 1280–1285. [Google Scholar] [CrossRef]
- Jung, Y.; Han, D.; Marschall, T. BWA-MEME: BWA-MEM emulated with a machine learning approach. Bioinformatics 2022, 38, 2404–2413. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Freed, D.; Aldana, R.; Weber, J.A.; Edwards, J.S. The Sentieon Genomics Tools—A fast and accurate solution to variant calling from next-generation sequence data. BioRxiv 2017. bioRxiv:115717. [Google Scholar] [CrossRef]
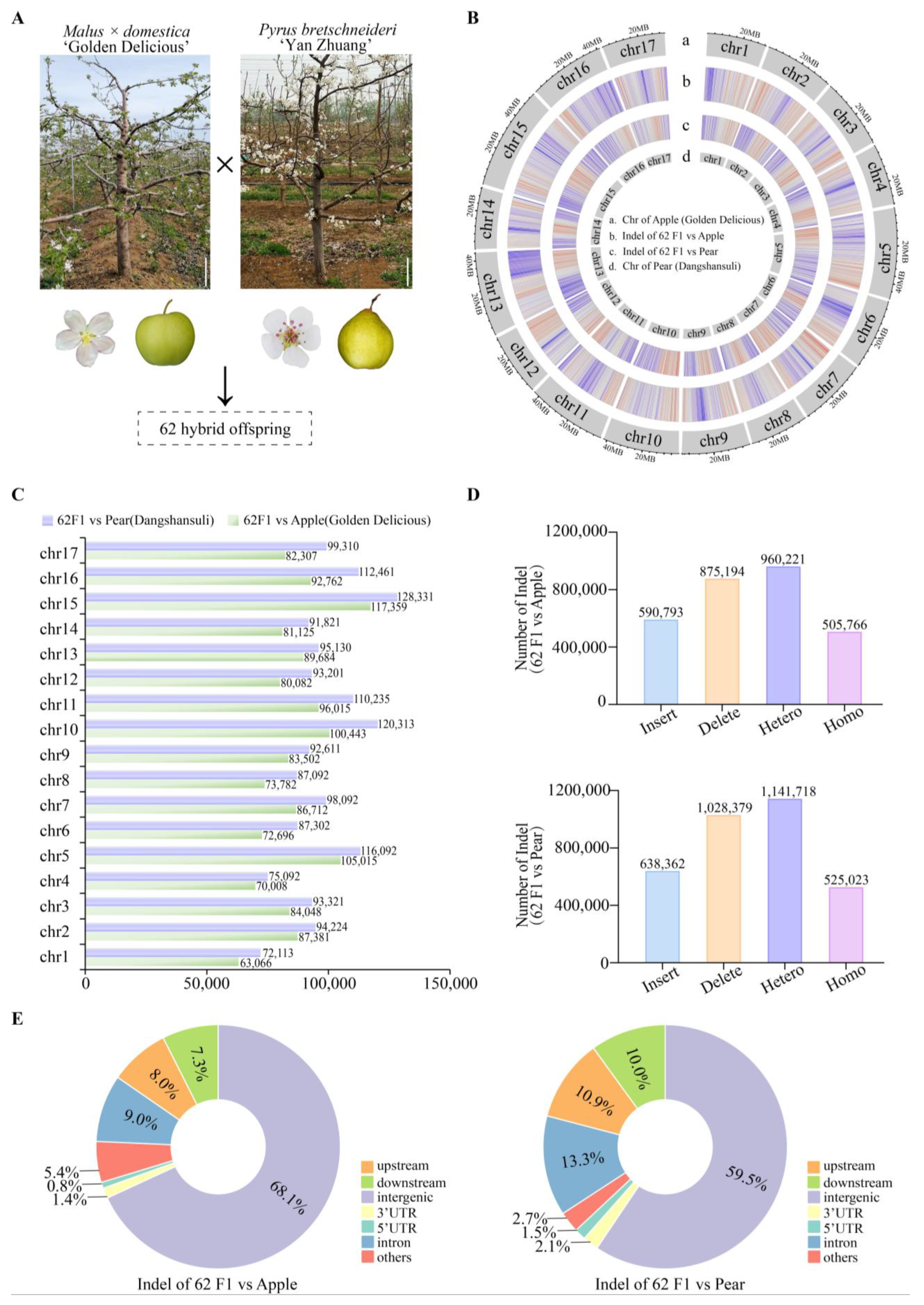
| Female (Malus × domestica) | Male (Pyrus brestschneideri) | No. of Flower | No. of Fruit | Fruit Set (%) | No. of Seed | No. of Seedling | Germination Rate (%) |
|---|---|---|---|---|---|---|---|
| Golden Delicous | Yan Zhuang | 500 | 126 | 25.2 | 170 | 62 | 36.5 |
| Golden Delicous | Jin Zhui | 500 | 110 | 22.0 | 141 | 46 | 32.6 |
| Fuji | Yan Zhuang | 500 | 132 | 26.4 | 178 | 76 | 42.7 |
| Fuji | Jin Zhui | 500 | 103 | 20.6 | 134 | 40 | 29.9 |
| Ref. Genome | Sample | Clean-Reads | Map Ratio (%) | Q30 | Depth | Cover Ratio | GC (%) |
|---|---|---|---|---|---|---|---|
| Malus × domestica ‘Golden Delicious’ | ‘Golden Delicious’ | 84,249,690 | 98.8 | 93.3 | 20.9 | 94.1% | 38.2% |
| Pyrus brestschneideri ‘Dangshansuli’ | ‘Yan Zhuang’ | 75,193,854 | 95.9 | 93.0 | 19.7 | 91.2% | 37.1% |
| Malus × domestica ‘Golden Delicious’ | 62 F1 | 46,661,113 | 98.9 | 97.3 | 19.4 | 89.4% | 38.1% |
| Pyrus brestschneideri ‘Dangshansuli’ | 62 F1 | 50,255,070 | 94.3 | 97.1 | 18.2 | 82.6% | 37.5% |
| Population | F1 | True Hybrid | Hybrid Rate | Total InDel | Effective InDel | Universality Rate |
|---|---|---|---|---|---|---|
| ‘Golden Delicious’ × ‘Jin Zhui’ | 46 | 38 | 82.6% | 51 | 45 | 88.2% |
| ‘Fuji’ × ‘Yan Zhuang’ | 76 | 61 | 80.3% | 51 | 39 | 76.5% |
| ‘Fuji’ × ‘Jin Zhui’ | 40 | 35 | 85.0% | 51 | 36 | 70.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.; Wang, B.; Xiao, Y.; Huang, J.; Wang, D.; Wang, S.; Li, W.; Zhang, Q.; Huang, F.; Shi, C.; et al. InDel Markers for Identifying Interspecific Hybrid Offspring of Apple and Pear. Plants 2025, 14, 646. https://doi.org/10.3390/plants14050646
Fan W, Wang B, Xiao Y, Huang J, Wang D, Wang S, Li W, Zhang Q, Huang F, Shi C, et al. InDel Markers for Identifying Interspecific Hybrid Offspring of Apple and Pear. Plants. 2025; 14(5):646. https://doi.org/10.3390/plants14050646
Chicago/Turabian StyleFan, Wenqi, Baoan Wang, Yao Xiao, Jinfeng Huang, Dongmei Wang, Shengyuan Wang, Wei Li, Qiulei Zhang, Fuli Huang, Chenxi Shi, and et al. 2025. "InDel Markers for Identifying Interspecific Hybrid Offspring of Apple and Pear" Plants 14, no. 5: 646. https://doi.org/10.3390/plants14050646
APA StyleFan, W., Wang, B., Xiao, Y., Huang, J., Wang, D., Wang, S., Li, W., Zhang, Q., Huang, F., Shi, C., & Li, T. (2025). InDel Markers for Identifying Interspecific Hybrid Offspring of Apple and Pear. Plants, 14(5), 646. https://doi.org/10.3390/plants14050646





