A Synthetic Review of Feedbacks and Drivers of Shrub–Grass Interaction in the Process of Grassland Shrub Encroachment
Abstract
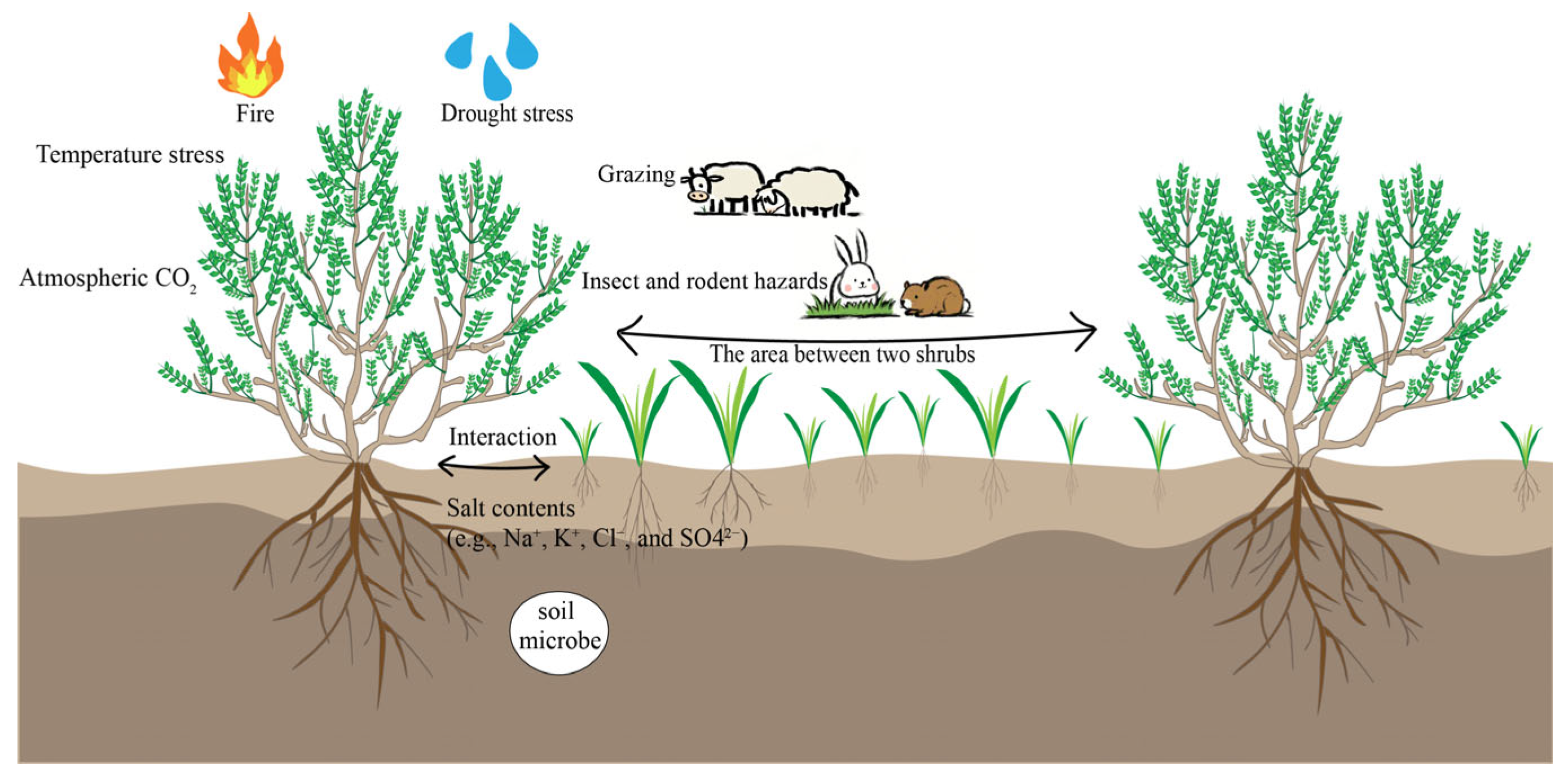
1. Introduction
2. Major Drivers Affecting Shrub–Grass Interactions
2.1. Biological Stress
2.1.1. Grazing Disturbance
2.1.2. Insects and Social, Burrowing, and Herbivorous Mammals
2.2. Abiotic Stress
2.2.1. Shrub–Grass Interactions Under Drought Stress
2.2.2. Shrub–Grass Interactions Under Temperature Stress
2.2.3. Shrub–Grass Interactions Under Salt Stress
2.2.4. Effects of Fire on Shrub–Grass Interactions
2.2.5. Effects of Atmospheric CO2 Concentration on Shrub–Grass Interactions
2.2.6. Species Specificity and Life-History Stages
3. Mechanisms of Shrub–Grass Interactions
3.1. Main Competition Mechanisms
Allelopathy and Competition
3.2. Main Facilitation Mechanisms
3.2.1. Habitat Amelioration
3.2.2. Resource Enrichment
3.2.3. Protection Against Herbivory
3.2.4. Introduction of Beneficial Organisms
3.2.5. Allelopathic Shrubs
| Potential Mechanisms That May Occur Within Shrub–Grass Interactions | References | |
|---|---|---|
| Habitat amelioration | Relieving solar radiation. | [56] |
| Reducing soil salinity. | [36,37] | |
| Reducing wind erosion and water erosion. | [57] | |
| Enhancing the soil infiltration capacity. | [80] | |
| Providing soil humus. | [57] | |
| Resource enrichment | Increasing the water content and nutrients in the soil in the microenvironment. | [9,58] |
| Changing the microbial processes that affect soil carbon storage and nutrient cycling. | [81] | |
| Association defense | Reducing eating and trampling by herbivores. | [70] |
| Introduction of beneficial organisms | Attracting pollinating animals and improving the pollination rate. | [82] |
| Increasing soil microorganisms, such as mycorrhizal fungi, etc. | [8,55,72,73,83] | |
| Attracting seed-spreading animals (birds, etc.). | [71] | |
| Competition for resources | Reducing soil moisture, reducing compaction, and enhancing aggregate stability. | [80] |
| Different plant traits. | [84] | |
| Allelopathy | Inhibitory effects of plants producing and releasing allelochemicals. | [49,53,54] |
4. Effects of Shrub–Grass Interactions on Community Succession
4.1. Effects of Shrub–Grass Interaction on Community Restoration Succession
4.2. Effects of Shrub–Grass Interaction on Community Retrograde Succession
5. Perspectives and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.J.; Anthelme, F.; et al. Facilitation in Plant Communities: The Past, the Present, and the Future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef]
- Auken, O.W.V. Shrub Invasions of North American Semiarid Grasslands. Annu. Rev. Ecol. Syst. 2000, 3, 197–215. [Google Scholar] [CrossRef]
- Maestre, F.T.; Bautista, S.; Cortina, J. Positive, Negative, and Net Effects in Grass-Shrub Interactions in Mediterranean Semiarid Grasslands. Ecology 2003, 84, 3186–3197. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R. Positive Interactions in Communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhang, Y.; Zhou, J.; Guo, Y.; Liu, H.; Li, Y.; Gao, S. Aridity and Soil Properties Drive the Shrub–Herb Interactions Along Drought Gradient in Desert Grassland in Inner Mongolia. Agronomy 2024, 14, 2588. [Google Scholar] [CrossRef]
- Hou, H.; Zhou, J.; Liu, H.; Li, Y.; Chang, C.; Wu, H.; Xiong, W.; Liu, X.; Gao, S. Advances in the Study of Shrub-Herbs Interaction of Shrub Encroachment in Grassland. Chin. J. Grassl. 2024, 46, 132–141. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. The Role of Nurse Plants in the Restoration of Degraded Environments. Front. Ecol. Environ. 2006, 4, 196–202. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Zhang, P.; Zhao, X.; Zhou, L.; Liu, T.; Hu, H.; Bai, Y.; Shen, H.; Fang, J. Climate and Native Grassland Vegetation as Drivers of the Community Structures of Shrub-Encroached Grasslands in Inner Mongolia, China. Landsc. Ecol. 2015, 30, 1627–1641. [Google Scholar] [CrossRef]
- Petri, L.; Ibañez, I. Assessing the Mechanisms and Impacts of Shrub Invasion in Forests: A Meta-Analysis. J. Appl. Ecol. 2023, 60, 2314–2326. [Google Scholar] [CrossRef]
- Maestre, F.T.; Valladares, F.; Reynolds, J.F. Is the Change of Plant–Plant Interactions with Abiotic Stress Predictable? A Meta-Analysis of Field Results in Arid Environments. J. Ecol. 2005, 93, 748–757. [Google Scholar] [CrossRef]
- He, Q.; Bertness, M.D.; Altieri, A.H. Global Shifts towards Positive Species Interactions with Increasing Environmental Stress. Ecol. Lett. 2013, 16, 695–706. [Google Scholar] [CrossRef]
- Xie, L.-N.; Guo, H.-Y.; Liu, Z.; Gabler, C.A.; Chen, W.-Z.; Gu, S.; Ma, C.-C. Shrubs Facilitate Recruitment of Caragana Stenophylla Pojark: Microhabitat Amelioration and Protection against Herbivory. Ann. For. Sci. 2017, 74, 70. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, Y.; Xu, D.; Xu, X.; Wang, C.; Wang, X.; Chen, J.; Xin, X.; Eldridge, D.J. The Fertile Island Effect Collapses under Extreme Overgrazing: Evidence from a Shrub-Encroached Grassland. Plant Soil. 2020, 448, 201–212. [Google Scholar] [CrossRef]
- Coetzee, B.W.T.; Tincani, L.; Wodu, Z.; Mwasi, S.M. Overgrazing and Bush Encroachment by Tarchonanthus Camphoratus in a Semi-Arid Savanna. Afr. J. Ecol. 2008, 46, 449–451. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, S.; Bian, J.; He, J.-S. Livestock Exclusion Enhances Shrub Encroachment in an Alpine Meadow on the Eastern Qinghai-Tibetan Plateau. Land. Degrad. Dev. 2023, 34, 1390–1402. [Google Scholar] [CrossRef]
- Davidson, A.D.; Detling, J.K.; Brown, J.H. Ecological Roles and Conservation Challenges of Social, Burrowing, Herbivorous Mammals in the World’s Grasslands. Front. Ecol. Environ. 2012, 10, 477–486. [Google Scholar] [CrossRef]
- Noble, J.C.; Hik, D.; Sinclair, A. Landscape Ecology of the Burrowing Bettong: Fire and Marsupial Biocontrol of Shrubs in Semi-Arid Australia. Rangel. J. 2007, 29, 107–119. [Google Scholar] [CrossRef]
- Weltzin, J.; Archer, S.; Heitschmidt, R. Small-Mammal Regulation of Vegetation Structure in a Temperate Savanna. Ecology 1997, 78, 751. [Google Scholar] [CrossRef]
- Limin, H.; Shouquan, C. Rodent Pest Control on Grasslands in China:Current State, Problems and Prospects. Zhiwu Baohu Xuebao 2022, 49, 415–423. [Google Scholar] [CrossRef]
- Kuechly, H.U.; Mueller, J.S.; Reinfelder, V.L.; Wiedemann, S.; Blaum, N. Rodent-Mediated Dispersal of Acacia Seeds in Kalahari Savannah Rangelands Implications for Bush Encroachment. Afr. J. Ecol. 2010, 49, 119. [Google Scholar] [CrossRef]
- Fickenscher, J.L.; Litvaitis, J.A.; Lee, T.D.; Johnson, P.C. Insect Responses to Invasive Shrubs: Implications to Managing Thicket Habitats in the Northeastern United States. For. Ecol. Manag. 2014, 322, 127–135. [Google Scholar] [CrossRef]
- Branson, D.H.; Joern, A.; Sword, G.A. Sustainable Management of Insect Herbivores in Grassland Ecosystems: New Perspectives in Grasshopper Control. BioScience 2006, 56, 743–755. [Google Scholar] [CrossRef]
- Otte, D.; Joern, A. On Feeding Patterns in Desert Grasshoppers and the Evolution of Specialized Diets. Proc. Acad. Nat. Sci. Phila. 1976, 128, 89–126. [Google Scholar]
- Laws, A.N.; Prather, C.M.; Branson, D.H.; Pennings, S.C. Effects of Grasshoppers on Prairies: Herbivore Composition Matters More than Richness in Three Grassland Ecosystems. J. Anim. Ecol. 2018, 87, 1727–1737. [Google Scholar] [CrossRef]
- Deraison, H.; Badenhausser, I.; Loeuille, N.; Scgrasser, C.; Gross, N. Functional Trait Diversity across Trophic Levels Determines Herbivore Impact on Plant Community Biomass. Ecol. Lett. 2015, 18, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, X.; Liu, W.; Lin, H.; Yang, C.; Zhang, T.; He, Y.; Sun, S.; Chen, L.; Xu, Y. Seed pests and their effect on quality of Caragana microphylla seeds in semi-arid sandy land. Pratacultural Sci. 2022, 39, 48–56. [Google Scholar] [CrossRef]
- Maestre, F.T.; Cortina, J. Do Positive Interactions Increase with Abiotic Stress? A Test from a Semi-Arid Steppe. Proc. R. Soc. Lond. B 2004, 271 (Suppl. S5), S331–S333. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Pugnaire, F.I.; Armas, C.; Rodríguez-Echeverría, S.; Schöb, C. The Shift from Plant-Plant Facilitation to Competition under Severe Water Deficit Is Spatially Explicit. Ecol. Evol. 2017, 7, 2441–2448. [Google Scholar] [CrossRef]
- Swanson, E.K.; Sheley, R.L.; James, J.J. Do Shrubs Improve Reproductive Chances of Neighbors across Soil Types in Drought? Oecologia 2020, 192, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shen, H.; Akinyemi, D.S.; Zhang, P.; Feng, Y.; Zhao, M.; Kang, J.; Zhao, X.; Hu, H.; Fang, J. Increased Precipitation Attenuates Shrub Encroachment by Facilitating Herbaceous Growth in a Mongolian Grassland. Funct. Ecol. 2022, 36, 2356–2366. [Google Scholar] [CrossRef]
- Guan, J.; Li, X.; Zhang, M.; Xie, L.; He, P.; Ma, C. Effects of Caragana microphylla Encroachment on Mineral Element Concentrations in Leaves and Aboveground Biomass Accumulation of Herbaceous Plants along an Aridity Gradient. Acta Ecol. Sin. 2023, 43, 8047–8056. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Pockman, W.T. The Vulnerability to Freezing-Induced Xylem Cavitation of Larrea Tridentata (Zygophyllaceae) in the Chihuahuan Desert. Am. J. Bot. 2002, 89, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Elorza, M.; Dana, E.D.; González, A.; Sobrino, E. Changes in the High-Mountain Vegetation of the Central Iberian Peninsula as a Probable Sign of Global Warming. Ann. Bot. 2003, 92, 273–280. [Google Scholar] [CrossRef]
- Liancourt, P.; Le Bagousse-Pinguet, Y.; Rixen, C.; Dolezal, J. SGH: Stress or Strain Gradient Hypothesis? Insights from an Elevation Gradient on the Roof of the World. Ann. Bot. 2017, 120, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Dona, A.J.; Galen, C. Nurse Effects of Alpine Willows (Salix) Enhance Over-Winter Survival at the Upper Range Limit of Fireweed, Chamerion Angustifolium. Arct. Antarct. Alp. Res. 2007, 39, 57–64. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, L.; Yang, Q.; Zhao, W.; Wang, X. Effect of Desert Shrubs on Fine-Scale Spatial Patterns of Understory Vegetation in a Dry-Land. Plant Ecol. 2016, 217, 1141–1155. [Google Scholar] [CrossRef]
- Rumbaugh, M.D.; Johnson, D.A.; Epps, G.A.V. Forage Yield and Quality in a Great Basin Shrub, Grass, and Legume Pasture Experiment. J. Range Manag. 1982, 35, 604. [Google Scholar] [CrossRef]
- Qi, M.; Sun, T.; Xue, S.; Yang, W.; Shao, D.; Martínez-López, J. Competitive Ability, Stress Tolerance and Plant Interactions along Stress Gradients. Ecology 2018, 99, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Smit, I.P.J.; Asner, G.P.; Govender, N.; Vaughn, N.R.; Van Wilgen, B.W. An Examination of the Potential Efficacy of High-Intensity Fires for Reversing Woody Encroachment in Savannas. J. Appl. Ecol. 2016, 53, 1623–1633. [Google Scholar] [CrossRef]
- D’Odorico, P.; Okin, G.S.; Bestelmeyer, B.T. A Synthetic Review of Feedbacks and Drivers of Shrub Encroachment in Arid Grasslands. Ecohydrology 2012, 5, 520–530. [Google Scholar] [CrossRef]
- Buitenwerf, R.; Bond, W.J.; Stevens, N.; Trollope, W.S.W. Increased Tree Densities in South African Savannas: >50 Years of Data Suggests CO2 as a Driver. Glob. Chang. Biol. 2012, 18, 675–684. [Google Scholar] [CrossRef]
- Morgan, J.A.; Milchunas, D.G.; LeCain, D.R.; West, M.; Mosier, A.R. Carbon Dioxide Enrichment Alters Plant Community Structure and Accelerates Shrub Growth in the Shortgrass Steppe. Proc. Natl. Acad. Sci. USA 2007, 104, 14724–14729. [Google Scholar] [CrossRef] [PubMed]
- Bond, W.J.; Parr, C.L. Beyond the Forest Edge: Ecology, Diversity and Conservation of the Grassy Biomes. Biol. Conserv. 2010, 143, 2395–2404. [Google Scholar] [CrossRef]
- Mooney, H.A.; Cleland, E.E. The Evolutionary Impact of Invasive Species. Proc. Natl. Acad. Sci. USA 2001, 98, 5446–5451. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.; Schimel, D.S.; Holland, E.A. Mechanisms of Shrubland Expansion: Land Use, Climate or CO2? Clim. Chang. 1995, 29, 91–99. [Google Scholar] [CrossRef]
- Rolo, V.; Plieninger, T.; Moreno, G. Facilitation of Holm Oak Recruitment through Two Contrasted Shrubs Species in Mediterranean Grazed Woodlands. J. Veg. Sci. 2013, 24, 344–355. [Google Scholar] [CrossRef]
- Iyengar, S.B.; Bagchi, S.; Barua, D.; Mishra, C.; Sankaran, M. A Dominant Dwarf Shrub Increases Diversity of Herbaceous Plant Communities in a Trans-Himalayan Rangeland. Plant Ecol. 2017, 218, 843–854. [Google Scholar] [CrossRef]
- Armas, C.; Pugnaire, F.I. Plant Interactions Govern Population Dynamics in a Semi-Arid Plant Community. J. Ecol. 2005, 93, 978–989. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Y.; Wei, S.; Yu, X. Isolation of Allelochemicals from Rhododendron Capitatum and Their Allelopathy on Three Perennial Herbaceous Plants. Plants 2024, 13, 2585. [Google Scholar] [CrossRef] [PubMed]
- Meiners, S.J.; Kong, C.-H.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an Ecological Context for Allelopathy. Plant Ecol. 2012, 213, 1861–1867. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent Advances in Allelopathy for Weed Control: From Knowledge to Applications. Pest. Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-H.; Xuan, T.D.; Khanh, T.D.; Tran, H.-D.; Trung, N.T. Allelochemicals and Signaling Chemicals in Plants. Molecules 2019, 24, 2737. [Google Scholar] [CrossRef]
- Fang, C.; Yang, L.; Chen, W.; Li, L.; Zhang, P.; Li, Y.; He, H.; Lin, W. MYB57 Transcriptionally Regulates MAPK11 to Interact with PAL2;3 and Modulate Rice Allelopathy. J. Exp. Bot. 2020, 71, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.G.; Oliveira, S.C.C.; Salles, K.A.; Sampaio, A.B.; Schmidt, I.B. Allelopathy of a Native Shrub Can Help Control Invasive Grasses at Sites under Ecological Restoration in a Neotropical Savanna. Plant Ecol. Divers. 2018, 11, 527–538. [Google Scholar] [CrossRef]
- Wagner, T.C.; Richter, J.; Joubert, D.F.; Fischer, C. A Dominance Shift in Arid Savanna: An Herbaceous Legume Outcompetes Local C4 Grasses. Ecol. Evol. 2018, 8, 6779–6787. [Google Scholar] [CrossRef] [PubMed]
- Kaye, M.W.; Hone, C.M. Removal of Invasive Shrubs Alters Light but Not Leaf Litter Inputs in a Deciduous Forest Understory. Restor. Ecol. 2016, 24, 617–625. [Google Scholar] [CrossRef]
- Sun, T.; Jia, Z.; Qian, Y.; Liu, H.; Tang, J. Comparison on Functions of Wind-Break and Sand-Fixation of Nitraria tangutorun Nebkhas at Different Developmental Stages in Minqin Desert-oasis Transition Zone. J. Yunnan Agric. Univ. (Nat. Sci.) 2019, 34, 713–724. [Google Scholar] [CrossRef]
- Tiantian, W.; Xuelin, Z.; Yan, L.; Lin, Z.; Jianbin, Z. Rainfall Interception Characteristics of Typical Shrubs in the Southwestern Edge of Maowusu Desert. Chin. J. Grassl. 2021, 43, 82–89. [Google Scholar] [CrossRef]
- Beltrán, E.; Valiente-Banuet, A.; Verdú, M. Trait Divergence and Indirect Interactions Allow Facilitation of Congeneric Species. Ann. Bot. 2012, 110, 1369–1376. [Google Scholar] [CrossRef]
- Yu, K.; D’Odorico, P. Hydraulic Lift as a Determinant of Tree-Grass Coexistence on Savannas. New Phytol. 2015, 207, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Barron-Gafford, G.A.; Sanchez-Cañete, E.P.; Minor, R.L.; Hendryx, S.M.; Lee, E.; Sutter, L.F.; Tran, N.; Parra, E.; Colella, T.; Murphy, P.C.; et al. Impacts of Hydraulic Redistribution on Grass–Tree Competition vs Facilitation in a Semi-Arid Savanna. New Phytol. 2017, 215, 1451–1461. [Google Scholar] [CrossRef]
- Fu, C.; Wang, G.; Goulden, M.L.; Scott, R.L.; Bible, K.; Cardon, Z. Combined Measurement and Modeling of the Hydrological Impact of Hydraulic Redistribution Using CLM 4.5 at Eight AmeriFlux Sites. Hydrol. Earth Syst. Sci. 2016, 20, 2001–2018. [Google Scholar] [CrossRef]
- Dohn, J.; Dembélé, F.; Karembé, M.; Moustakas, A.; Amévor, K.A.; Hanan, N.P. Tree Effects on Grass Growth in Savannas: Competition, Facilitation and the Stress-Gradient Hypothesis. J. Ecol. 2013, 101, 202–209. [Google Scholar] [CrossRef]
- Rodríguez, A.; Durán, J.; Gallardo, A. Influence of Legumes on N Cycling in a Heathland in Northwest Spain. Web Ecol. 2007, 7, 87–93. [Google Scholar] [CrossRef]
- Mb, C. Graminoid Responses to Grazing by Large Herbivores: Adaptations, Exaptations, and Interacting Processes. Ann. Mo. Bot. Gard. 1985, 72, 852–863. [Google Scholar] [CrossRef]
- Quiroga, R.E.; Golluscio, R.A.; Blanco, L.J.; Fernández, R.J. Aridity and Grazing as Convergent Selective Forces: An Experiment with an Arid Chaco Bunchgrass. Ecol. Appl. 2010, 20, 2369. [Google Scholar] [CrossRef]
- Adler, P.; Milchunas, D.; Lauenroth, W.; Sala, O.; Burke, I. Functional Traits of Graminoids in Semi-Arid Steppes: A Test of Grazing Histories. J. Appl. Ecol. 2004, 41, 653–663. [Google Scholar] [CrossRef]
- Hanley, M.E.; Lamont, B.B.; Fairbanks, M.M.; Rafferty, C.M. Plant Structural Traits and Their Role in Anti-Herbivore Defence. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 157–178. [Google Scholar] [CrossRef]
- Hanley, M.E.; Lamont, B.B. Herbivory, Serotiny and Seedling Defence in Western Australian Proteaceae. Oecologia 2001, 126, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Saixiyala; Yang, D.; Zhang, S.; Liu, G.; Yang, X.; Huang, Z.; Ye, X. Facilitation by a Spiny Shrub on a Rhizomatous Clonal Herbaceous in Thicketization-Grassland in Northern China: Increased Soil Resources or Shelter from Herbivores. Front. Plant Sci. 2017, 8, 809. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Esteban, A.; Delibes, M.; Fedriani, J.M. Unpaved Road Verges as Hotspots of Fleshy-Fruited Shrub Recruitment and Establishment. Biol. Conserv. 2013, 167, 50–56. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Maitra, P.; Babalola, B.J.; Zhao, Y. Temporal Variations in Root-Associated Fungal Communities of Potaninia Mongolica, an Endangered Relict Shrub Species in the Semi-Arid Desert of Northwest China. Front. Plant Sci. 2022, 13, 975369. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Qi, D.; Liu, X.; Kong, X.; Gao, Y.; Zhou, Z.; Wu, Q. Proteomic Analysis of Symbiotic Proteins of Glomus mosseae and Amorpha fruticosa. Sci. Rep. 2015, 5, 18031. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, M.A.; Calvo-Magro, E.; Valentine, A. Benefits of the Symbiotic Association of Shrubby Legumes for the Rehabilitation of Degraded Soils under Mediterranean Climatic Conditions. Land. Degrad. Dev. 2016, 27, 395–405. [Google Scholar] [CrossRef]
- Camara, A.S. Do Woody Plants Facilitate Herbaceous Plants in Dryland New Zealand? AJEE 2022, 18, 1–13. [Google Scholar] [CrossRef]
- Song, F.; Kong, X.; Li, J.; Chang, W. Screening the Related Genes in the AM Fungi and Amorpha fruticosa Symbiosis with the Suppression Subtractive Hybridization Technique. Sci. Silvae Sin. 2014, 50, 64–74. [Google Scholar] [CrossRef]
- Gorzelak, M.A.; Hambleton, S.; Massicotte, H.B. Community Structure of Ericoid Mycorrhizas and Root-Associated Fungi of Vaccinium membranaceum across an Elevation Gradient in the Canadian Rocky Mountains. Fungal Ecol. 2012, 5, 36–45. [Google Scholar] [CrossRef]
- Hamim, A.; Boukeskasse, A.; Ouhdouch, Y.; Farrouki, A.; Barrijal, S.; Miché, L.; Mrabet, R.; Duponnois, R.; Hafidi, M. Phosphate Solubilizing and PGR Activities of Ericaceous Shrubs Microorganisms Isolated from Mediterranean Forest Soil. Biocatal. Agric. Biotechnol. 2019, 19, 101128. [Google Scholar] [CrossRef]
- Ehlers, B.K.; Charpentier, A.; Grøndahl, E. An Allelopathic Plant Facilitates Species Richness in the Mediterranean Garrigue. J. Ecol. 2014, 102, 176–185. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Zhang, Z.; Liu, Y.; Cui, Z.; Leite, P.A.M.; Shi, J.; Wang, Y.; Wu, G.-L. Shrub Encroachment Enhances the Infiltration Capacity of Alpine Meadows by Changing the Community Composition and Soil Conditions. Catena 2022, 213, 106222. [Google Scholar] [CrossRef]
- Connell, R.K.; O’Connor, R.C.; Nippert, J.B.; Blair, J.M. Spatial Variation in Soil Microbial Processes as a Result of Woody Encroachment Depends on Shrub Size in Tallgrass Prairie. Plant Soil. 2021, 460, 359–373. [Google Scholar] [CrossRef]
- Ruttan, A.; Lortie, C.J.; Haas, S.M. Shrubs as Magnets for Pollination: A Test of Facilitation and Reciprocity in a Shrub-Annual Facilitation System. Curr. Res. Insect Sci. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Holzapfel, C.; Mahall, B.E. Bidirectional Facilitation and Interference Between Shrubs and Annuals in the Mojave Desert. Ecology 1999, 80, 1747–1761. [Google Scholar] [CrossRef]
- Holden, E.M.; Cahill Jr, J.F. Plant Trait Dissimilarity Increases Competitive Interactions among Co-Occurring Plants. Funct. Ecol. 2024, 38, 1464–1474. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, A.; Yang, Y.; Ma, Q.; Li, X.; Zhao, C. Long-Term Effects of Xerophytic Shrub Haloxylon ammodendron Plantations on Soil Properties and Vegetation Dynamics in Northwest China. PLoS ONE 2016, 11, e0168000. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shen, X.; Huang, Q.; Sun, F.; Zhou, J.; Ma, X.; Ran, Z.; Chen, Y.; Li, Z.; Yan, Y.; et al. Resource Islands of Salix Cupularis Facilitating Seedling Emergence of the Companion Herbs in the Restoration Process of Desertified Alpine Meadow, the Tibetan Plateau. J. Environ. Manag. 2021, 289, 112434. [Google Scholar] [CrossRef]
- Ding, W.; Wang, Y.-B.; Xiang, G.-H.; Chi, Y.-G.; Lu, S.-B.; Zheng, S.-X. Effects of Caragana microphylla Encroachment on Community Structure and Ecosystem Function of a Typical Steppe. Chin. J. Plant Ecol. 2020, 44, 33–43. [Google Scholar] [CrossRef]
- Maestre, F.T.; Bautista, S.; Cortina, J.; Bellot, J. Potential for Using Facilitation by Grasses to Establish Shrubs on a Semiarid Degraded Steppe. Ecol. Appl. 2001, 11, 1641–1655. [Google Scholar] [CrossRef]
- Knapp, A.K.; Briggs, J.M.; Collins, S.L.; Archer, S.R.; Bret-Harte, M.S.; Ewers, B.E.; Peters, D.P.; Young, D.R.; Shaver, G.R.; Pendall, E.; et al. Shrub Encroachment in North American Grasslands: Shifts in Growth Form Dominance Rapidly Alters Control of Ecosystem Carbon Inputs. Glob. Chang. Biol. 2008, 14, 615–623. [Google Scholar] [CrossRef]
- Báez, S.; Collins, S.L. Shrub Invasion Decreases Diversity and Alters Community Stability in Northern Chihuahuan Desert Plant Communities. PLoS ONE 2008, 3, e2332. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.-F.; Cui, Z.; Huang, Z.; Liu, Y.; Leite, P.A.M.; Zhao, J.; Wu, G.-L. Shrub Encroachment Impaired the Structure and Functioning of Alpine Meadow Communities on the Qinghai–Tibetan Plateau. Land. Degrad. Dev. 2022, 33, 2454–2463. [Google Scholar] [CrossRef]
- Xiong, X.G.; Han, X.-G.; Bao, Y.J. Discussion on the Research into Sandy Desertification, Accompanying by Thicketization of Semiarid Grasslands in Inner Mongolia, China. Acta Prataculturae Sin. 2005, 14, 1–5. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, H.; Yan, H.; Bai, X.; Zhang, Y.; Guo, Y.; Zhou, J.; Gao, S. A Synthetic Review of Feedbacks and Drivers of Shrub–Grass Interaction in the Process of Grassland Shrub Encroachment. Plants 2025, 14, 605. https://doi.org/10.3390/plants14040605
Hou H, Yan H, Bai X, Zhang Y, Guo Y, Zhou J, Gao S. A Synthetic Review of Feedbacks and Drivers of Shrub–Grass Interaction in the Process of Grassland Shrub Encroachment. Plants. 2025; 14(4):605. https://doi.org/10.3390/plants14040605
Chicago/Turabian StyleHou, Huiyang, Haoran Yan, Xue Bai, Yuzhen Zhang, Yanjun Guo, Jianwei Zhou, and Shaobo Gao. 2025. "A Synthetic Review of Feedbacks and Drivers of Shrub–Grass Interaction in the Process of Grassland Shrub Encroachment" Plants 14, no. 4: 605. https://doi.org/10.3390/plants14040605
APA StyleHou, H., Yan, H., Bai, X., Zhang, Y., Guo, Y., Zhou, J., & Gao, S. (2025). A Synthetic Review of Feedbacks and Drivers of Shrub–Grass Interaction in the Process of Grassland Shrub Encroachment. Plants, 14(4), 605. https://doi.org/10.3390/plants14040605







