Chemical Characterization and Antibiotic-Enhancing Activity of the Essential Oils of Propolis of Melipona quadrifasciata quadrifasciata
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils of Propolis from Melipona quadrifasciata quadrifasciata
2.1.1. Extraction of Propolis Essential Oil
2.1.2. Chemical Composition Analysis
2.2. Antibacterial Activity
2.2.1. Bacterial Strains
2.2.2. Bacterial Inoculum Preparation
2.2.3. Substances Used
2.2.4. Minimum Inhibitory Concentration (MIC)
2.2.5. Evaluation of Antibiotic-Enhancing Activity
2.2.6. Statistical Analysis
3. Results and Discussion
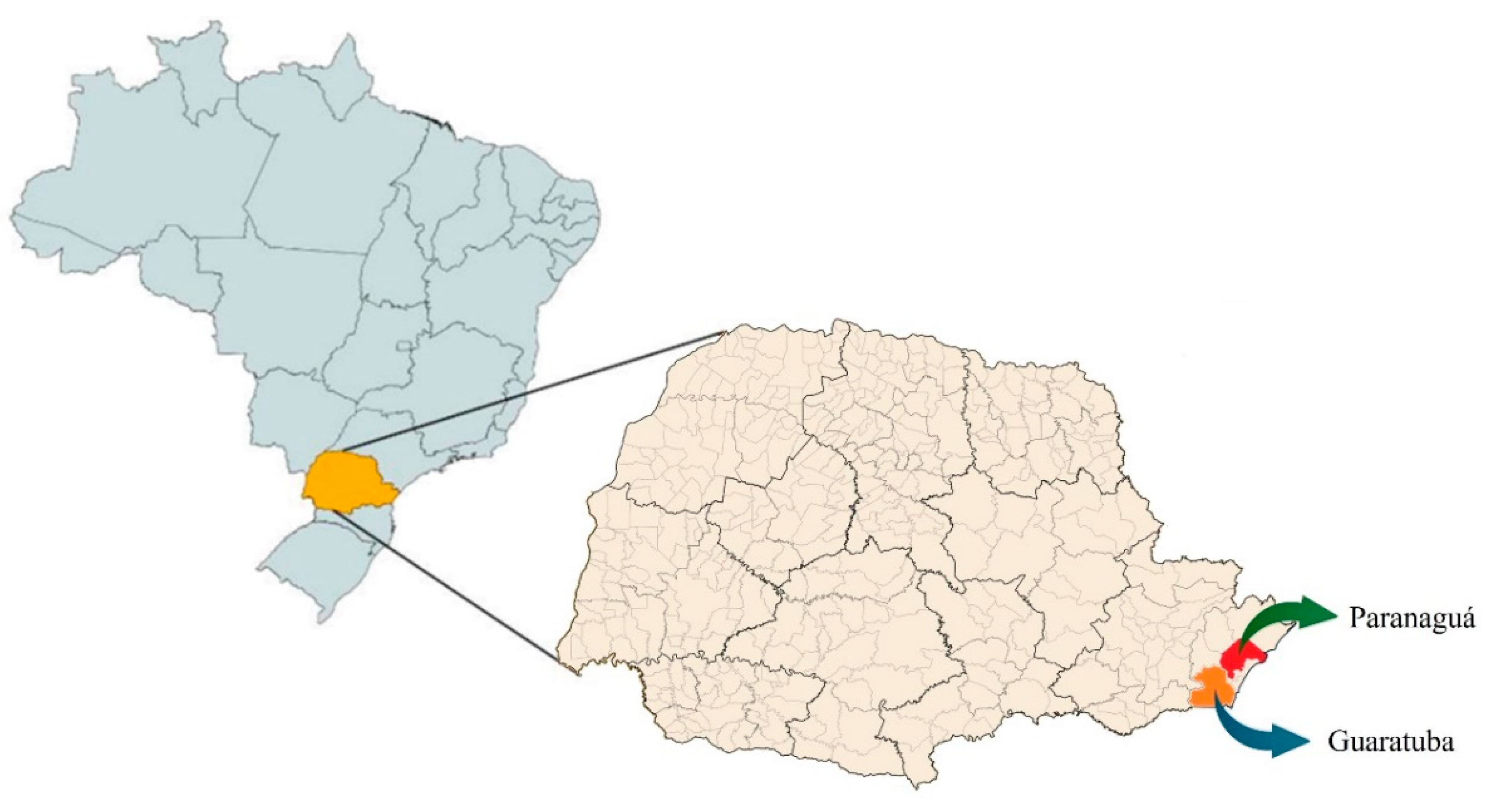
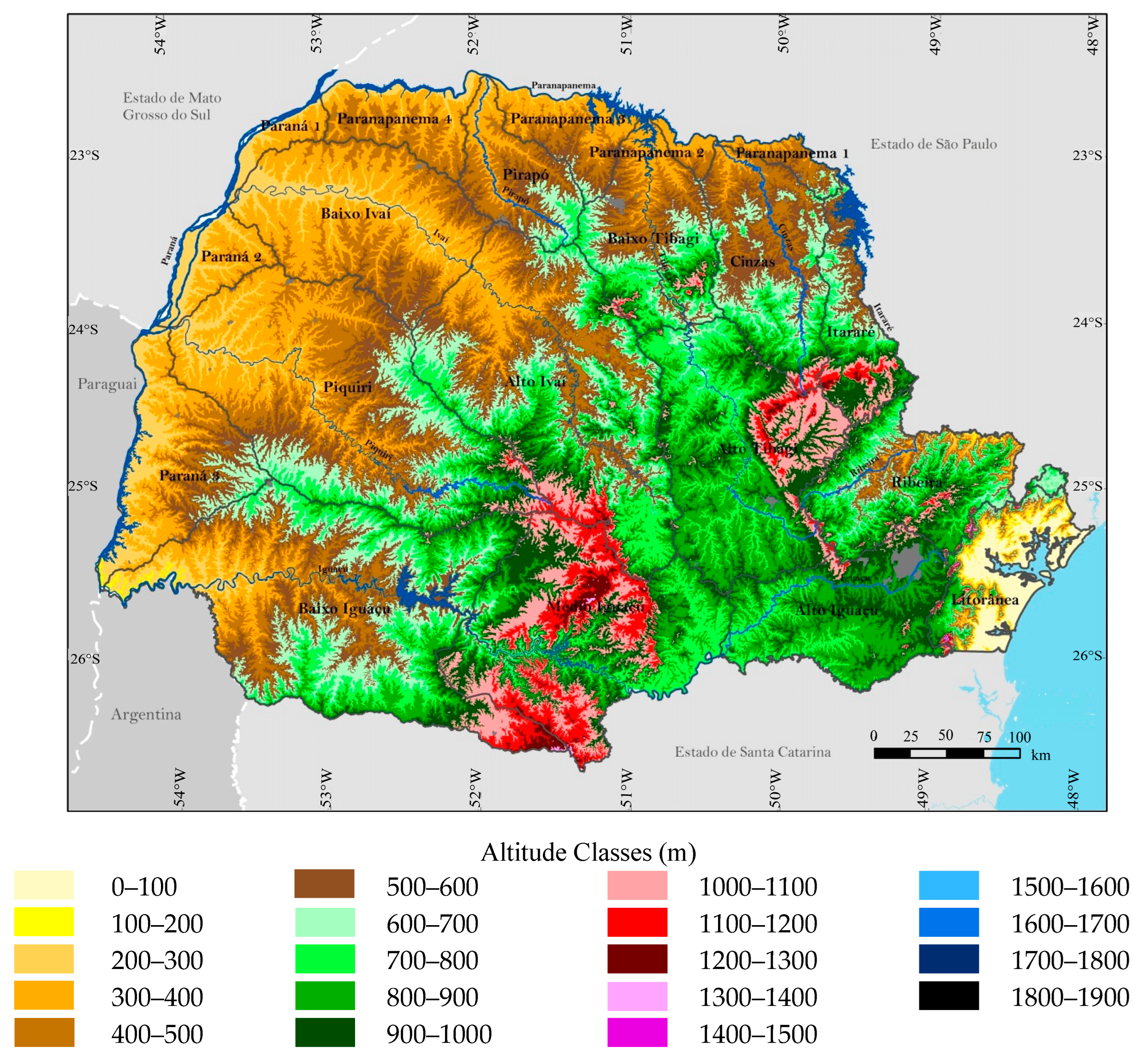
3.1. Relief Characteristics and Climates of the Cities of Paranaguá and Guaratuba
3.2. Chemical Composition of Essential Oils
3.3. Minimum Inhibitory Concentration of Essential Oils
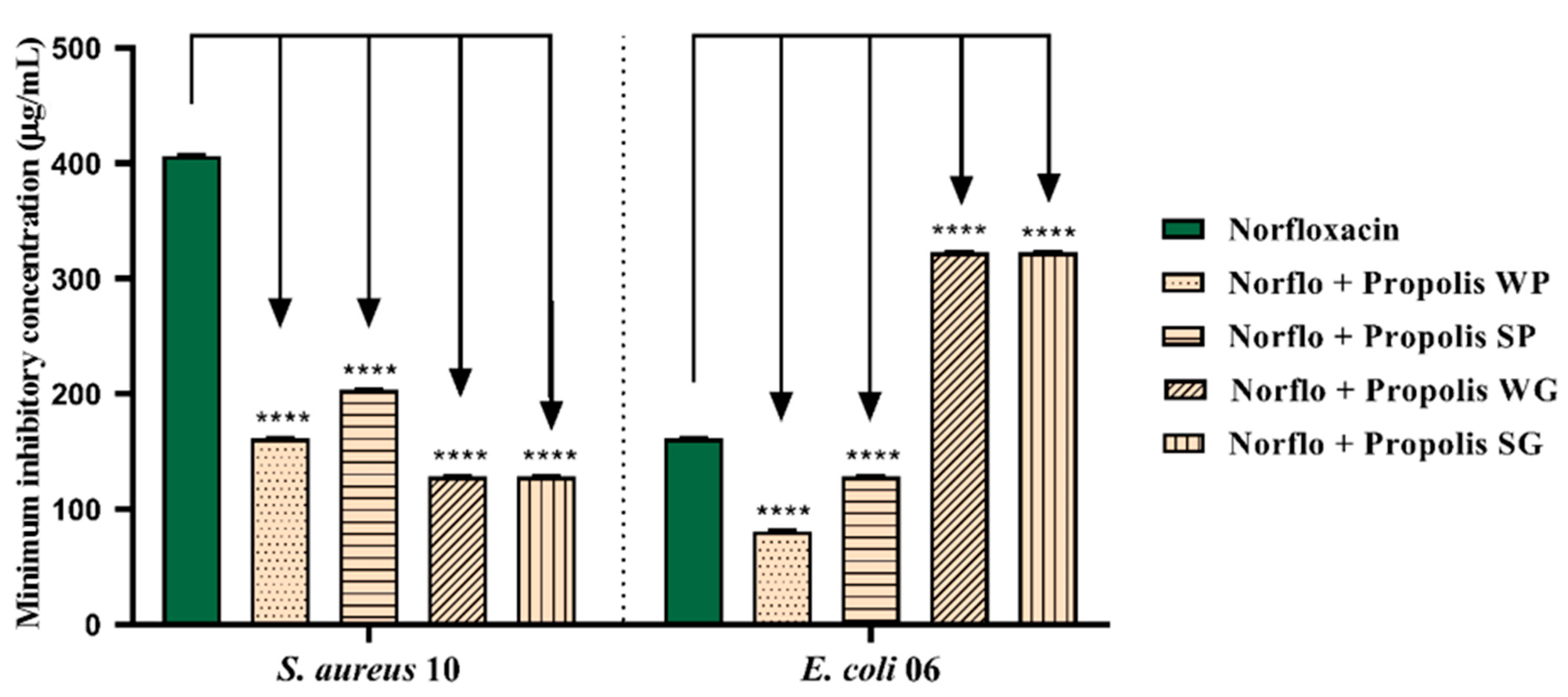
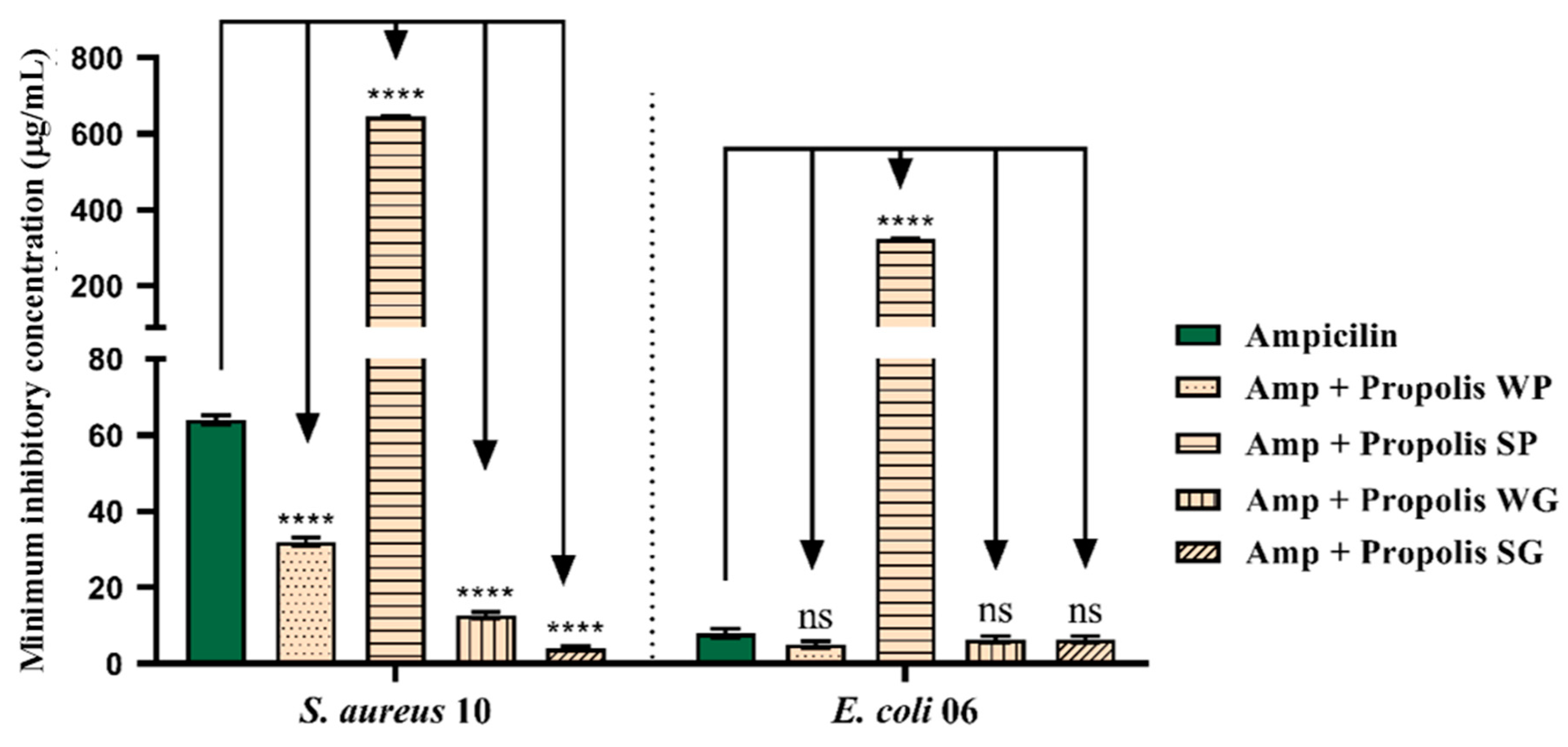
3.4. Antibiotic Potentiating Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farrell, J.M.; Zhao, C.Y.; Tarquinio, K.M.; Brown, S.P. Causes and Consequences of COVID-19-Associated Bacterial Infections. Front. Microbiol. 2021, 12, 682571. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Swetschinski, L.R.; Robles Aguilar, G.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Davis Weaver, N.; Wool, E.E.; Han, C.; Gershberg Hayoon, A.; et al. Global Mortality Associated with 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance, Wellcome Trust and HM Government. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 3 May 2024).
- WHO—World Health Organization. World Antimicrobial Awareness Week 2020. Available online: https://www.who.int/campaigns/world-antimicrobial-awareness-week/2020 (accessed on 5 June 2024).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- WHO, World Health Organization. 2020 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. 2020. Available online: https://www.who.int/publications-detail-redirect/9789240021303 (accessed on 7 June 2024).
- Valli, M.; Russo, H.M.; Bolzani, V.S. The Potential Contribution of the Natural Products from Brazilian Biodiversity to Bioeconomy. An. Acad. Bras. Ciênc. 2018, 90 (Suppl. S1), 763–778. [Google Scholar] [CrossRef] [PubMed]
- Teotonio, R.A. Farmacognosia. Editora e Distribuidora Educacional S.A.: Londrina, Brazil, 2018. [Google Scholar]
- Sampaio, M.A.L.; Araújo, A.C.J.; Borges, J.A.O.; Lima, C.M.G.; Coutinho, H.D.M.; Freitas, P.R.; Emran, T.B.; Obaidullah, A.J.; Silva, R.O.M. Antibacterial and Antibiotic-Modifying Activity of the Commercialized Essential Oil of Copaifera spp. Associated with LED Lights Against a Staphylococcus aureus Strain. J. Funct. Foods 2024, 114, 106075. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of Innovation in Health and Disease. Chem.-Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Frantianni, F.; de Martino, L.; Coppola, R.; de Feo, V. Efeito dos óleos essenciais em bactérias patogênicas. Farmacêuticos 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Gaines-Day, H.R.; Gratton, C. Do Managed Bees Have Negative Effects on Wild Bees?: A Systematic Review of the Literature. PLoS ONE 2017, 12, e0189268. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Salatino, M.L.F. Scientific note: Often quoted, but not factual data about propolis composition. Apidologie 2021, 52, 312–314. [Google Scholar] [CrossRef]
- Santos, F.D.A.D.; Nunes, L.E. Propólis: Aspectos Químicos e Propriedades Terapêuticas. Rev. Eletrônica Multidiscip. Investig. Cient. 2023, 2, 1–13. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [PubMed]
- Drescher, N.; Klein, A.-M.; Schmitt, T.; Leonhardt, S.D. A Clue on Bee Glue: New Insight into the Sources and Factors Driving Resin Intake in Honeybees (Apis mellifera). PLoS ONE 2019, 14, e0210594. [Google Scholar] [CrossRef] [PubMed]
- Lepeletier de Saint Fargeau, A.L.M.; Brullé, A. Histoire Naturelle des Insectes. Hyménoptères; Librairie Encyclopédique de Roret: Paris, France, 1836. [Google Scholar] [CrossRef]
- dos Santos, C.M.; Campos, J.F.; Santos, H.F.; Balestieri, J.B.P.; Silva, D.B.; de Picoli Souza, K.; dos Santos, E.L. Chemical Composition and Pharmacological Effects of Geopropolis Produced by Melipona quadrifasciata anthidioides. Oxidative Med. Cell. Longev. 2017, 2017, 8320804. [Google Scholar] [CrossRef]
- Torres, A.R.; Sandjo, L.P.; Friedemann, M.T.; Tomazzoli, M.M.; Maraschin, M.; Mello, C.F.; Santos, A.R.S. Chemical Characterization, Antioxidant and Antimicrobial Activity of Propolis Obtained from Melipona quadrifasciata quadrifasciata and Tetragonisca angustula Stingless Bees. Braz. J. Med. Biol. Res. 2018, 51, e7118. [Google Scholar] [CrossRef] [PubMed]
- Matos, F.J.A. Plantas Medicinais: Guia de Seleção e Emprego de Plantas Usadas na Fitoterapia no Nordeste do Brasil, 3rd ed.; Imprensa Universitária UFC: Fortaleza, Brazil, 2007. [Google Scholar]
- Adams, R. Identification of Essential Oils Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Bezerra, C.M.; Camilo, C.J.; Silva, M.K.N.; Freitas, T.S.; Ribeiro-Filho, J.; Coutinho, H.D.M. Vanillin Selectively Modulates the Action of Antibiotics Against Resistant Bacteria. Microb. Pathog. 2017, 113, 265–268. [Google Scholar] [CrossRef] [PubMed]
- CLSI—Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Coutinho, H.D.; Costa, J.G.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of the Antibiotic Activity Against a Multiresistant Escherichia coli by Mentha arvensis L. and Chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef]
- Maack, R. Geografia Física do Estado do Paraná; Banco de Desenvolvimento do Paraná: Curitiba, Brazil, 1968. [Google Scholar]
- Silva, L.E.; Araújo, J.P.; Amaral, W.; Menezes, E.C.O. Espécies Nativas Potenciais da Floresta Atlântica do Litoral Paranaense: Gestão de Recursos de Uso Comum no Contexto do Desenvolvimento Territorial Sustentável. Rev. Bras. Meio Ambiente Sustentabilidade 2021, 1, 33–58. Available online: https://rbmaes.emnuvens.com.br/revista/article/view/110 (accessed on 6 May 2024).
- Silva, L.E.; Dotto, A.R.F.; Rebelo, R.A. Bioprospecção e Inovação na Floresta Atlântica: A atuação da REBIFLORA no Litoral do Paraná e Santa Catarina. Rev. Fitos 2022, 16 (Suppl. S2), 227–237. [Google Scholar] [CrossRef]
- IPARDES—Instituto Paranaense de Desenvolvimento Econômico e Social. Uso da Terra Remanescente da Cobertura Vegetal—Paraná (2010–2014). 2015. Available online: https://www.ipardes.pr.gov.br/sites/ipardes/arquivos_restritos/files/documento/2020-09/Uso%20da%20terra%20e%20Remanescentes%20.pdf (accessed on 3 June 2024).
- IPARDES—Instituto Paranaense de Desenvolvimento Econômico e Social. Hipsometria—Paraná. 2019. Available online: http://www.ipardes.pr.gov.br/sites/ipardes/arquivos_restritos/files/documento/2019-09/Hipsometria%20-%20Paraná.pdf (accessed on 3 June 2024).
- Nitsche, P.R.; Caramori, P.H.; Ricce, W.S.; Pinto, L.F.D. Atlas Climático do Estado do Paraná; IAPAR: Londrina, Brazil, 2019. [Google Scholar]
- Climate Data—Paranaguá Climate. Average Temperature by Month, Paranaguá Water Temperature. 2024. Available online: https://en.climate-data.org/south-america/brazil/parana/paranagua-3457 (accessed on 12 June 2024).
- Climate Data—Guaratuba Climate. Average Temperature by Month, Guaratuba Water Temperature. 2024. Available online: https://en.climate-data.org/south-america/brazil/parana/guaratuba-34671 (accessed on 12 June 2024).
- Gerginova, D.; Popova, M.; Chimshirova, R.; Trusheva, B.; Shanahan, M.; Guzmán, M.; Solorzano-Gordillo, E.; López-Roblero, E.; Spivak, M.; Simova, S.; et al. The Chemical Composition of Scaptotrigona mexicana Honey and Propolis Collected in Two Locations: Similarities and Differences. Foods 2023, 12, 3317. [Google Scholar] [CrossRef] [PubMed]
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Naggar, Y.A.; Algethami, A.F.; Shou, Q.; Alsharif, S.M.; et al. Bee Pollen: Clinical Trials and Patent Applications. Nutrients 2022, 14, 2858. [Google Scholar] [CrossRef]
- Zapata-Hernández, G.; Gajardo-Rojas, M.; Calderón-Seguel, M.; Muñoz, A.A.; Yáñez, K.P.; Requier, F.; Fontúrbel, F.E.; Ormeño-Arriagada, P.I.; Arrieta, H. Advances and knowledge gaps on climate change impacts on honey bees and beekeeping: A systematic review. Glob. Change Biol. 2024, 30, e17219. [Google Scholar] [CrossRef]
- de Jongh, E.J.; Harper, S.L.; Yamamoto, S.S.; Wright, C.J.; Wilkinson, C.W.; Ghosh, S.; Otto, S.J.G. One Health, One Hive: A Scoping Review of Honey Bees, Climate Change, Pollutants, and Antimicrobial Resistance. PLoS ONE 2022, 17, e0242393. [Google Scholar] [CrossRef]
- Shehata, M.G.; Ahmad, F.T.; Badr, A.N.; Masry, S.H.; El-Sohaimy, S.A. Chemical Analysis, Antioxidant, Cytotoxic and Antimicrobial Properties of Propolis from Different Geographic Regions. Ann. Agric. Sci. 2020, 65, 209–217. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Shu, H.-Z.; Peng, C.; Bu, L.; Guo, L.; Liu, F.; Xiong, L. Bisabolane-type Sesquiterpenoids: Structural Diversity and Biological Activity. Phytochemistry 2021, 192, 112927. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Askari, V.R. A Mechanistic Review on Immunomodulatory Effects of Selective Type Two Cannabinoid Receptor Β-caryophyllene. BioFactors 2022, 48, 857–882. [Google Scholar] [CrossRef] [PubMed]
- Li, D.S.; Shi, L.L.; Guo, K.; Luo, S.H.; Liu, Y.C.; Chen, Y.G.; Liu, Y.; Li, S.H. A New Sesquiterpene Synthase Catalyzing the Formation of (R)-β-Bisabolene from Medicinal Plant Colquhounia coccinea var. mollis and Its Anti-adipogenic and Antibacterial Activities. Phytochemistry 2023, 211, 113681. [Google Scholar] [CrossRef] [PubMed]
- Ricardi, C.; Barachini, S.; Consoli, G.; Marazziti, D.; Polini, B.; Chiellini, G. Beta-Caryophyllene, a Cannabinoid Receptor Type 2 Selective Agonist, in Emotional and Cognitive Disorders. Int. J. Mol. Sci. 2024, 25, 3203. [Google Scholar] [CrossRef]
- Pereira, R.L.S.; de Freitas, T.S.; Freitas, P.R.; de Araújo, A.C.J.; Campina, F.F.; Fidelis, K.R.; dos Santos, H.S. Seasonality Effects on Antibacterial and Antibiotic Potentiating Activity against Multidrug-Resistant Strains of Escherichia coli and Staphylococcus aureus and ATR-FTIR Spectra of Essential Oils from Vitex gardneriana Leaves. Curr. Microbiol. 2020, 77, 3969–3977. [Google Scholar] [CrossRef] [PubMed]
- Silveira, R.M.; Carvalho, A.F.; Bünger, M.D.O.; Francisca, M.D.O.; da Costa, I.R. Meta-Analysis of the Influence of Seasonality on the Chemical Composition of Essential Oils from Myrtaceae Species. South Afr. J. Bot. 2022, 150, 1096–1101. [Google Scholar] [CrossRef]
- de Souza, E.A.; Inoue, H.T.; Fernandes Júnior, A.; Veiga, N.; Orsi, R.D.O. Influence of Seasonality and Production Method on the Antibacterial Activity of Propolis. Acta Sci. Anim. Sci. 2014, 36, 49–53. [Google Scholar] [CrossRef]
- Calegari, M.A.; Prasniewski, A.; Silva, C.D.; Sado, R.Y.; Maia, F.M.C.; Tonial, L.M.S.; Oldoni, T.L.C. Propolis from Southwest of Paraná Produced by Selected Bees: Influence of Seasonality and Food Supplementation on Antioxidant Activity and Phenolic Profile. An. Acad. Bras. Ciênc. 2017, 89, 45–55. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Martins, O.A.; Ripoll, M.K.; Waller, S.B.; Osório, L.G.; Gomes, A.R.; Faria, R.O.; Meireles, M.C.A.; de Mello, J.R.B. Métodos de Avaliação Antimicrobiana de Extratos de Diferentes Variedades de Olea europaea L.: Revisão de Literatura. Sci. Anim. Health 2021, 9, 180–199. [Google Scholar] [CrossRef]
- Yasir, M.; Nawaz, A.; Ghazanfar, S.; Okla, M.K.; Chaudhary, A.; Al, W.H.; Ajmal, M.N.; AbdElgawad, H.; Ahmad, Z.; Abbas, F.; et al. Anti-bacterial Activity of Essential Oils Against Multidrug-Resistant Foodborne Pathogens Isolated from Raw Milk. Braz. J. Biol. 2024, 84, e259449. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.-J.; Jwa, S.-K. Inhibitory Effects of β-Caryophyllene on Streptococcus mutans Biofilm. Arch. Oral Biol. 2018, 88, 42–46. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Anusha, E.; Manogem, E.M. Extraction and Characterization of Bioactive Compounds from Plant, Michelia champaca (L.) in Polar Solvents: GC-MS and Antibacterial Properties. Ecol. Environ. Conserv. 2024, 30, S436–S446. [Google Scholar] [CrossRef]
- Moradlou, O.; Rabiei, Z.; Delavari, N. Antibacterial Effects of Carbon Quantum Dots@Hematite Nanostructures Deposited on Titanium Against Gram-Positive and Gram-Negative Bacteria. J. Photochem. Photobiol. A Chem. 2019, 379, 144–149. [Google Scholar] [CrossRef]
- Sahu, M.C.; Dubey, D.; Rath, S.; Panda, T.; Padhy, R.N. Monograph: In Vitro Efficacy of 30 Ethnomedicinal Plants Used by Indian Aborigines Against 6 Multidrug Resistant Gram-Positive Pathogenic Bacteria. Asian Pac. J. Trop. Dis. 2015, 5, 136–150. [Google Scholar] [CrossRef]
- Verma, R.; Pavithra, P.; Janani, V.; Charumathi, K.; Indumathy, R.; Potala, S. Antibacterial Activity of Plants Used in Indian Herbal Medicine. Int. J. Green Pharm. 2010, 4, 22. [Google Scholar] [CrossRef]
- Firat, Z.; Demirci, F.; Demirci, B.; Kırmızıbekmez, H.; Baser, K.H.C. Microbial Transformation of (–)-α-Bisabolol Towards Bioactive Metabolites. Rec. Nat. Prod. 2021, 15, 593–601. [Google Scholar] [CrossRef]
- Kazemi, M.; Rostami, H. Composição Química, Atividades Antimicrobiana e Antioxidante do Óleo Essencial de Psammogeton canescens. Nat. Prod. Res. 2014, 29, 277–280. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Magalhães, L.G.; Martins, C.H.G.; Santiago, M.B.; Crotti, A.E.M.; Andrade, P.M.D.; Santos, T.C.L.D.; Miranda, M.L.D. Avaliação In Vitro das Atividades Anticárie, Antimicobacteriana, Antileishmania e Citotóxica dos Óleos Essenciais de Eremanthus erythropappus e do α-Bisabolol, Seu Principal Sesquiterpeno. Aust. J. Crop Sci. 2020, 14, 236–243. [Google Scholar] [CrossRef]
- do Nascimento, P.R.S.; da Silva Júnior, E.L.; Branco, A.C.d.S.C. Aplicações farmacológicas da Cúrcuma longa L. como planta medicinal: Uma revisão. Res. Soc. Dev. 2020, 9, e2629108430. [Google Scholar] [CrossRef]
- Al-Dhahli, A.S.; Al-Hassani, F.A.; Alarjani, K.M.; Yehia, H.M.; Al Lawati, W.M.; Hejaz Azmi, S.N.; Khan, S.A. Essential oil from the rhizomes of the Saudi and Chinese Zingiber officinale cultivars: Comparison of chemical composition, antibacterial and molecular docking studies. J. King Saud Univ.-Sci. 2020, 32, 3343–3350. [Google Scholar] [CrossRef]
- Levinson, W.; Chin-Hong, P.; Joyce, E.A.; Nussbaum, J.; Schwartz, B. Review of Medical Microbiology & Immunology: A Guide to Clinical Infectious Diseases; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Reddy, D.N. Essential Oils Extracted from Medicinal Plants and Their Applications. In Natural Bio-Active Compounds; Springer: Singapore, 2019; pp. 237–283. [Google Scholar] [CrossRef]
- Almeida, R.S.; Freitas, P.R.; Araújo, A.C.J.; Menezes, I.R.A.; Santos, E.L.; Tintino, S.R.; Moura, T.F.; Filho, J.R.; Ferreira, V.A.; Silva, A.C.A.; et al. GC-MS Profile and Enhancement of Antibiotic Activity by the Essential Oil of Ocotea odorífera and Safrole: Inhibition of Staphylococcus aureus Efflux Pumps. Antibiotics 2020, 9, 247. [Google Scholar] [CrossRef]
- do Prado, G.S.B.; da Costa, C.F.; Carneiro, E.N.A.; Silva, A.J.A.; Siqueira, T.S.; Caetano, L.P.C.; de Oliveira Vago, L. Mecanismos de resistência a antibióticos em bactérias Gram-negativas: Novas abordagens terapêuticas. Rev. Ibero-Am. Humanidades Ciênc. Educ. 2023, 9, 1920–1930. [Google Scholar] [CrossRef]
- Valcanaia, C.P. Estudo Sobre o Óleo Volátil de Própolis de Abelhas Nativas sem Ferrão Melipona q. quadrifasciata e Tetragonisca angustula e Avaliação do seu Potencial Biológico. Master’s Thesis, Programa de Pós-Graduação em Química, Centro de Ciências Exatas e Naturais, Universidade Regional de Blumenau, Blumenau, Brazil, 2020. Available online: http://bu.furb.br//docs/DS/2020/367527_1_1.pdf (accessed on 21 June 2024).
- Valcanaia, C.P.; Masote, J.B.B.; Sommer, H.F.; Schiquet, S.; Padilha, B.; Krepsky, L.; Paganelli, C.J.; Borges, P.P.; Danielli, L.J.; Apel, M.A.; et al. Antimicrobial Activity of Volatile Oils from Brazilian Stingless Bees Melipona quadrifasciata quadrifasciata and Tetragonisca angustula Propolis. Chem. Biodivers. 2022, 19, e202200369. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.; de Sousa, M.H.O.; dos Santos Diniz Freitas, M.; Cesca, K.; de Moura, N.F. Composição química e atividade biológica da própolis de Melipona quadrifasciata. Res. Soc. Dev. 2022, 11, e193111234175. [Google Scholar] [CrossRef]


| Sigla | Descrição |
|---|---|
| OPMQ WP | Oil extracted in Winter from Paranaguá |
| OPMQ SP | Oil extracted in Summer from Paranaguá |
| OPMQ WG | Oil extracted in Winter from Guaratuba |
| OPMQ SG | Oil extracted in Summer from Guaratuba |
| Bacteria | Origin | Resistance Profile |
|---|---|---|
| Escherichia coli 06 | Urine culture | Cephalexin, Cefoxitin, Cefadroxil, Ceftriaxone, Cefepime, Ampicillin/Sulbactam |
| Staphylococcus aureus 10 | Rectal swab culture | Cefadroxil, Cephalexin, Cefalotin, Oxacillin, Penicillin, Ampicillin, Amoxicillin, Moxifloxacin, Ciprofloxacin, Levofloxacin, Ampicillin-Sulbactam, Amoxicillin/Clavulanic Acid, Erythromycin, Clarithromycin, Azithromycin, Clindamycin |
| Constituents | RT (min)/FID | RT (min)/EM | RIexp/IAlit | WP [%] | SP [%] | WG [%] | SG [%] |
|---|---|---|---|---|---|---|---|
| α-pinene | 9330 | 7125 | 936/932 | 0.20 | 0.93 | 8.74 | 1.54 |
| α-copaene | 28,088 | 27,183 | 1383/1374 | 7.23 | 6.28 | 4.73 | 5.47 |
| β-caryophyllene | 29,999 | 29,092 | 1428/1417 | 24.55 | 18.88 | tr | 25.69 |
| α-humulene | 31,350 | 30,475 | 1462/1452 | 3.30 | 3.00 | tr | 4.74 |
| ar-curcumene | 32,383 | 31,708 | 1487/1479 | 4.64 | 6.91 | 11.95 | 4.12 |
| caryophyllene oxide | 33,155 | 32,375 | 1507/1501 | 2.19 | 1.70 | - | 3.57 |
| β-bisabolene | 33,478 | 32,800 | 1515/1505 | 22.87 | 35.99 | 44.56 | 21.19 |
| β-curcumene | 33,583 | 32,933 | 1518/1514 | 8.56 | 7.03 | 10.95 | 4.20 |
| δ-cadinene | 34,070 | 33,367 | 1530/1522 | 2.85 | 2.47 | 1.23 | 0.70 |
| Others constituents | 17.44 | 12.44 | 10.40 | 20.82 | |||
| Total Characterization | 93.83% | 95.63% | 95.56% | 92.04% | |||
| Bacteria | MIC (μg/mL) of OPMQ | |||
|---|---|---|---|---|
| WP | SP | WG | SG | |
| Escherichia coli ATCC 25922 | ≥1024 | ≥1024 | ≥1024 | ≥1024 |
| Staphylococcus aureus ATCC 25923 | ||||
| Escherichia coli 06 | ||||
| Staphylococcus aureus 10 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albuquerque, E.S.; Paulo, C.L.R.; de Oliveira Borges, J.A.; Gonçalves, S.A.; Alencar, G.G.; Ferreira do Carmo, J.; de Morais Almeida, A.I.; Santos, M.L.d.; Almeida-Bezerra, J.W.; da Silva, L.E.; et al. Chemical Characterization and Antibiotic-Enhancing Activity of the Essential Oils of Propolis of Melipona quadrifasciata quadrifasciata. Plants 2025, 14, 587. https://doi.org/10.3390/plants14040587
Albuquerque ES, Paulo CLR, de Oliveira Borges JA, Gonçalves SA, Alencar GG, Ferreira do Carmo J, de Morais Almeida AI, Santos MLd, Almeida-Bezerra JW, da Silva LE, et al. Chemical Characterization and Antibiotic-Enhancing Activity of the Essential Oils of Propolis of Melipona quadrifasciata quadrifasciata. Plants. 2025; 14(4):587. https://doi.org/10.3390/plants14040587
Chicago/Turabian StyleAlbuquerque, Emílio Sousa, Cicera Laura Roque Paulo, João Arthur de Oliveira Borges, Sheila Alves Gonçalves, Gabriel Gonçalves Alencar, Judith Ferreira do Carmo, Angelica Isabely de Morais Almeida, Maura Lins dos Santos, José Weverton Almeida-Bezerra, Luiz Everson da Silva, and et al. 2025. "Chemical Characterization and Antibiotic-Enhancing Activity of the Essential Oils of Propolis of Melipona quadrifasciata quadrifasciata" Plants 14, no. 4: 587. https://doi.org/10.3390/plants14040587
APA StyleAlbuquerque, E. S., Paulo, C. L. R., de Oliveira Borges, J. A., Gonçalves, S. A., Alencar, G. G., Ferreira do Carmo, J., de Morais Almeida, A. I., Santos, M. L. d., Almeida-Bezerra, J. W., da Silva, L. E., Oliveira-Tintino, C. D. d. M., & Coutinho, H. D. M. (2025). Chemical Characterization and Antibiotic-Enhancing Activity of the Essential Oils of Propolis of Melipona quadrifasciata quadrifasciata. Plants, 14(4), 587. https://doi.org/10.3390/plants14040587









