Three New Records of Pathogens Causing Stem Blight on Vaccinium corymbosum in China
Abstract
1. Introduction
2. Results
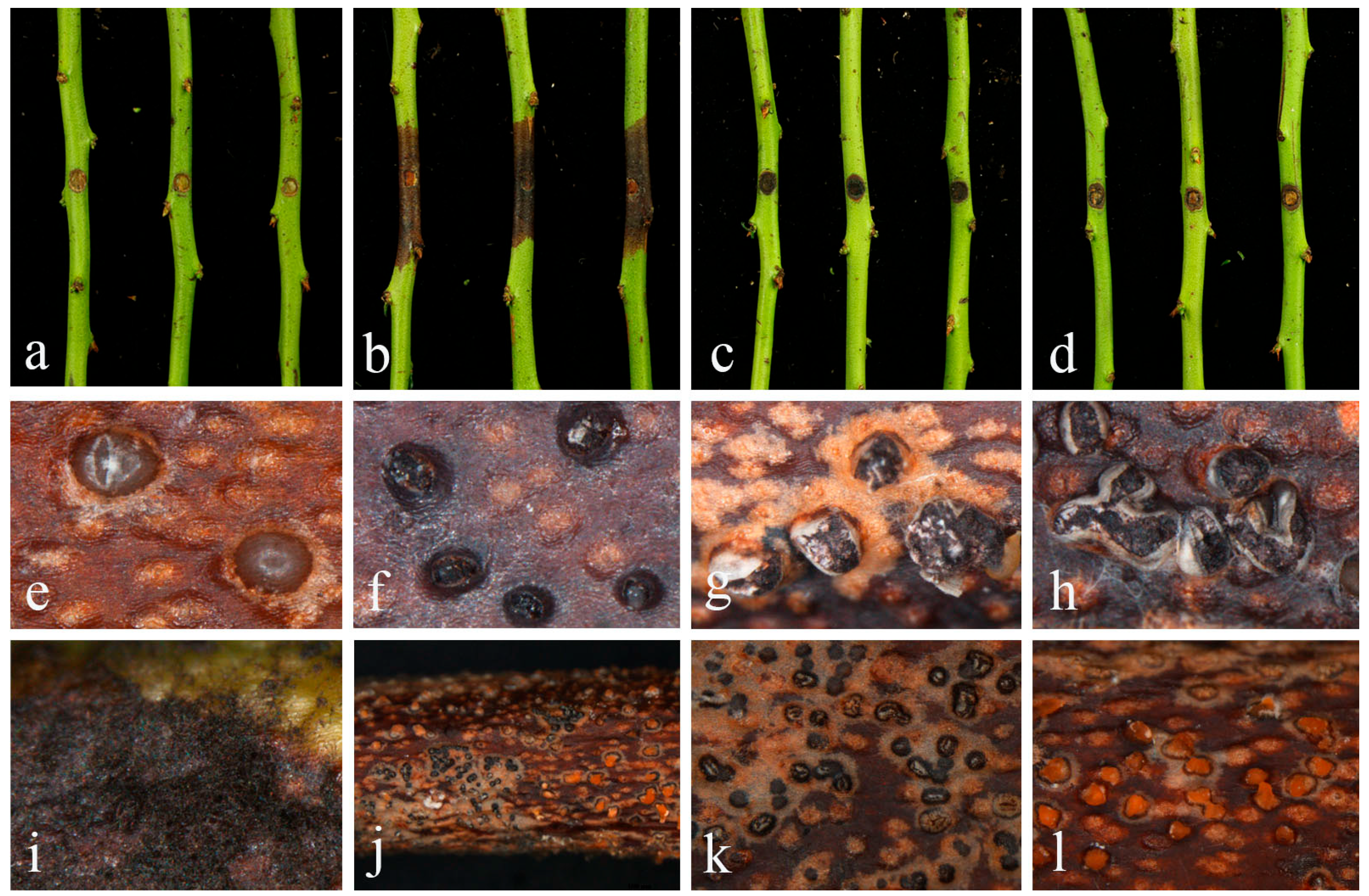
2.1. Field Symptoms and Fungal Isolation
2.2. Fungal Identification and Description
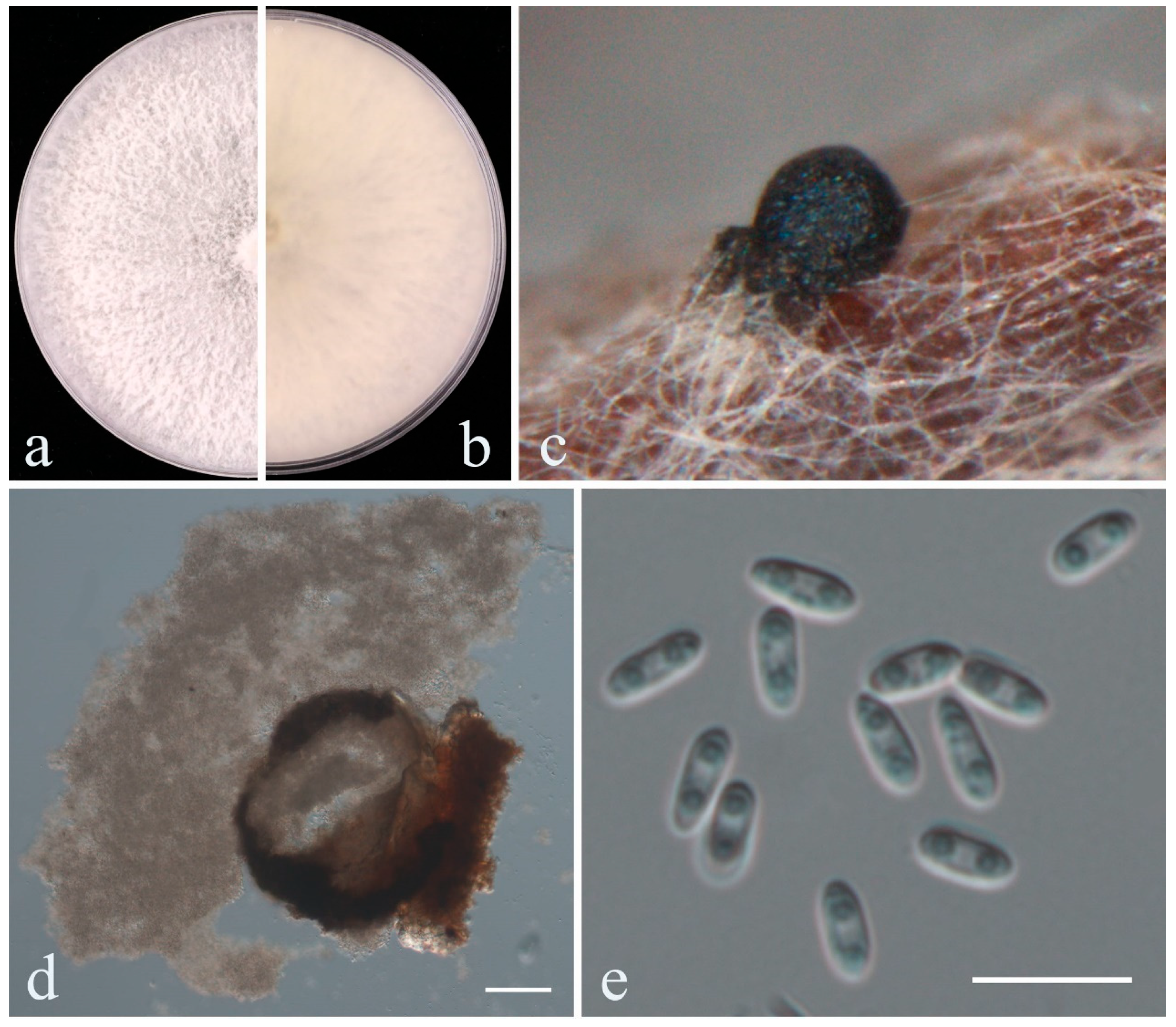
2.2.1. Colletotrichum
- Phylogeny
- Taxonomy
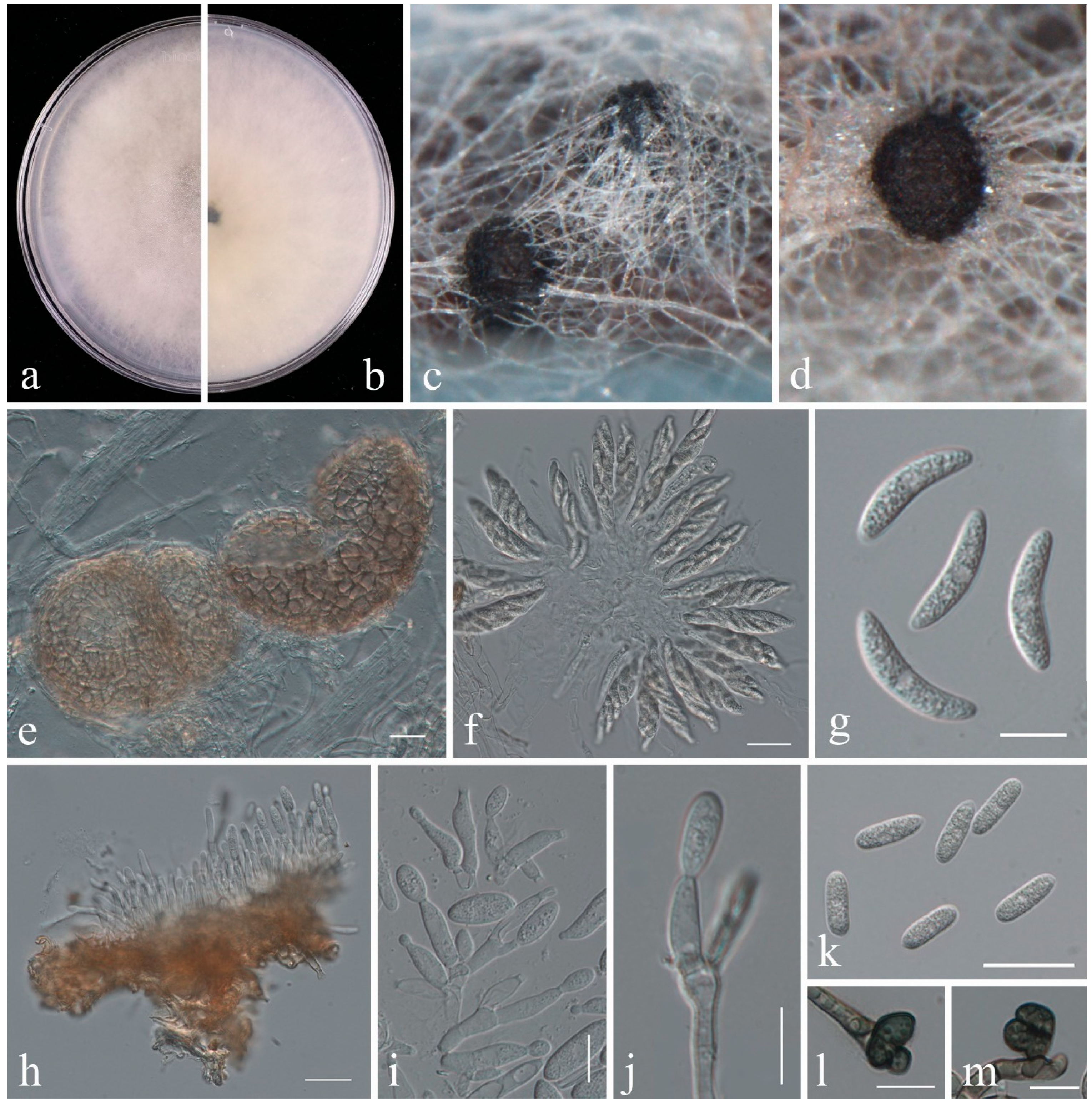
2.2.2. Curvularia
- Phylogeny
- Taxonomy
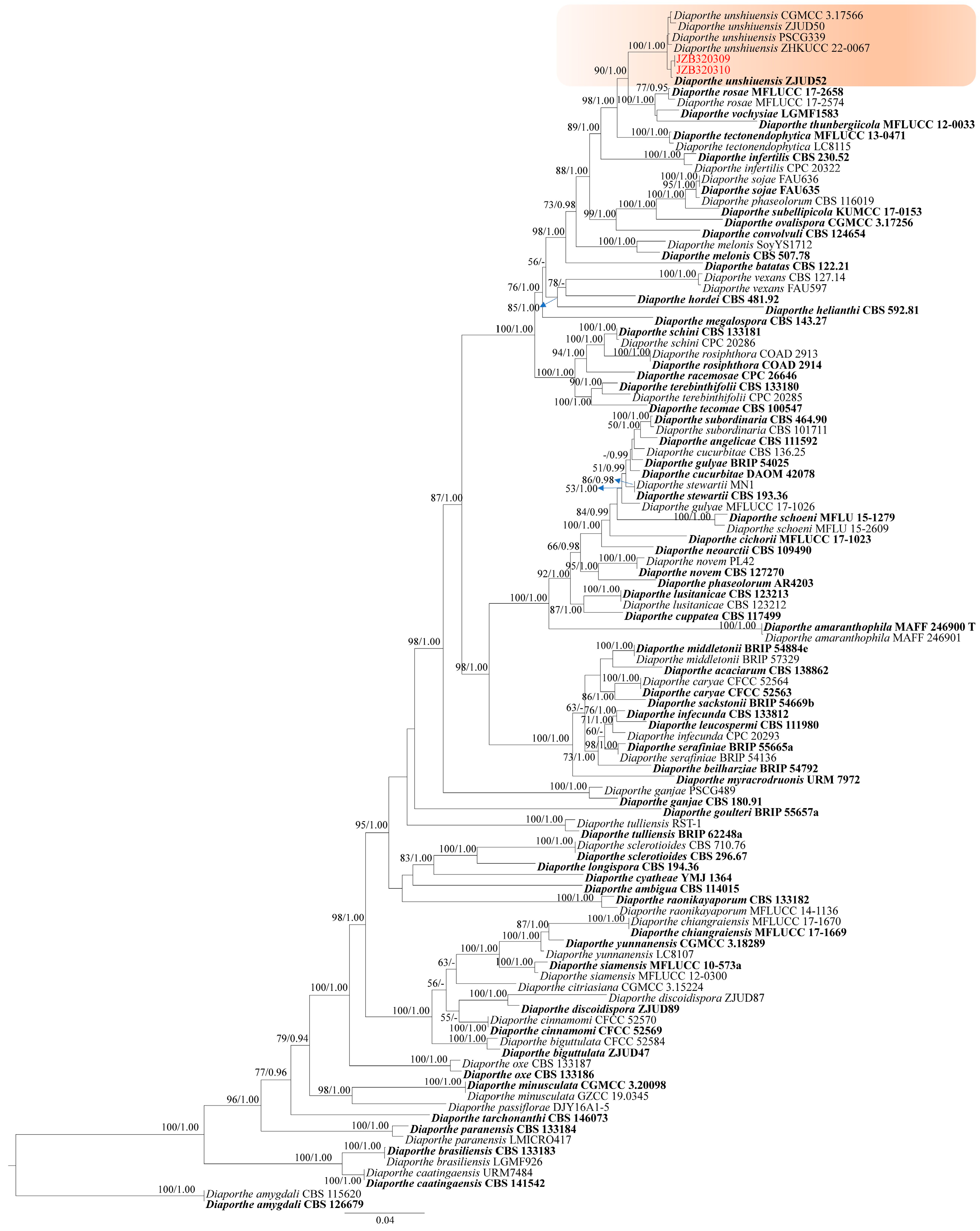
2.2.3. Diaporthe
- Phylogeny
- Taxonomy
2.3. Pathogenetic Assay
3. Discussion
4. Materials and Methods
4.1. Fungal Isolation and Morphology Characterization
4.2. DNA Extraction and PCR Amplification
4.3. Phylogenetic Analyses
4.4. Pathogenicity Tests
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- International Blueberry Organization. Global State of the Blueberry Industry Report 2024. Available online: https://www.internationalblueberry.org/2024-report/ (accessed on 18 October 2024).
- U.S. Department of Agriculture. China: Blueberry Annual Voluntary 2023. Available online: https://fas.usda.gov/data/china-blueberry-annual-voluntary-2023 (accessed on 18 November 2023).
- Polashock, J.J.; Caruso, F.L.; Averill, A.L.; Schilder, A.C. Compendium of Blueberry, Cranberry, and Lingonberry Diseases and Pests, 2nd ed.; APS Press: St. Paul, MN, USA, 2017; pp. 154–196. [Google Scholar]
- Martino, I.; Lione, G.; Garbelotto, M.; Gonthier, P.; Guarnaccia, V. Modeling the effect of temperature on the severity of blueberry stem blight and dieback with a focus on Neofusicoccum parvum and cultivar susceptibility. Horticulturae 2024, 10, 363. [Google Scholar] [CrossRef]
- Sammonds, J.; Billones, R.; Ridgway, H.J.; Walter, M.; Jaspers, M.V. Survey of blueberry farms for Botryosphaeria dieback and crown rot pathogens. N. Z. Plant Prot. 2009, 62, 238–242. [Google Scholar] [CrossRef]
- Espinoza, J.G.; Briceño, E.X.; Chávez, E.R.; Úrbez-Torres, J.R.; Latorre, B.A. Neofusicoccum spp. associated with stem canker and dieback of blueberry in Chile. Plant Dis. 2009, 93, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; He, W.; Zhang, Y. Stem blight of blueberry caused by Lasiodiplodia vaccinii sp. nov. in China. Plant Dis. 2019, 103, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Ru, S.; Ding, S.; Oliver, J.E.; Amodu, A. A review of Botryosphaeria stem blight disease of blueberry from the perspective of plant breeding. Agriculture 2023, 13, 100. [Google Scholar] [CrossRef]
- Hilário, S.; Santos, L.; Alves, A. Diversity and pathogenicity of Diaporthe species revealed from a survey of blueberry orchards in Portugal. Agriculture 2021, 11, 1271. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, W.; Zhao, L.; Zhang, L.; Yin, Y.; Zhang, Y. Neofusicoccum vaccinii: A novel species causing stem blight and dieback of blueberries in China. Plant Dis. 2022, 106, 2338–2347. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, X.; Li, X.; Quan, C.; Li, P.; Chang, X.; Gu, J.; Khaskheli, M.I.; Gong, G. Pestalotiopsis species associated with blueberry leaf spots and stem cankers in Sichuan Province of China. Plant Dis. 2023, 107, 149–156. [Google Scholar] [CrossRef]
- Santos, J.; Hilário, S.; Pinto, G.; Alves, A. Diversity and pathogenicity of pestalotioid fungi associated with blueberry plants in portugal, with description of three novel species of Neopestalotiopsis. Eur. J. Plant. Pathol. 2022, 162, 539–555. [Google Scholar] [CrossRef]
- Wright, E.R.; Folgado, M.; Rivera, M.C.; Crelier, A.; Vasquez, P.; Lopez, S.E. Nigrospora sphaerica causing leaf spot and twig and shoot blight on blueberry: A new host of the pathogen. Plant Dis. 2008, 92, 171. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.N.; Zhou, Z.S.; Wu, Y.X.; Chi, F.M.; Ji, Z.R.; Zhang, H.J. First report of Colletotrichum acutatum associated with stem blight of blueberry plants in China. Plant Dis. 2013, 97, 422. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hildebrand, P.D.; Renderos, W.E.; Abbasi, P.A. Identification and characterization of Sphaerulina vaccinii sp. nov. as the cause of leaf spot and stem canker in lowbush blueberry and its epidemiology. Phytopathology 2021, 111, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liang, X.; Lin, Y.; Hsiang, T.; Xiang, M.; Zhang, Y. First report of leaf spot and stem blight on blueberry (Vaccinium corymbosum ‘Bluerain’) caused by Calonectria pseudoreteaudii in China. Plant Dis. 2023, 107, 1951. [Google Scholar] [CrossRef]
- Dugan, F.; Glawe, D.; Attanayake, R.; Chen, W. The importance of reporting new host-fungus records for ornamental and regional crops. Plant Health. Prog. 2009, 10, 34. [Google Scholar] [CrossRef]
- Hyde, K.D.; Noorabadi, M.T.; Thiyagaraja, V.; He, M.Q.; Johnston, P.R.; Wijesinghe, S.N.; Armand, A.; Biketova, A.Y.; Chethana, K.W.T.; Erdoğdu, M.; et al. The 2024 outline of fungi and fungus-like taxa. Mycosphere 2024, 15, 5146–6239. [Google Scholar] [CrossRef]
- Index Fungorum. Available online: https://www.indexfungorum.org/Names/Names.asp (accessed on 16 February 2025).
- Liu, F.; Ma, Z.Y.; Hou, L.W.; Diao, Y.Z.; Wu, W.P.; Damm, U.; Song, S.; Cai, L. Updating species diversity of Colletotrichum, with a phylogenomic overview. Stud. Mycol. 2022, 101, 1–56. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Chen, Y.J.; Phukhamsakda, C.; Boekhout, T.; Groenewald, J.Z.; McKenzi, E.H.C.; Francisco, E.C.; Frisvad, J.C.; Groenewald, M.; Hurdeal, V.G.; et al. What are the 100 most cited fungal genera? Stud. Mycol. 2024, 108, 1–412. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Fu, M.; Crous, P.W.; Bai, Q.; Zhang, P.F.; Xiang, J.; Guo, Y.S.; Zhao, F.F.; Yang, M.M.; Hong, N.; Xu, W.X.; et al. Colletotrichum species associated with anthracnose of Pyrus spp. in China. Persoonia 2019, 42, 1–35. [Google Scholar] [CrossRef]
- Doyle, V.P.; Oudemans, P.V.; Rehner, S.A.; Litt, A. Habitat and host indicate lineage identity in Colletotrichum gloeosporioides s.l. from wild and agricultural landscapes in north america. PLoS ONE 2013, 8, e62394. [Google Scholar] [CrossRef] [PubMed]
- Cosseboom, S.D.; Hu, M. Ontogenic susceptibility of grapevine clusters to ripe rot, caused by the Colletotrichum acutatum and C. gloeosporioides species complexes. Phytopathology 2022, 112, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Zhang, W.; Li, Y.M.; Ji, S.X.; Li, X.H.; Hyde, K.D.; Zhang, K.C.; Phillips, A.J.L.; Manawasinghe, I.S.; Yan, J.Y. Identification and characterization of Colletotrichum species associated with cherry leaf spot disease in China. Plant Dis. 2023, 107, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Crous, P.W. Multi-locus phylogeny of the genus Curvularia and description of ten new species. Mycol. Prog. 2020, 19, 559–588. [Google Scholar] [CrossRef]
- Gomes, R.R.; Glienke, C.; Videira, S.I.R.; Lombard, L.; Groenewald, J.Z.; Crous, P.W. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 2013, 31, 1–41. [Google Scholar] [CrossRef]
- Norphanphoun, C.; Gentekaki, E.; Hongsanan, S.; Jayawardena, R.; Senanayake, I.; Manawasinghe, I.; Abeywickrama, P.; Bhunjun, C.; Hyde, K. Diaporthe: Formalizing the species-group concept. Mycosphere 2022, 13, 752–819. [Google Scholar] [CrossRef]
- Hongsanan, S.; Norphanphoun, C.; Senanayake, I.C.; Jayawardena, R.S.; Manawasinghe, I.S.; Abeywickrama, P.D.; Khuna, S.; Suwannarach, N.; Senwanna, C.; Monkai, J.; et al. Annotated notes on Diaporthe species. Mycosphere 2023, 14, 918–1189. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Zhu, J.T.; Chen, Y.Y.; Maharachchikumbura, S.S.N.; Hyde, K.D.; Liu, J.K. A re-evaluation of Diaporthe: Refining the boundaries of species and species complexes. Fungal Divers. 2024, 126, 1–125. [Google Scholar] [CrossRef]
- Huang, F.; Udayanga, D.; Wang, X.H.; Hou, X.; Mei, X.F.; Fu, Y.S.; Hyde, K.D.; Li, H.Y. Endophytic Diaporthe associated with Citrus: A phylogenetic reassessment with seven new species from China. Fungal Biol. 2015, 119, 331–347. [Google Scholar] [CrossRef]
- Yang, Q.; Fan, X.L.; Guarnaccia, V.; Tian, C.M. High diversity of Diaporthe species associated with dieback diseases in China, with twelve new species described. Mycokeys 2018, 39, 97–149. [Google Scholar] [CrossRef]
- Luo, M.; Guo, W.; Zhao, M.P.; Manawasinghe, I.S.; Guarnaccia, V.; Liu, J.W.; Hyde, K.D.; Dong, Z.Y.; You, C.P. Endophytic Diaporthe associated with Morinda officinalis in China. J. Fungi 2022, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Manawasinghe, I.S.; Dissanayake, A.J.; Li, X.H.; Liu, M.; Wanasinghe, D.N.; Xu, J.P.; Zhao, W.S.; Zhang, W.; Zhou, Y.Y.; Hyde, K.D.; et al. High genetic diversity and species complexity of Diaporthe associated with grapevine dieback in China. Front. Microbiol. 2019, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.S.; Crous, P.W.; Bai, Q.; Fu, M.; Yang, M.M.; Wang, X.H.; Du, Y.M.; Hong, N.; Xu, W.X.; Wang, G.P. High diversity of Diaporthe species associated with pear shoot canker in China. Persoonia 2020, 45, 132–162. [Google Scholar] [CrossRef]
- Du, Y.M.; Wang, X.H.; Guo, Y.S.; Xiao, F.; Peng, Y.H.; Hong, N.; Wang, G.P. Biological and molecular characterization of seven Diaporthe species associated with kiwifruit shoot blight and leaf spot in China. Phytopathol. Mediterr. 2021, 60, 177–198. [Google Scholar] [CrossRef]
- Wang, X.H.; Guo, Y.S.; Du, Y.M.; Yang, Z.L.; Huang, X.Z.; Hong, N.; Xu, W.X.; Wang, G.P. Characterization of Diaporthe species associated with peach constriction canker, with two novel species from China. MycoKeys 2021, 80, 77–90. [Google Scholar] [CrossRef]
- Xiao, X.E.; Liu, Y.D.; Zheng, F.; Xiong, T.; Zeng, Y.T.; Wang, W.; Zheng, X.L.; Wu, Q.; Xu, J.P.; Crous, P.W.; et al. High species diversity in Diaporthe associated with Citrus diseases in China. Persoonia 2023, 51, 229–256. [Google Scholar] [CrossRef]
- Petrović, K.; Skaltsas, D.; Castlebury, L.A.; Kontz, B.; Allen, T.W.; Chilvers, M.I.; Gregory, N.; Kelly, H.M.; Koehler, A.M.; Kleczewski, N.M.; et al. Diaporthe seed decay of soybean [Glycine Max (L.) Merr.] is endemic in the United States, but new fungi are involved. Plant Dis. 2021, 105, 1621–1629. [Google Scholar] [CrossRef]
- Elfar, K.; Torres, R.; Díaz, G.A.; Latorre, B.A. Characterization of Diaporthe australafricana and Diaporthe spp. associated with stem canker of blueberry in Chile. Plant Dis. 2013, 97, 1042–1050. [Google Scholar] [CrossRef]
- Lombard, L.; van Leeuwen, G.C.M.; Guarnaccia, V.; Polizzi, G.; van Rijswick, P.C.J.; Rosendahl, K.C.H.M.; Gabler, J.; Crous, P.W. Diaporthe species associated with Vaccinium, with specific reference to Europe. Phytopathol. Mediterr. 2014, 53, 287–299. [Google Scholar] [CrossRef]
- Sessa, L.; Abreo, E.; Lupo, S. Diversity of fungal latent pathogens and true endophytes associated with fruit trees in Uruguay. J. Phytopatho. 2018, 166, 633–647. [Google Scholar] [CrossRef]
- Yu, C.; Wu, C.; Li, G.; Wang, C. First report of Diaporthe nobilis causing postharvest rot of blueberry in Shandong Province, China. Plant Dis. 2018, 102, 1856. [Google Scholar] [CrossRef]
- Hilário, S.; Amaral, I.A.; Gonçalves, M.F.M.; Lopes, A.; Santos, L.; Alves, A. Diaporthe species associated with twig blight and dieback of Vaccinium Corymbosum in Portugal, with description of four new species. Mycologia 2020, 112, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Hu, Y.; Wu, Q.X.; Wu, Y.Y.; Zhang, Z.L.; Yi, R.; Song, X.H. First report of Diaporthe sojae causing stem canker on blueberry in China. Plant Dis. 2023, 107, 2851. [Google Scholar] [CrossRef]
- Tsushima, A.; Shirasu, K. Genomic resources of Colletotrichum fungi: Development and application. J. Gen. Plant. Pathol. 2022, 88, 349–357. [Google Scholar] [CrossRef]
- Neugebauer, K.A.; Mattupalli, C.; Hu, M.; Oliver, J.E.; VanderWeide, J.; Lu, Y.; Sullivan, K.; Stockwell, V.O.; Oudemans, P.; Miles, T.D. Managing fruit rot diseases of Vaccinium corymbosum. Front. Plant Sci. 2024, 15, 1428769. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, X.J.; Khaskheli, M.I.; Sun, X.F.; Chang, X.L.; Gong, G.S. Identification of Colletotrichum species associated with blueberry anthracnose in Sichuan, China. Pathogens 2020, 9, 718. [Google Scholar] [CrossRef]
- Bell, S.R.; Hernández Montiel, L.G.; González Estrada, R.R.; Gutiérrez Martínez, P. Main diseases in postharvest blueberries, conventional and eco-friendly control methods: A review. LWT 2021, 149, 112046. [Google Scholar] [CrossRef]
- Kusai, N.A.; Mior Zakuan Azmi, M.; Zulkifly, S.; Yusof, M.T.; Mohd Zainudin, N.A.I. Morphological and molecular characterization of Curvularia and related species associated with leaf spot disease of rice in Peninsular Malaysia. Rend. Fis. Acc. Lincei 2016, 27, 205–214. [Google Scholar] [CrossRef]
- Ram, D.; Devi, T.P.; Koti, P.S.; Jeevan, B.; Kamil, D.; Vanapalli, C.S.; Raghu, S.; Sunani, S.K.; Kashyap, A.S. Exploring the taxonomic classification of Curvularia genera: Enhancing understanding of phytopathogenic species in Poaceae through morphological and molecular approaches. J. Plant Pathol. 2024, 106, 539–551. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phukhamsakda, C.; Hyde, K.D.; McKenzie, E.H.C.; Saxena, R.K.; Li, Q.R. Do all fungi have ancestors with endophytic lifestyles? Fungal Divers. 2024, 125, 73–98. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Venturi, V. Synergisms between microbial pathogens in plant disease complexes: A growing trend. Front. Plant Sci. 2015, 6, 385. [Google Scholar] [CrossRef] [PubMed]
- Manawasinghe, I.S.; Phillips, A.J.L.; Xu, J.; Balasuriya, A.; Hyde, K.D.; Stępień, Ł.; Harischandra, D.L.; Karunarathna, A.; Yan, J.Y.; Weerasinghe, J.; et al. Defining a species in fungal plant pathology: Beyond the species level. Fungal Divers. 2021, 109, 267–282. [Google Scholar] [CrossRef]
- Chethana, K.W.T.; Manawasinghe, I.S.; Hurdeal, V.G.; Bhunjun, C.S.; Appadoo, M.A.; Gentekaki, E.; Raspé, O.; Promputtha, I.; Hyde, K.D. What are fungal species and how to delineate them? Fungal Divers. 2021, 109, 1–25. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; Volume 38, pp. 315–322. [Google Scholar]
- Myllys, L.; Stenroos, S.; Thell, A. New genes for phylogenetic studies of lichenized fungi: Glyceraldehyde-3-phosphate dehydrogenase and beta-tubulin genes. The Lichenologist 2002, 34, 237–246. [Google Scholar] [CrossRef]
- Guerber, J.C.; Liu, B.; Correll, J.C.; Johnston, P.R. Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia 2003, 95, 872–895. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic species in Lasiodiplodia theobromae. Fungal Diver. 2008, 28, 1–13. [Google Scholar]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-a sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the Fusarium are nonorthologous. Mol. Phylogenet. and Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree Version 1.4. 2012. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 1 January 2023).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wu, L.; Ren, K.; Wang, M.; Wang, N.; Tangtrakulwanich, K.; Li, X.; Chethana, K.W.T.; Hyde, K.D.; Zhang, W.; et al. Three New Records of Pathogens Causing Stem Blight on Vaccinium corymbosum in China. Plants 2025, 14, 647. https://doi.org/10.3390/plants14050647
Zhou Y, Wu L, Ren K, Wang M, Wang N, Tangtrakulwanich K, Li X, Chethana KWT, Hyde KD, Zhang W, et al. Three New Records of Pathogens Causing Stem Blight on Vaccinium corymbosum in China. Plants. 2025; 14(5):647. https://doi.org/10.3390/plants14050647
Chicago/Turabian StyleZhou, Yueyan, Linna Wu, Kaixuan Ren, Meng Wang, Nannan Wang, Khanobporn Tangtrakulwanich, Xinghong Li, Kandawatte Wedaralalage Thilini Chethana, Kevin D. Hyde, Wei Zhang, and et al. 2025. "Three New Records of Pathogens Causing Stem Blight on Vaccinium corymbosum in China" Plants 14, no. 5: 647. https://doi.org/10.3390/plants14050647
APA StyleZhou, Y., Wu, L., Ren, K., Wang, M., Wang, N., Tangtrakulwanich, K., Li, X., Chethana, K. W. T., Hyde, K. D., Zhang, W., & Yan, J. (2025). Three New Records of Pathogens Causing Stem Blight on Vaccinium corymbosum in China. Plants, 14(5), 647. https://doi.org/10.3390/plants14050647







