Adaptive Benefits of Antioxidant and Hormone Fluctuations in Wedelia trilobata Under Simulated Salt Stress with Nutrient Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Method and Design
3. Data Collection
3.1. Phenotypic Growth Indicators
3.2. Biochemical Indicators
3.3. Endogenous Hormones
4. Data Analysis
5. Results and Analysis
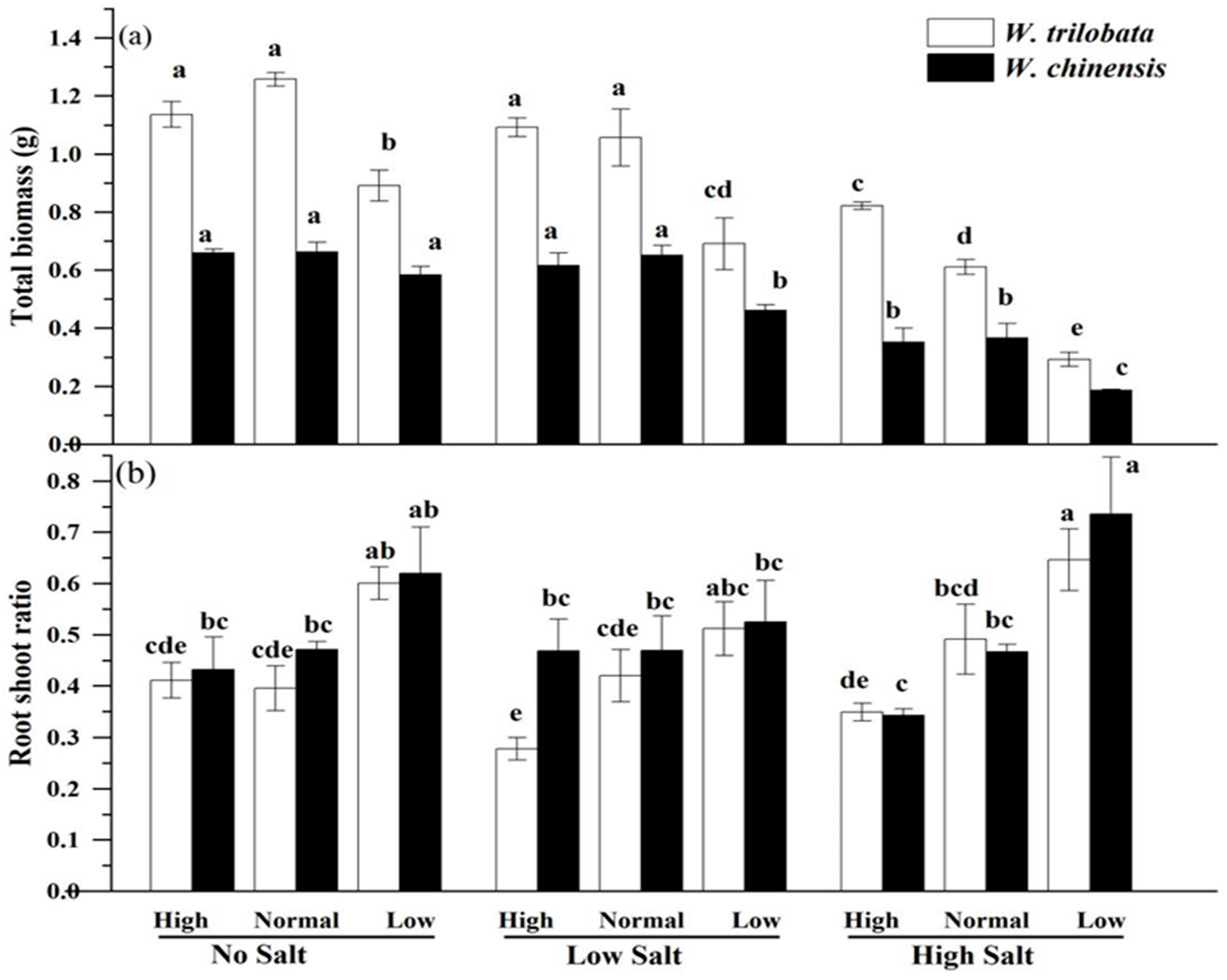
5.1. Effects of Different Salinity Level on the Growth of Wedelia trilobata and W. chinensis Under Different Nutrient Conditions
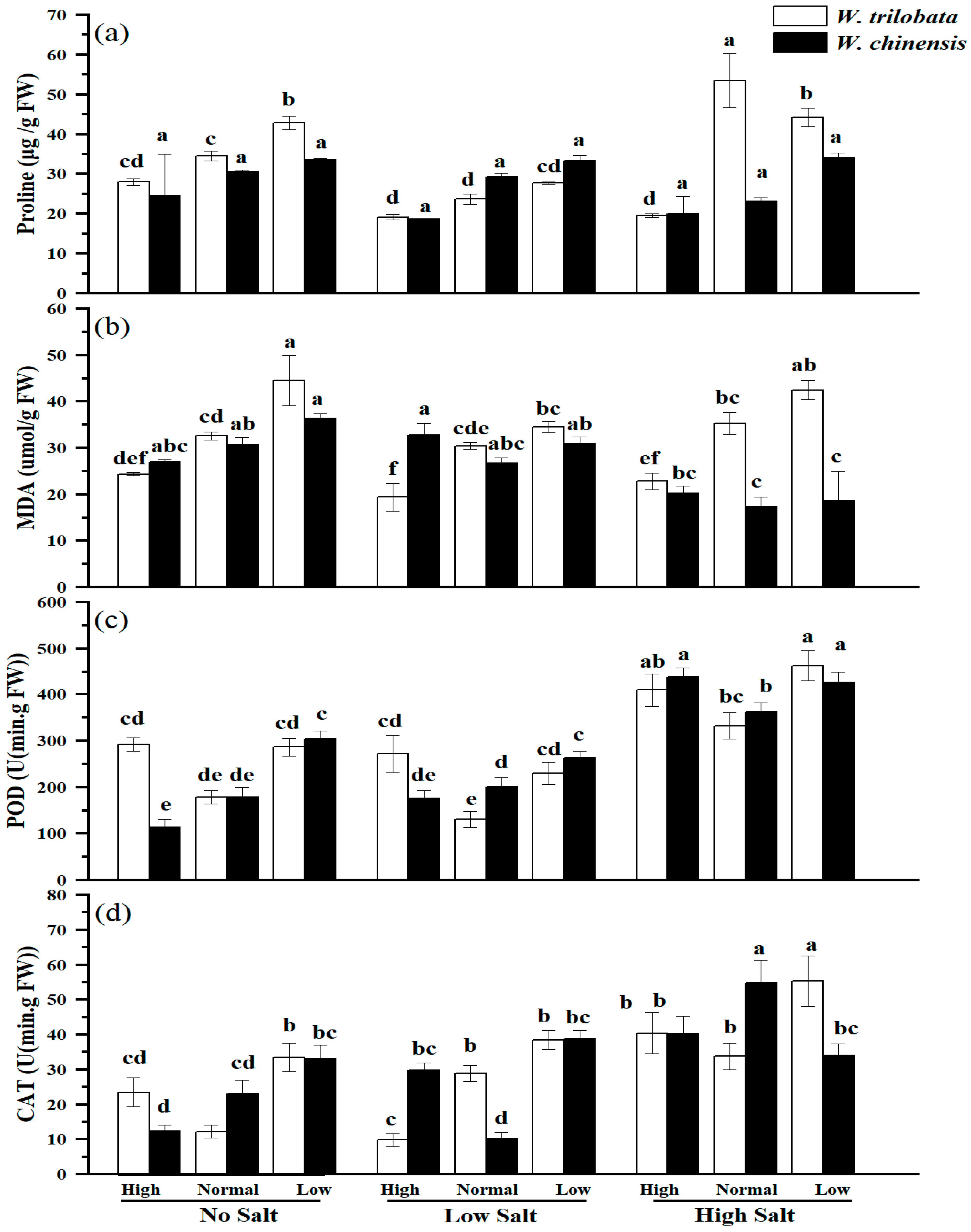
5.2. Effects of Different Salinity Levels on the Antioxidant Enzyme Activities of Wedelia trilobata and W. chinensis Under Different Nutrient Conditions
5.3. Effects of Simulated Salinity Stress on Endogenous Hormone Ratio of Wedelia trilobata Under Different Nutrient Conditions
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Umair Hassan, M.; Chattha, M.U.; Khan, I.; Khan, T.A.; Nawaz, M.; Tang, H.; Noor, M.A.; Asseri, T.A.Y.; Hashem, M.; Guoqin, H. Zinc seed priming alleviates salinity stress and enhances sorghum growth by regulating antioxidant activities, nutrient homeostasis, and osmolyte synthesis. Agronomy 2024, 14, 1815. [Google Scholar] [CrossRef]
- Manjunath, M.S.; Koshariya, A.K.; Sharma, N.; Rajput, A.; Pandey, S.K.; Singh, S.; Kumar, R.; Singh, B.V. Exploring the Use of Aromatic Compounds in Crop Growth and Protection. Int. J. Plant Soil Sci. 2023, 35, 78–89. [Google Scholar] [CrossRef]
- Bouzroud, S.; Henkrar, F.; Fahr, M.; Smouni, A. Salt stress responses and alleviation strategies in legumes: A review of the current knowledge. 3 Biotech 2023, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Bailey-Serres, J.; Brodersen, C.; Buckley, T.N.; Conti, L.; Christmann, A.; Dinneny, J.R.; Grill, E.; Hayes, S.; Heckman, R.W.; et al. Burning questions for a warming and changing world: 15 unknowns in plant abiotic stress. Plant Cell 2023, 35, 67–108. [Google Scholar] [CrossRef]
- Kumari, A.; Ahlawat, P.; Kiran; Rani, B.; Goyal, A.; Pooja; Pazhany, A.S.; Kumar, A.; Devi, S.; Kumari, N. An Overview of Phytohormones Mediated Drought and Salinity Tolerance in Plants. In Salinity and Drought Tolerance in Plants: Physiological Perspectives; Springer: Singapore, 2023; pp. 387–417. [Google Scholar]
- Soto, I.; Balzani, P.; Carneiro, L.; Cuthbert, R.N.; Macedo, R.; Serhan Tarkan, A.; Ahmed, D.A.; Bang, A.; Bacela-Spychalska, K.; Bailey, S.A.; et al. Taming the terminological tempest in invasion science. Biol. Rev. 2024, 99, 1357–1390. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Zhou, L.; Yang, K.; Fang, J.; Biere, A.; Callaway, R.M.; Wu, M.; Yu, H.; Shi, Y.; Ding, J. Rapid evolutionary trade-offs between resistance to herbivory and tolerance to abiotic stress in an invasive plant. Ecol. Lett. 2023, 26, 942–954. [Google Scholar] [CrossRef]
- Huang, P.; Hameed, R.; Abbas, M.; Balooch, S.; Alharthi, B.; Du, Y.; Abbas, A.; Younas, A.; Du, D. Integrated omic techniques and their genomic features for invasive weeds. Funct. Integr. Genom. 2023, 23, 44. [Google Scholar] [CrossRef]
- Pan, L.; He, F.; Liang, Q.; Bo, Y.; Lin, X.; Javed, Q.; Ullah, M.S.; Sun, J. Allelopathic effects of caffeic acid and its derivatives on seed germination and growth competitiveness of native plants (Lantana indica) and invasive plants (Solidago canadensis). Agriculture 2023, 13, 1719. [Google Scholar] [CrossRef]
- Shen, C.; Chen, P.; Zhang, K.; He, M.; Wan, J.; Wang, Y.; Tao, Z.; Huang, W.; Siemann, E. Dynamics and mechanisms of secondary invasion following biological control of an invasive plant. New Phytol. 2023, 238, 2594–2606. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.C.; Basso Júnior, I.J.; Navarro, V.L.; Silva, W.C.; Silverio, J.M.; Scalon, S.D.P.Q. Silicon Alleviates Damages on Photosynthetic Apparatus and Increases Resilience in Young Inga vera Plants Exposed to Water Deficit. J. Soil. Sci. Plant Nutr. 2023, 23, 3219–3231. [Google Scholar] [CrossRef]
- Jianfan, S.; Qaiser, J.; Yizhou, D.; Ahmad, A.; Adeel, A.; Babar, I.; Yuhan, H.; Yan, X.; Daolin, D. Invasive Alternanthera philoxeroides has performance advantages over natives under flooding with high amount of nitrogen. Aquat. Ecol. 2022, 56, 891–903. [Google Scholar]
- Mariyam, S.; Bhardwaj, R.; Khan, N.A.; Sahi, S.V.; Seth, C.S. Review on nitric oxide at the forefront of rapid systemic signaling in mitigation of salinity stress in plants: Crosstalk with calcium and hydrogen peroxide. Plant Sci. 2023, 336, 111835. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J. Phytochemistry of Australia’s Tropical Rainforest: Medicinal Potential of Ancient Plants; Csiro Publishing: Clayton, Australia, 2021. [Google Scholar]
- Chaudhry, U.K.; Gökçe, Z.N.Ö.; Gökçe, A.F. Drought and salt stress effects on biochemical changes and gene expression of photosystem II and catalase genes in selected onion cultivars. Biologia 2021, 76, 3107–3121. [Google Scholar] [CrossRef]
- Ma, J.; Islam, F.; Ayyaz, A.; Fang, R.; Hannan, F.; Farooq, M.A.; Ali, B.; Huang, Q.; Sun, R.; Zhou, W. Wood vinegar induces salinity tolerance by alleviating oxidative damages and protecting photosystem ii in rapeseed cultivars. Ind. Crop. Prod. 2022, 189, 115763. [Google Scholar] [CrossRef]
- Moraes, G.; De Almeida, L.C. Nutrition and functional aspects of digestion in fish. In Biology and Physiology of Freshwater Neotropical Fish; Academic Press: Cambridge, MA, USA, 2020; pp. 251–271. [Google Scholar]
- Zhang, H.; Goncalves, P.; Copeland, E.; Qi, S.; Dai, Z.; Li, G.; Wang, C.; Du, D.; Thomas, T. Invasion by the weed conyza canadensis alters soil nutrient supply and shifts microbiota structure. Soil Biol. Biochem. 2020, 143, 107739. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, H.; Kaur, H.; Srivastava, S. The beneficial roles of trace and ultratrace elements in plants. Plant Growth Regul. 2023, 100, 219–236. [Google Scholar] [CrossRef]
- Jun, S.E.; Shim, J.S.; Park, H.J. Beyond npk: Mineral nutrient-mediated modulation in orchestrating flowering time. Plants 2023, 12, 3299. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Parets, S.; Fernández-Díaz, J.; Beteta-Göbel, R.; Rodríguez-Lorca, R.; Román, R.; Lladó, V.; Rosselló, C.A.; Fernández-García, P.; Escribá, P.V. Lipids in pathophysiology and development of the membrane lipid therapy: New bioactive lipids. Membranes 2021, 11, 919. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Fan, Y.; Li, X.; Li, Y.; Mao, H.; Zuo, Z.; Zou, Z. Effects of daily light integral on tomato (Solanum lycopersicon L.) Grafting and quality in a controlled environment. Int. J. Agric. Biol. Eng. 2022, 15, 44–50. [Google Scholar] [CrossRef]
- Qin, H.; Pandey, B.K.; Li, Y.; Huang, G.; Wang, J.; Quan, R.; Zhou, J.; Zhou, Y.; Miao, Y.; Zhang, D.; et al. Orchestration of ethylene and gibberellin signals determines primary root elongation in rice. Plant Cell 2022, 34, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Tiwari, S.; Kumawat, K.C.; Cardinale, M. Nano-biofertilizers as bio-emerging strategies for sustainable agriculture development: Potentiality and their limitations. Sci. Total Environ. 2023, 860, 160476. [Google Scholar] [CrossRef]
- Kaniganti, S.; Bhattacharya, J.; Petla, B.P.; Reddy, P.S. Strigolactone, a neglected plant hormone, with a great potential for crop improvement: Crosstalk with other plant hormones. Environ. Exp. Bot. 2022, 204, 105072. [Google Scholar] [CrossRef]
- Shahbani, Z.; Kosh-Khui, M.; Salehi, H.; Kafi, M.; Kamgar Haghighi, A.A.; Eshghi, S.; Omidi, M. Hormonal and Physiological Changes in Miniature Roses (Rosa chinensis Jacq. var. minima Rehd.) Exposed to Water Deficit and Salinity Stress Conditions. Gesunde Pflanz. 2023, 75, 1781–1797. [Google Scholar] [CrossRef]
- Khan, I.U.; Zhang, Y.; Shi, X.; Qi, S.; Zhang, H.; Du, D.; Gul, F.; Wang, J.; Naz, M.; Shah, S.W.A.; et al. Dose dependent effect of nitrogen on the phyto extractability of Cd in metal contaminated soil using Wedelia trilobata. Ecotoxicol. Environ. Saf. 2023, 264, 115419. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, H.; Xu, Z.; Yang, H.; Du, Y.; Chen, S.; Abbas, A.; Yin, H.; Sun, P.; Du, D. The responses of invasive Wedelia trilobata and native Wedelia chinensis to levofloxacin hydrochloride: Implication for biological invasion. Pol. J. Environ. Stud. 2023, 32, 3605–3615. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Manoharan, B.; Dhandapani, V.; Jegadeesan, S.; Rutherford, S.; Wan, J.S.H.; Huang, P.; Dai, Z.; Du, D. Pathogen resistance in Sphagneticola trilobata (Singapore daisy): Molecular associations and differentially expressed genes in response to disease from a widespread fungus. Genetica 2022, 150, 13–26. [Google Scholar] [CrossRef]
- Sun, S.W.; Lin, Y.C.; Weng, Y.M.; Chen, M.J. Efficiency improvements on ninhydrin method for amino acid quantification. J. Food Compos. Anal. 2006, 19, 112–117. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Morales Hernandez, C.E.; Padilla Guerrero, I.E.; Gonzalez Hernandez, G.A.; Salazar Solis, E.; Torres Guzman, J.C. Catalase overexpression reduces the germination time and increases the pathogenicity of the fungus Metarhizium anisopliae. Appl. Microbiol. Biotechnol. 2010, 87, 1033–1044. [Google Scholar] [CrossRef]
- Konstantinou, G.N. Enzyme-linked immunosorbent assay (ELISA). In Food Allergens: Methods and Protocols; Humana Press: New York, NY, USA, 2017; pp. 79–94. [Google Scholar]
- Singh, A.; Mehta, S.; Yadav, S.; Nagar, G.; Ghosh, R.; Roy, A.; Chakraborty, A.; Singh, I.K. How to cope with the challenges of environmental stresses in the era of global climate change: An update on ROS stave off in plants. Int. J. Mol. Sci. 2022, 23, 1995. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.; Pradhan, S.; Bhattacharjee, A.; Dubey, S.; Sharma, S. Management of abiotic stresses by microbiome-based engineering of the rhizosphere. J. Appl. Microbiol. 2022, 133, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.R.; Frank, D.A.; Fridley, J.D. Contrasting mycorrhizal growth responses in native and invasive woody species are associated with distinct root trait syndromes. Funct. Ecol. 2023, 37, 2312–2324. [Google Scholar] [CrossRef]
- Huang, P.; He, L.; Abbas, A.; Hussain, S.; Hussain, S.; Du, D.; Hafeez, M.-B.; Balooch, S.; Zahra, N.; Ren, X.; et al. Seed priming with sorghum water extract improves the performance of camelina (Camelina sativa (L.) Crantz.) Under Salt Stress. Plants 2021, 10, 749. [Google Scholar] [CrossRef]
- Sun, J.; Liu, M.; Tang, K.; Tang, E.; Cong, J.; Lu, X.; Liu, Z.; Feng, Y. Advantages of growth and competitive ability of the invasive plant Solanum rostratum over two co-occurring natives and the effects of nitrogen levels and forms. Front. Plant Sci. 2023, 14, 1169317. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, V.; Sharma, J.; Saini, S.; Sharma, P.; Kumar, S.; Sinhmar, Y.; Kumar, D.; Sharma, A. Silicon supplementation alleviates the salinity stress in wheat plants by enhancing the plant water status, photosynthetic pigments, proline content and antioxidant enzyme activities. Plants 2022, 11, 2525. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, Z.; Su, Y.; Wang, T. Associations between population epigenetic differentiation and environmental factors in the exotic weed mile-a-minute (Mikania micrantha). Weed Sci. 2021, 69, 307–332. [Google Scholar] [CrossRef]
- Lekberg, Y.; Arnillas, C.A.; Borer, E.T.; Bullington, L.S.; Fierer, N.; Kennedy, P.G.; Leff, J.W.; Luis, A.D.; Seabloom, E.W.; Henning, J.A. Nitrogen and phosphorus fertilization consistently favor pathogenic over mutualistic fungi in grassland soils. Nat. Commun. 2021, 12, 3484. [Google Scholar] [CrossRef]
- Guo, Z.; Miao, W.; Lyu, Y.; Wang, X. Soil fungi lead to stronger ‘diminishing returns’ in fine-root length versus mass allometry towards earlier successional tropical forests. Funct. Ecol. 2024, 38, 2406–2420. [Google Scholar] [CrossRef]
- Zhang, A.; Yin, J.; Zhang, Y.; Wang, R.; Zhou, X.; Guo, H. Plants alter their aboveground and belowground biomass allocation and affect community-level resistance in response to snow cover change in Central Asia, Northwest China. Sci. Total Environ. 2023, 902, 166059. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef] [PubMed]
- Young, S.L.; Anderson, J.V.; Baerson, S.R.; Bajsa-Hirschel, J.; Blumenthal, D.M.; Boyd, C.S.; Boyette, C.D.; Brennan, E.B.; Cantrell, C.L.; Chao, W.S.; et al. Agricultural Research Service Weed Science Research: Past, Present, and Future. Weed Sci. 2023, 71, 312–327. [Google Scholar] [CrossRef]
- Bowen, J.L.; Spivak, A.C.; Bernhard, A.E.; Fulweiler, R.W.; Giblin, A.E. Salt marsh nitrogen cycling: Where land meets sea. Trends Microbiol. 2023, 32, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Bisht, N.; Mishra, S.K.; Chauhan, P.S. Bacillus amyloliquefaciens inoculation alters physiology of rice (Oryza sativa L. var. IR-36) through modulating carbohydrate metabolism to mitigate stress induced by nutrient starvation. Int. J. Biol. Macromol. 2020, 143, 937–951. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.; Li, S.; Hui, L.; Li, Y.; Chen, K.; Meng, T.; Yu, C.; Leng, F.; Ma, J. Comparative analysis of carbon and nitrogen metabolism, antioxidant indexes, polysaccharides and lobetyolin changes of different tissues from Codonopsis pilosula co-inoculated with Trichoderma. J. Plant Physiol. 2021, 267, 153546. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Naeem, M.; Ali, H.; Alabbosh, K.F.; Hussain, H.; Khan, I.; Siddiqui, S.A.; Khan, A.A.; Iqbal, B. From challenges to solutions: The impact of melatonin on abiotic stress synergies in horticultural plants via redox regulation and epigenetic signaling. Sci. Hortic-Amst. 2023, 321, 112369. [Google Scholar] [CrossRef]
- Tomar, R.S.; Kataria, S.; Jajoo, A. Behind the scene: Critical role of reactive oxygen species and reactive nitrogen species in salt stress tolerance. J. Agron. Crop Sci. 2021, 207, 577–588. [Google Scholar] [CrossRef]
- Fatokun, K.; Beckett, R.P.; Varghese, B.; Cloete, J.; Pammenter, N.W. Influence of cathodic water invigoration on the emergence and subsequent growth of controlled deteriorated pea and pumpkin seeds. Plants 2020, 9, 955. [Google Scholar] [CrossRef]
- Huang, P.; Xu, Z.; He, W.; Yang, H.; Li, B.; Ding, W.; Lei, Y.; Abbas, A.; Hameed, R.; Wang, C.; et al. The Cooperation Regulation of Antioxidative System and Hormone Contents on Physiological Responses of Wedelia trilobata and Wedelia chinensis under Simulated Drought Environment. Plants 2024, 13, 472. [Google Scholar] [CrossRef]
- Hossain, A.; Pamanick, B.; Venugopalan, V.K.; Ibrahimova, U.; Rahman, M.A.; Siyal, A.L.; Maitra, S.; Chatterjee, S.; Aftab, T. Emerging roles of plant growth regulators for plants adaptation to abiotic stress–induced oxidative stress. In Emerging Plant Growth Regulators in Agriculture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–72. [Google Scholar]
- Sahbeni, G.; Ngabire, M.; Musyimi, P.K.; Szekely, B. Challenges and opportunities in remote sensing for soil salinization mapping and monitoring: A review. Remote Sens. 2023, 15, 2540. [Google Scholar] [CrossRef]
- Geng, G.; Li, R.; Stevanato, P.; Lv, C.; Lu, Z.; Yu, L.; Wang, Y. Physiological and transcriptome analysis of sugar beet reveals different mechanisms of response to neutral salt and alkaline salt stresses. Front. Plant Sci. 2020, 11, 571864. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Gong, L.; Nie, Z.; Li, F.; Ahammed, G.J.; Fang, X. ABA-induced stomatal movements in vascular plants during dehydration and rehydration. Environ. Exp. Bot. 2021, 186, 104436. [Google Scholar] [CrossRef]


| Wt Parameters | Salt | Nutrition | Salt × Nutrition | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| PH | 9.653 | 0.001 | 42.472 | <0.001 | 3.427 | 0.03 |
| LN | 0.419 | 0.664 | 32.012 | <0.001 | 1.882 | 0.157 |
| NN | 2.188 | 0.141 | 10.256 | 0.001 | 0.251 | 0.905 |
| RL | 15.22 | <0.001 | 2.436 | 0.116 | 1.209 | 0.341 |
| TB | 76.457 | <0.001 | 49.404 | <0.001 | 2.871 | 0.053 |
| RSR | 3.289 | 0.061 | 21.522 | <0.001 | 1.173 | 0.356 |
| Proline | 29.772 | <0.001 | 37.071 | <0.001 | 10.639 | <0.001 |
| MDA | 5.597 | 0.013 | 45.933 | <0.001 | 0.851 | 0.511 |
| POD | 41.974 | <0.001 | 17.317 | <0.001 | 0.821 | 0.529 |
| CAT | 20.634 | <0.001 | 18.081 | <0.001 | 3.917 | 0.019 |
| GA/ABA | 46.861 | <0.001 | 36.61 | <0.001 | 12.993 | <0.001 |
| GA/IAA | 7.473 | 0.004 | 43.59 | <0.001 | 0.219 | 0.924 |
| IAA/ABA | 48.753 | <0.001 | 25.182 | <0.001 | 12.206 | <0.001 |
| Wc Parameters | Salt | Nutrition | Salt × Nutrition | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| PH | 7.339 | 0.005 | 33.814 | <0.001 | 2.838 | 0.055 |
| LN | 4.41 | 0.028 | 6.304 | 0.008 | 1.675 | 0.199 |
| NN | 17.397 | <0.001 | 10.691 | 0.001 | 2.132 | 0.119 |
| RL | 22.25 | <0.001 | 7.416 | 0.004 | 1.879 | 0.158 |
| TB | 84.638 | <0.001 | 17.916 | <0.001 | 0.966 | 0.45 |
| RSR | 0.135 | 0.875 | 8.182 | 0.003 | 1.656 | 0.204 |
| Proline | 0.895 | 0.426 | 9.356 | 0.002 | 0.555 | 0.698 |
| MDA | 21.139 | <0.001 | 1.558 | 0.238 | 1.731 | 0.187 |
| POD | 114.331 | <0.001 | 20.27 | <0.001 | 7.878 | 0.001 |
| CAT | 24.65 | <0.001 | 3.56 | 0.05 | 13.604 | <0.001 |
| GA/ABA | 9.234 | 0.002 | 3.158 | 0.067 | 1.23 | 0.333 |
| GA/IAA | 32.582 | <0.001 | 9.064 | 0.002 | 14.803 | <0.001 |
| IAA/ABA | 17.049 | <0.001 | 0.451 | 0.644 | 10.295 | <0.001 |
| PH | LN | NN | RL | TB | RSR | Pro | MDA | POD | CAT | G/A | G/I | I/A | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH | 1 | 0.737 ** | 0.755 ** | 0.368 | 0.767 ** | −0.728 ** | −0.419 * | −0.584 ** | −0.288 | −0.686 ** | 0.680 ** | 0.555 ** | 0.700 ** |
| LN | 1 | 0.658 ** | 0.007 | 0.517 ** | −0.651 ** | −0.288 | −0.590 ** | −0.150 | −0.490 ** | 0.291 | 0.233 | 0.341 | |
| NN | 1 | 0.316 | 0.694 ** | −0.557 ** | −0.414 * | −0.594 ** | −0.144 | −0.553 ** | 0.504 ** | 0.419 * | 0.563 ** | ||
| RL | 1 | 0.610 ** | −0.296 | −0.355 | −0.265 | −0.460 * | −0.577 ** | 0.474 * | 0.306 | 0.536 ** | |||
| TB | 1 | −0.552 ** | −0.494 ** | −0.520 ** | −0.653 ** | −0.792 ** | 0.778 ** | 0.513 ** | 0.809 ** | ||||
| RSR | 1 | 0.601 ** | 0.779 ** | 0.327 | 0.626 ** | −0.501 ** | −0.504 ** | −0.532 ** | |||||
| Pro | 1 | 0.684 ** | 0.275 | 0.374 | −0.273 | −0.480 * | −0.323 | ||||||
| MDA | 1 | 0.075 | 0.496 ** | −0.387 * | −0.422 * | −0.467 * | |||||||
| POD | 1 | 0.547 ** | −0.450 * | −0.083 | −0.489 ** | ||||||||
| CAT | 1 | −0.762 ** | −0.374 | −0.801 ** | |||||||||
| G/A | 1 | 0.588 ** | 0.949 ** | ||||||||||
| G/I | 1 | 0.390 * | |||||||||||
| I/A | 1 |
| PH | LN | NN | RL | TB | RSR | Pro | MDA | POD | CAT | G/A | G/I | I/A | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH | 1 | 0.639 ** | 0.465 * | 0.400 * | 0.701 ** | −0.417 * | −0.415 * | 0.175 | −0.507 ** | −0.380 | 0.509 ** | 0.366 | 0.608 ** |
| LN | 1 | 0.694 ** | 0.362 | 0.674 ** | −0.389 * | −0.232 | 0.183 | −0.632 ** | −0.310 | 0.501 ** | 0.123 | 0.652 ** | |
| NN | 1 | 0.379 | 0.742 ** | −0.354 | −0.225 | 0.485 * | −0.800 ** | −0.440 * | 0.595 ** | 0.355 | 0.692 ** | ||
| RL | 1 | 0.672 ** | −0.29 | 0.127 | 0.321 | −0.684 ** | −0.482 * | 0.255 | −0.134 | 0.476 * | |||
| TB | 1 | −0.293 | −0.050 | 0.649 ** | −0.831 ** | −0.587 ** | 0.589 ** | 0.306 | 0.711 ** | ||||
| RSR | 1 | 0.437 * | 0.130 | 0.180 | 0.007 | −0.258 | −0.322 | −0.293 | |||||
| Pro | 1 | 0.141 | 0.097 | −0.099 | −0.384 * | −0.516 ** | −0.259 | ||||||
| MDA | 1 | −0.487 ** | −0.268 | 0.301 | 0.192 | 0.281 | |||||||
| POD | 1 | 0.674 ** | −0.702 ** | −0.253 | −0.829 ** | ||||||||
| CAT | 1 | −0.496 ** | −0.187 | −0.707 ** | |||||||||
| G/A | 1 | 0.753 ** | 0.901 ** | ||||||||||
| G/I | 1 | 0.484 * | |||||||||||
| I/A | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Li, B.; Huang, P.; Zhang, B.; Abbas, A.; Xu, Z.; Yin, H.; Du, D. Adaptive Benefits of Antioxidant and Hormone Fluctuations in Wedelia trilobata Under Simulated Salt Stress with Nutrient Conditions. Plants 2025, 14, 303. https://doi.org/10.3390/plants14030303
Yang H, Li B, Huang P, Zhang B, Abbas A, Xu Z, Yin H, Du D. Adaptive Benefits of Antioxidant and Hormone Fluctuations in Wedelia trilobata Under Simulated Salt Stress with Nutrient Conditions. Plants. 2025; 14(3):303. https://doi.org/10.3390/plants14030303
Chicago/Turabian StyleYang, Hong, Bin Li, Ping Huang, Bin Zhang, Adeel Abbas, Zhiwei Xu, Huilei Yin, and Daolin Du. 2025. "Adaptive Benefits of Antioxidant and Hormone Fluctuations in Wedelia trilobata Under Simulated Salt Stress with Nutrient Conditions" Plants 14, no. 3: 303. https://doi.org/10.3390/plants14030303
APA StyleYang, H., Li, B., Huang, P., Zhang, B., Abbas, A., Xu, Z., Yin, H., & Du, D. (2025). Adaptive Benefits of Antioxidant and Hormone Fluctuations in Wedelia trilobata Under Simulated Salt Stress with Nutrient Conditions. Plants, 14(3), 303. https://doi.org/10.3390/plants14030303






