Mite Infestation Induces a Moderate Oxidative Stress in Short-Term Soybean Exposure
Abstract
1. Introduction
2. Results
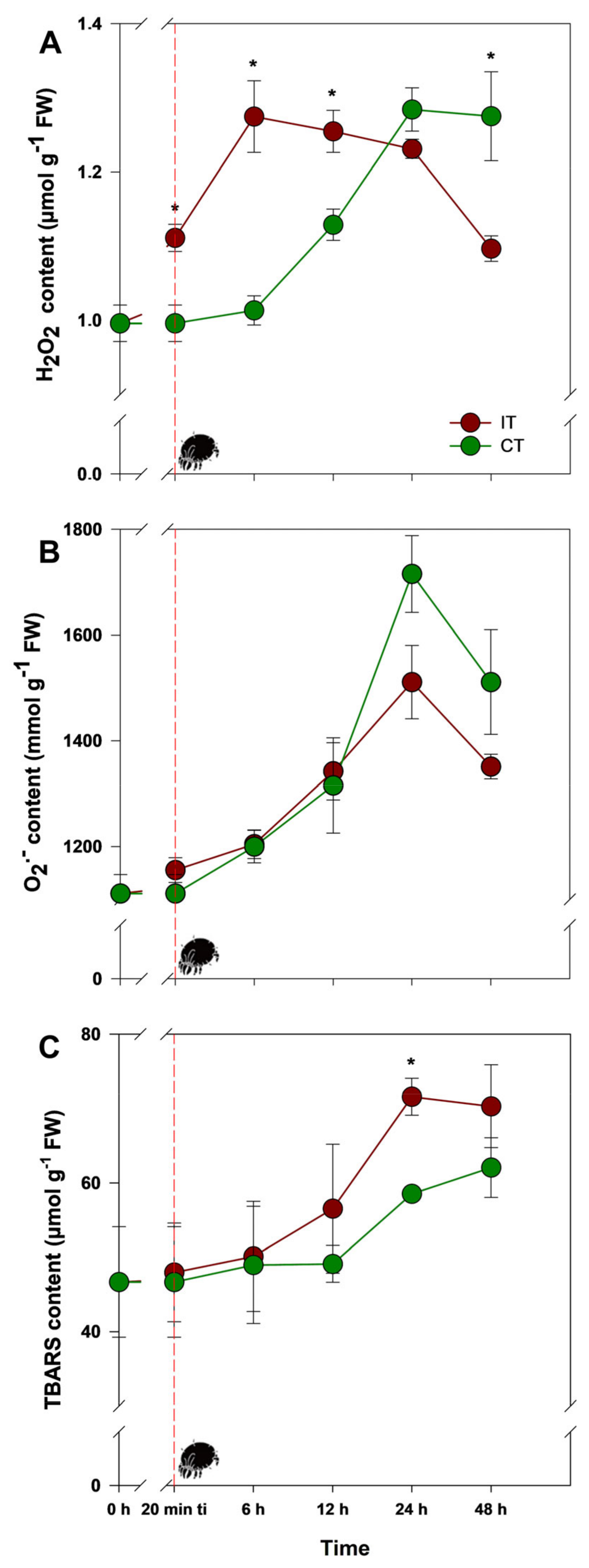
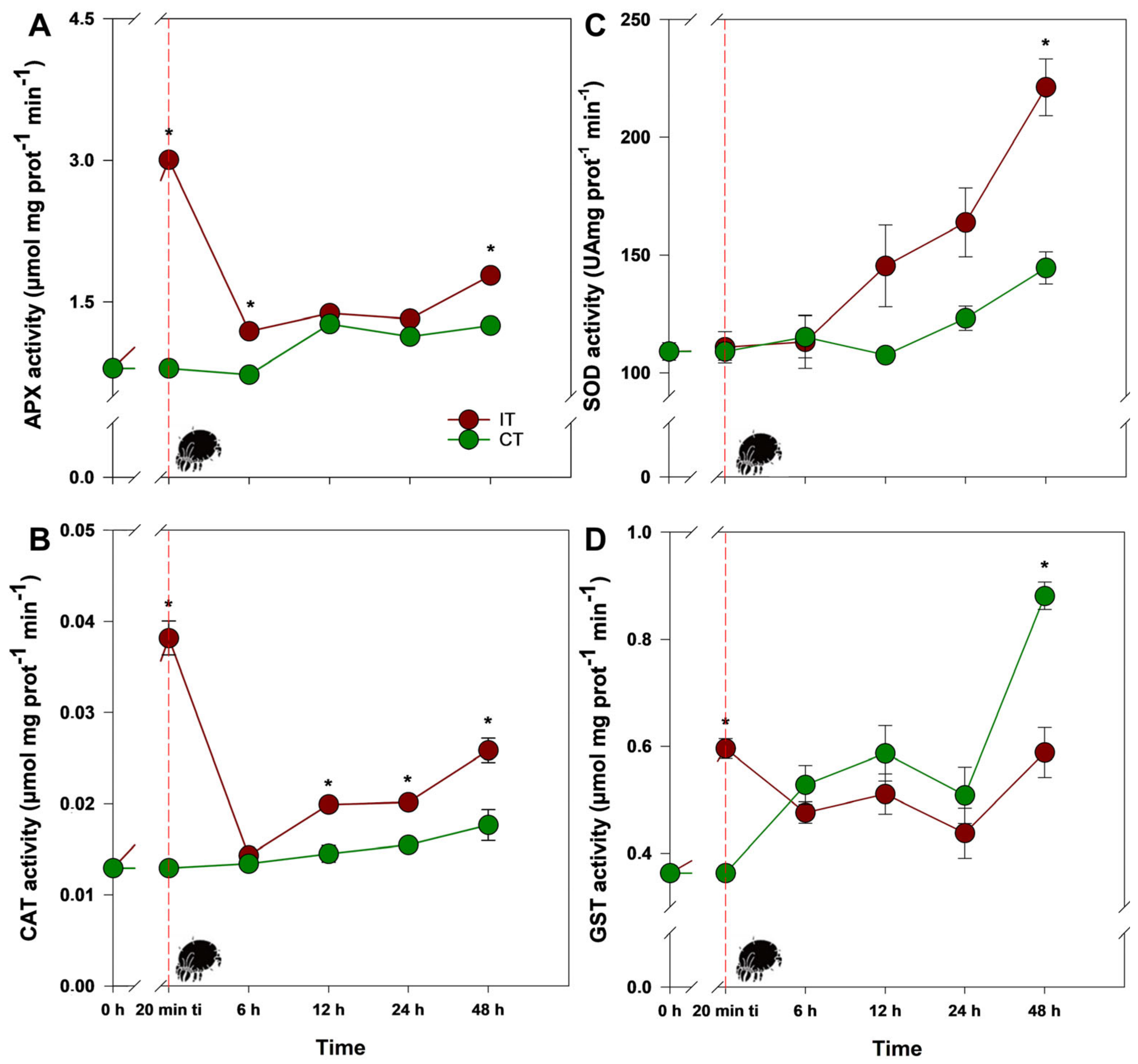
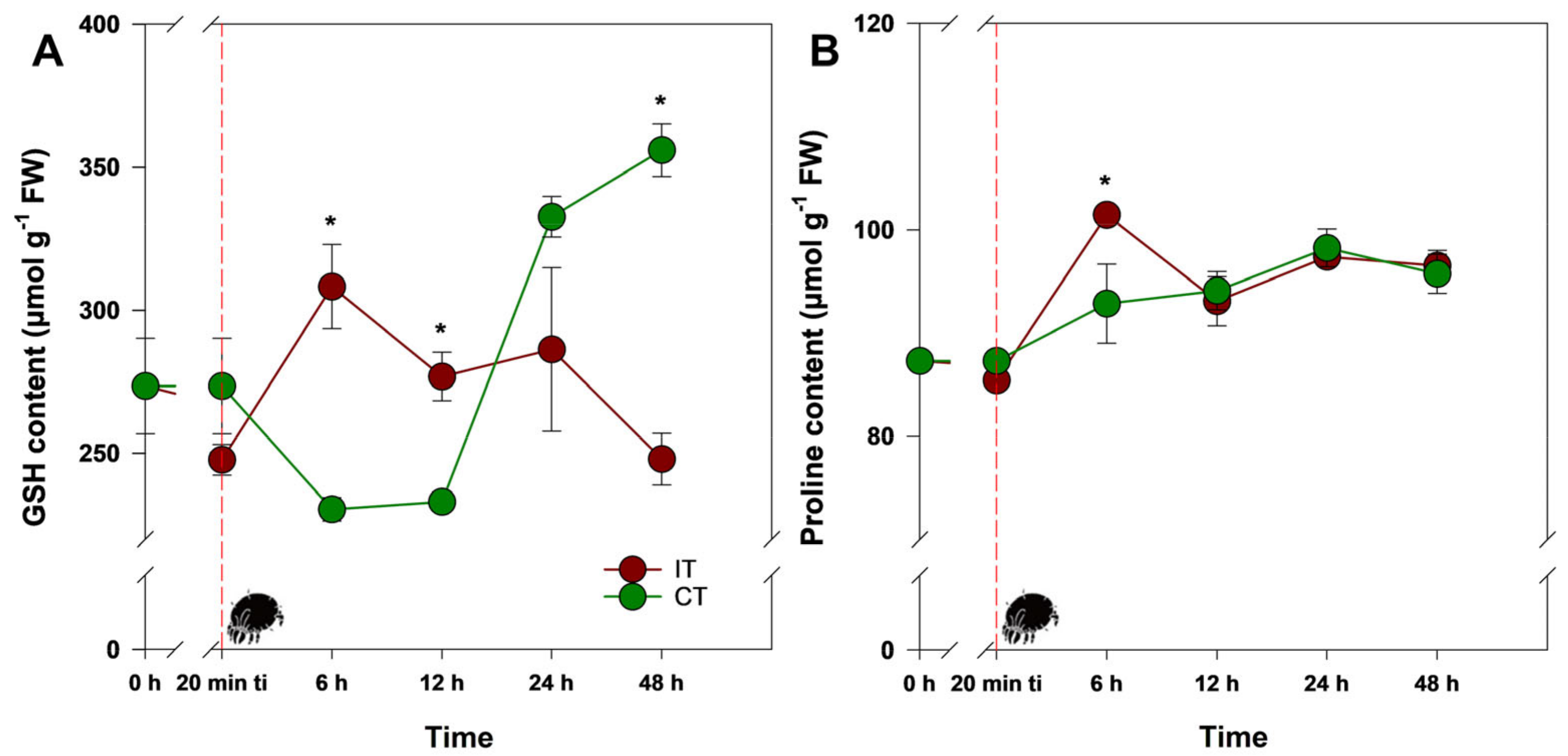
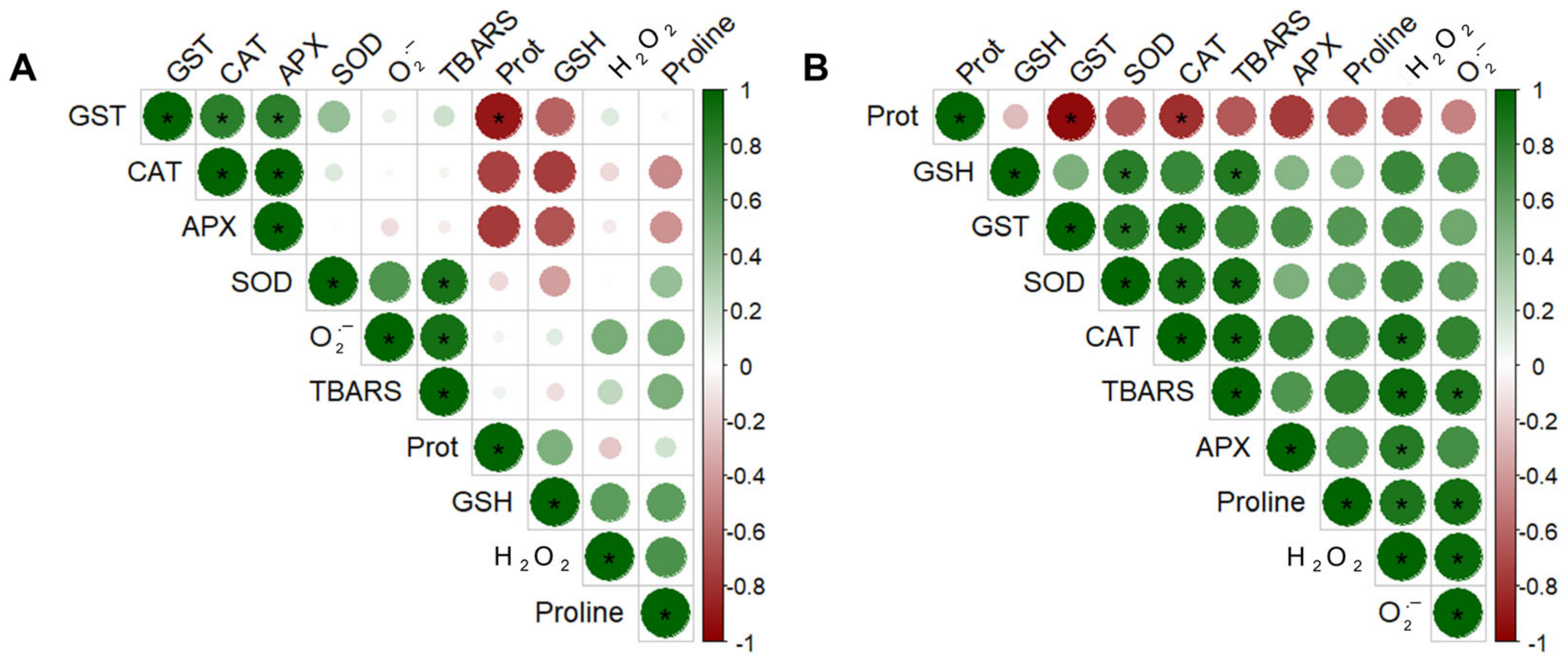
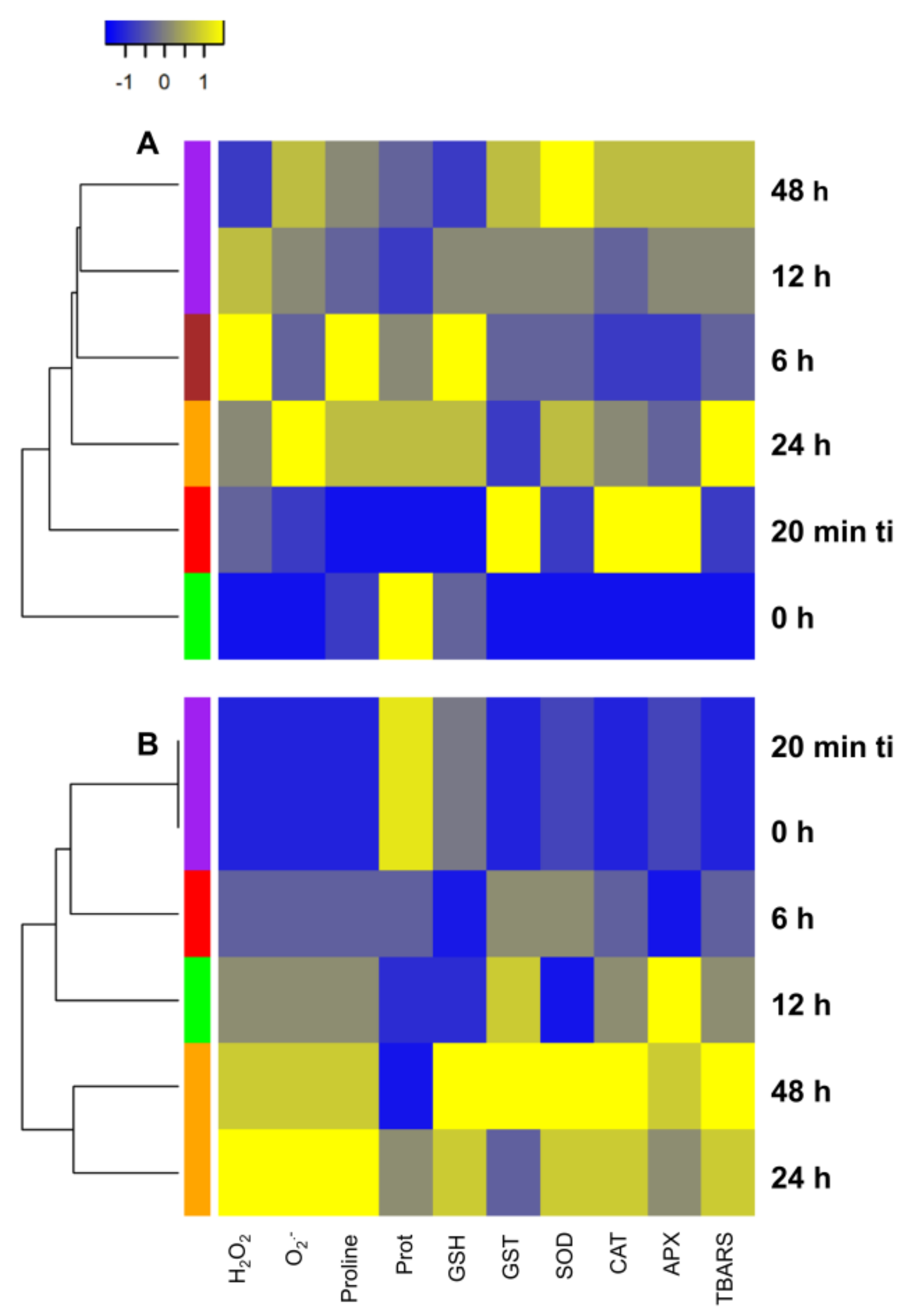
2.1. Tetranychus ludeni Triggers an Oxidative Burst in Soybean Plants at the Onset of Infestation
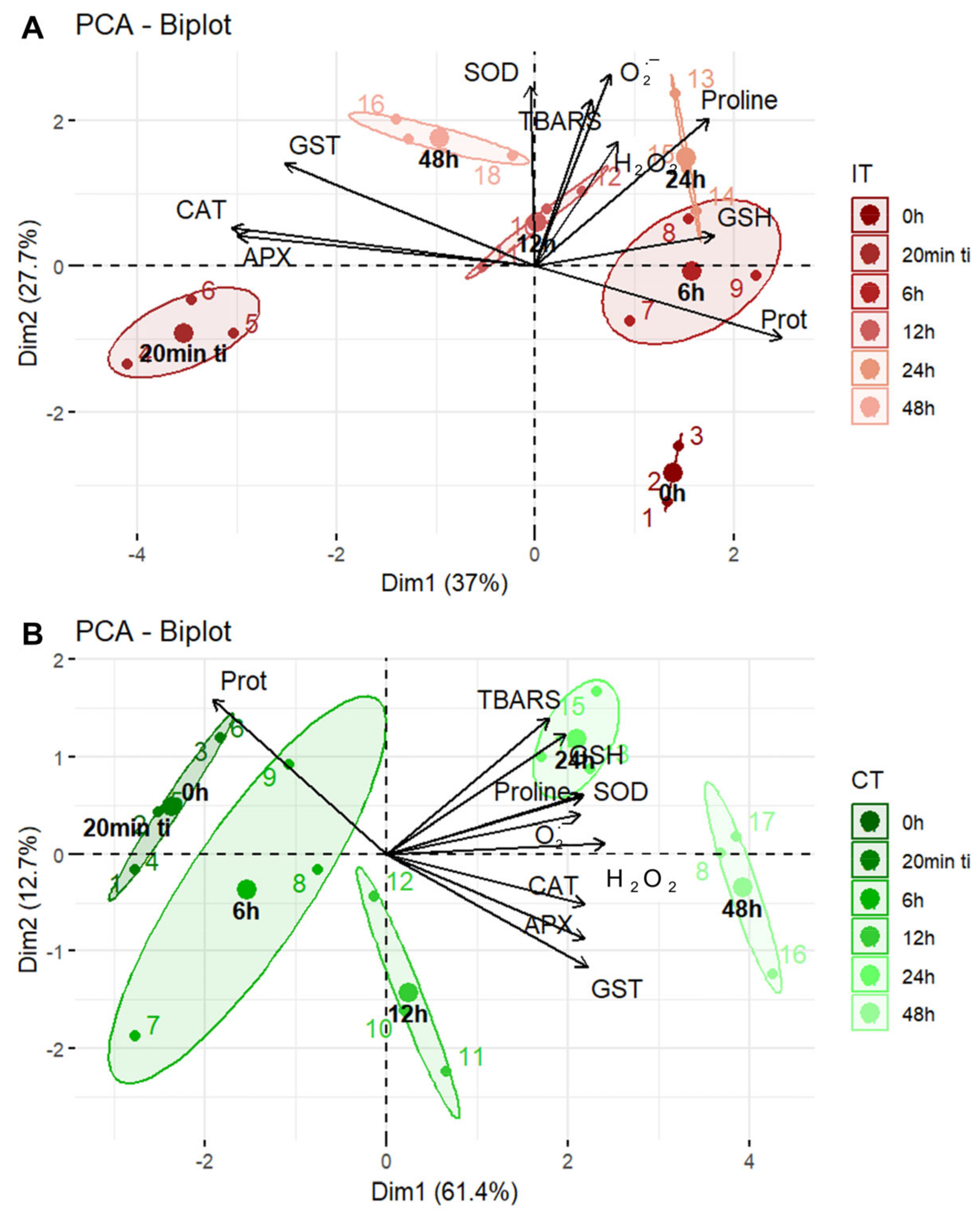
2.2. A General Perspective
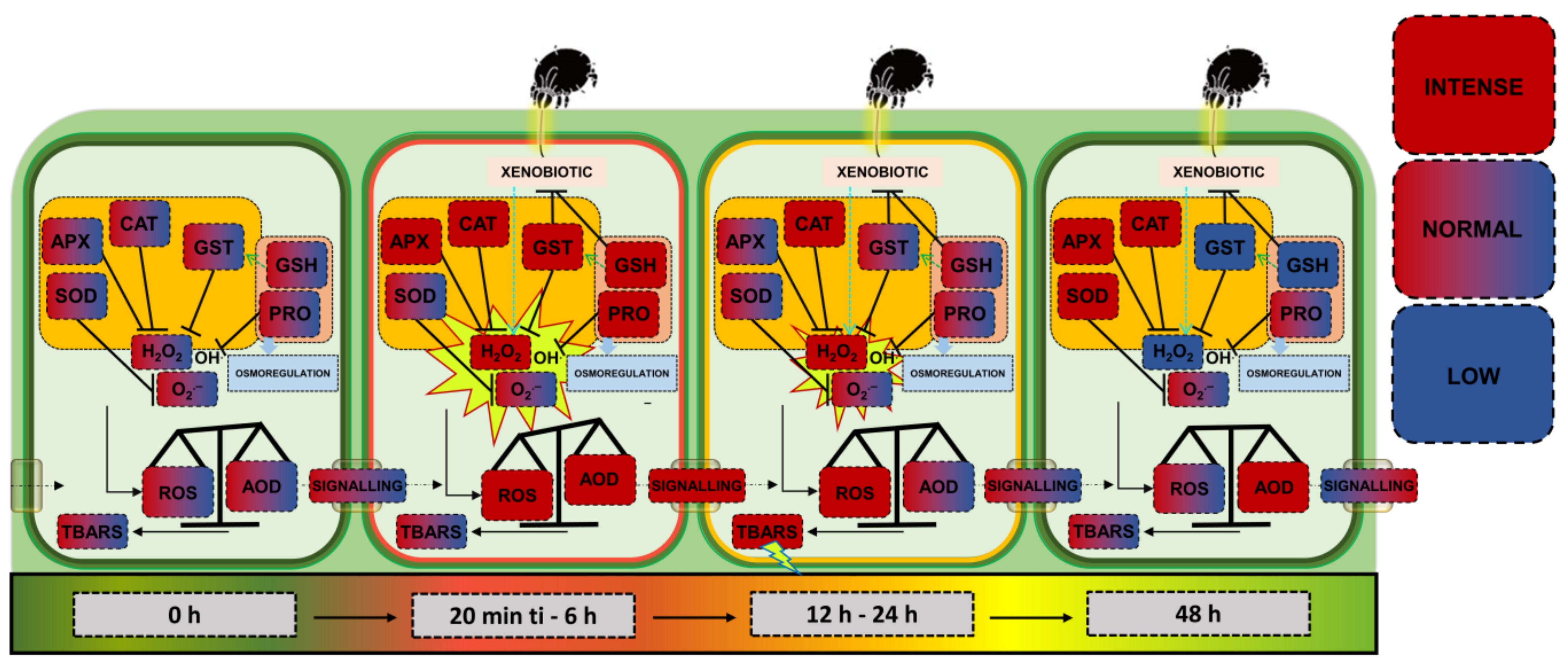
3. Discussion
3.1. Could Soybean Plants Tolerate Tetranychus ludeni Infestation?
3.2. Who Regulates Whom?
4. Materials and Methods
4.1. Stock Colonies of T. ludeni
4.2. Plant Growth and Treatment Conditions
4.3. Time-Course Experiment Design
4.4. Obtaining Leaf Extract
4.5. Levels of ROS and Lipid Peroxidation
4.6. Antioxidant Enzyme Analysis
4.7. Non-Enzymatic Antioxidant Analysis
4.8. Determination of Total Soluble Protein Concentration
4.9. Statistical Analysis
5. Conclusions and Perspectives
- This study demonstrates that soybean plants under infestation by phytophagous mites exhibit a moderate redox stress or a new redox state characterized as a non-significant stressful oxidative. This condition reflects the plants’ intrinsic physiological ability to withstand infestation without significant damage. This finding is extremely relevant in the current agricultural context, as it opens the possibility of reducing the use of pesticides in soybean management. Thus, producers can adopt strategies based on cultural practices to control these phytophagous mites, promoting a more sustainable and cost-effective approach to agricultural production.
- Additionally, it was observed that antioxidant enzymes, particularly APX (ascorbate peroxidase) and CAT (catalase), displayed extremely rapid responses within the first minutes following infestation. Notably, this is the first study to demonstrate such immediate antioxidant defense mechanisms related to the redox metabolism of soybean plants under a biotic factor. These findings have significant potential to support biotechnological and breeding programs aimed at developing more tolerant cultivars. Exploring these rapid antioxidant responses may become a critical tool for enhancing plant tolerance to biotic factors, contributing to food security and the sustainability of modern agriculture.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antoniou, C.; Fragkoudi, I.; Martinou, A.; Stavrinides, M.C.; Fotopoulos, V. Spatial Response of Medicago truncatula Plants to Drought and Spider Mite Attack. Plant Physiol. Biochem. 2018, 130, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate Change Alters Plant-Herbivore Interactions. New Phytol. 2021, 229, 1894–1910. [Google Scholar] [CrossRef] [PubMed]
- Migeon, A.; Auger, P.; Fossati-Gaschignard, O.; Hufbauer, R.A.; Miranda, M.; Zriki, G.; Navajas, M. Climate of Origin Influences How a Herbivorous Mite Responds to Drought-Stressed Host Plants. Peer Community J. 2023, 3, e43. [Google Scholar] [CrossRef]
- Subedi, B.; Poudel, A.; Aryal, S. The Impact of Climate Change on Insect Pest Biology and Ecology: Implications for Pest Management Strategies, Crop Production, and Food Security. J. Agric. Food Res. 2023, 14, 100733. [Google Scholar] [CrossRef]
- Reichert, M.B.; Schneider, J.R.; Wurlitzer, W.B.; Ferla, N.J. Impacts of Cultivar and Management Practices on the Diversity and Population Dynamics of Mites in Soybean Crops. Exp. Appl. Acarol. 2024, 92, 41–59. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Recognition of Herbivory-Associated Molecular Patterns. Plant Physiol. 2008, 146, 825. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Parise, A.G.; Gagliano, M.; Souza, G.M. Extended Cognition in Plants: Is It Possible? Plant Signal Behav. 2020, 15, 1710661. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.A.G.; Sousa, R.H.V. Looking for a Systemic Concept and Physiological Diagnosis of a Plant Stress State. Theor. Exp. Plant Physiol. 2024, 36, 523–533. [Google Scholar] [CrossRef]
- Snoeck, S.; Guayazán-Palacios, N.; Steinbrenner, A.D. Molecular Tug-of-War: Plant Immune Recognition of Herbivory. Plant Cell 2022, 34, 1497–1513. [Google Scholar] [CrossRef] [PubMed]
- Iida, J.; Desaki, Y.; Hata, K.; Uemura, T.; Yasuno, A.; Islam, M.; Maffei, M.E.; Ozawa, R.; Nakajima, T.; Galis, I.; et al. Tetranins: New Putative Spider Mite Elicitors of Host Plant Defense. New Phytol. 2019, 224, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Mittler, R.; Jones, D.P. The Redox Code of Plants. Plant Cell Environ. 2024, 47, 2821–2829. [Google Scholar] [CrossRef]
- Wurlitzer, W.B.; Labudda, M.; Silveira, J.A.G.; Matthes, R.D.; Schneider, J.R.; Ferla, N.J. From Signaling to Stress: How Does Plant Redox Homeostasis Behave under Phytophagous Mite Infestation? Int. J. Plant Biol. 2024, 15, 561–585. [Google Scholar] [CrossRef]
- Villarroel, C.A.; Jonckheere, W.; Alba, J.M.; Glas, J.J.; Dermauw, W.; Haring, M.A.; Van Leeuwen, T.; Schuurink, R.C.; Kant, M.R. Salivary Proteins of Spider Mites Suppress Defenses in Nicotiana benthamiana and Promote Mite Reproduction. Plant J. 2016, 86, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, K.; Gogoi, H.; Borgohain, M.; Saikia, R.; Chikkaputtaiah, C.; Hiremath, S.; Basu, U. The Molecular Dynamics between Reactive Oxygen Species (ROS), Reactive Nitrogen Species (RNS) and Phytohormones in Plant’s Response to Biotic Stress. Plant Cell Rep. 2024, 43, 263. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Peng, Z.; Tong, H.; Xie, W.; Wang, S.; Wu, Q.; Zhang, J.; Li, C.; Zhang, Y. A Salivary Ferritin in the Whitefly Suppresses Plant Defenses and Facilitates Host Exploitation. J. Exp. Bot. 2019, 70, 3343–3355. [Google Scholar] [CrossRef]
- Wurlitzer, W.B.; Schneider, J.R.; Silveira, J.A.G.; de Almeida Oliveira, M.G.; Labudda, M.; Chavarria, G.; Weber, A.C.; Hoehne, L.; Pinheiro, G.M.; Vinhas, N.N.; et al. Tetranychus ludeni (Acari: Tetranychidae) Infestation Triggers a Spatiotemporal Redox Response Dependent on Soybean Genotypes. Planta 2024, 260, 130. [Google Scholar] [CrossRef] [PubMed]
- Galviz, Y.; Souza, G.M.; Lüttge, U. The Biological Concept of Stress Revisited: Relations of Stress and Memory of Plants as a Matter of Space–Time. Theor. Exp. Plant Physiol. 2022, 34, 239–264. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Plant Responses to Insect Herbivory: Interactions between Photosynthesis, Reactive Oxygen Species and Hormonal Signalling Pathways. Plant Cell Environ. 2012, 35, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.W.; Tuttle, D.M. A Guide to the Spider Mites (Tetranychidae) of the United States; Indira Publishing House: West Bloomfield, MI, USA, 1994; p. 347. [Google Scholar]
- Bolland, H.R.; Gutierrez, J.; Flechtmann Brill, C.H.W.; Boston· Koln, L. World Catalogue of the Spider Mite Family (Acari: Tetranychidae); Brill: Leiden, The Netherlands, 1998. [Google Scholar]
- Peláez-Vico, M.Á.; Fichman, Y.; Zandalinas, S.I.; Foyer, C.H.; Mittler, R. ROS Are Universal Cell-to-Cell Stress Signals. Curr. Opin. Plant Biol. 2024, 79, 102540. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Mitra, R.; Dasgupta, D.; Ganguly, R.; Acharya, K.; Minkina, T.; Popova, V.; Churyukina, E.; Keswani, C. Unraveling Lipid Peroxidation-Mediated Regulation of Redox Homeostasis for Sustaining Plant Health. Plant Physiol. Biochem. 2024, 206, 108272. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.H.V.; Carvalho, F.E.L.; Ribeiro, C.W.; Passaia, G.; Cunha, J.R.; Lima-Melo, Y.; Margis-Pinheiro, M.; Silveira, J.A.G. Peroxisomal APX Knockdown Triggers Antioxidant Mechanisms Favourable for Coping with High Photorespiratory H2O2 Induced by CAT Deficiency in Rice. Plant Cell Environ. 2015, 38, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Dang, J.; Zhang, J.; Wang, L.; Zheng, H.; Li, G.; Li, J.; Zhou, F.; Khan, A.; Zhang, Z.; et al. A Glutathione S-Transferase Regulates Lignin Biosynthesis and Enhances Salt Tolerance in Tomato. Plant Physiol. 2024, 196, 2989–3006. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liang, X.; Chen, Q.; Liu, Y.; Wu, C.; Wu, M.; Shui, J.; Qiao, Y.; Zhang, Y.; Geng, Y. MePAL6 Regulates Lignin Accumulation to Shape Cassava Resistance against Two-Spotted Spider Mite. Front. Plant Sci. 2023, 13, 1067695. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline Metabolism as Regulatory Hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef]
- Signorelli, S.; Coitiño, E.L.; Borsani, O.; Monza, J. Molecular Mechanisms for the Reaction between •OH Radicals and Proline: Insights on the Role as Reactive Oxygen Species Scavenger in Plant Stress. J. Phys. Chem. B 2014, 118, 37–47. [Google Scholar] [CrossRef]
- Sarmento, R.A.; Lemos, F.; Bleeker, P.M.; Schuurink, R.C.; Pallini, A.; Oliveira, M.G.A.; Lima, E.R.; Kant, M.; Sabelis, M.W.; Janssen, A. A Herbivore That Manipulates Plant Defence. Ecol. Lett. 2011, 14, 229. [Google Scholar] [CrossRef]
- Garcia, A.; Talavera-Mateo, L.; Petrik, I.; Oklestkova, J.; Novak, O.; Santamaria, M.E. Spider Mite Infestation Triggers Coordinated Hormonal Trade-Offs Enabling Plant Survival with a Fitness Cost. Physiol. Plant 2024, 176, e14479. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Felton, G.W.; Tumlinson, J.H. The Dual Function of Elicitors and Effectors from Insects: Reviewing the “arms Race” against Plant Defenses. Plant Mol. Biol. 2022, 109, 427–445. [Google Scholar] [CrossRef]
- PAS, C. Manual de Segurança e Qualidade Para a Cultura Da Soja; Série Qualidade e Segurança Dos Alimentos; EMBRAPA: Brasilia, Brazil, 2005. [Google Scholar]
- Fehr, W.; Caviness, C. Stages of Soybean Development; Special Report 80, Iowa Agricultural Experiment Station, Iowa Cooperative External Series; Iowa State University: Ames, IO, USA, 1977. [Google Scholar]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.R.; De Bona, A.C.; Müller, M.; Chavarria, G. Mancozeb Associated with Water Deficit: Physiological and Biochemical Responses of Soybean Plants. Plant Stress 2023, 10, 100284. [Google Scholar] [CrossRef]
- Chaitanya, K.S.K.; Naithani, S.C. Role of Superoxide, Lipid Peroxidation and Superoxide Dismutase in Membrane Perturbation during Loss of Viability in Seeds of Shorea robusta Gaertn.f. New Phytol. 1994, 126, 623–627. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Anderson, M.D.; Prasad, T.K.; Stewart, C.R. Changes in Isozyme Profiles of Catalase, Peroxidase, and Glutathione Reductase during Acclimation to Chilling in Mesocotyls of Maize Seedlings. Plant Physiol. 1995, 109, 1247–1257. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Del Longo, O.T.; González, C.A.; Pastori, G.M.; Trippi, V.S. Antioxidant Defences under Hyperoxygenic and Hyperosmotic Conditions in Leaves of Two Lines of Maize with Differential Sensitivity to Drought. Plant Cell Physiol. 1993, 34, 1023–1028. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Dalton, D.A.; Boniface, C.; Turner, Z.; Lindahl, A.; Kim, H.J.; Jelinek, L.; Govindarajulu, M.; Finger, R.E.; Taylor, C.G. Physiological Roles of Glutathione S-Transferases in Soybean Root Nodules. Plant Physiol. 2009, 150, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, R.S. Drought Stress, Enzymes of Glutathione Metabolism, Oxidation Injury, and Protein Synthesis in Tortula ruralis. Plant Physiol. 1991, 95, 648–651. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Shabnam, N.; Tripathi, I.; Sharmila, P.; Pardha-Saradhi, P. A Rapid, Ideal, and Eco-Friendlier Protocol for Quantifying Proline. Protoplasma 2016, 253, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Determination of Glutathione and Glutathione Disulfide Using Glutathione Reductase and 2-Vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wei, T.; Simko, V. Visualization of a Correlation Matrix, R Package Corrplot Version 0.95; CRAN: Contributed Packages: Vienna, Austria, 2024. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Extract and Visualize the Results of Multivariate Data Analyses, R Package Factoextra Version 1.0.7; CRAN: Contributed Packages: Vienna, Austria, 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurlitzer, W.B.; Schneider, J.R.; Silveira, J.A.G.; de Almeida Oliveira, M.G.; Ferla, N.J. Mite Infestation Induces a Moderate Oxidative Stress in Short-Term Soybean Exposure. Plants 2025, 14, 590. https://doi.org/10.3390/plants14040590
Wurlitzer WB, Schneider JR, Silveira JAG, de Almeida Oliveira MG, Ferla NJ. Mite Infestation Induces a Moderate Oxidative Stress in Short-Term Soybean Exposure. Plants. 2025; 14(4):590. https://doi.org/10.3390/plants14040590
Chicago/Turabian StyleWurlitzer, Wesley Borges, Julia Renata Schneider, Joaquim A. G. Silveira, Maria Goreti de Almeida Oliveira, and Noeli Juarez Ferla. 2025. "Mite Infestation Induces a Moderate Oxidative Stress in Short-Term Soybean Exposure" Plants 14, no. 4: 590. https://doi.org/10.3390/plants14040590
APA StyleWurlitzer, W. B., Schneider, J. R., Silveira, J. A. G., de Almeida Oliveira, M. G., & Ferla, N. J. (2025). Mite Infestation Induces a Moderate Oxidative Stress in Short-Term Soybean Exposure. Plants, 14(4), 590. https://doi.org/10.3390/plants14040590






