A Simple and Scalable Chopped-Thallus Transformation Method for Marchantia polymorpha
Abstract
1. Introduction
2. Materials and Methods
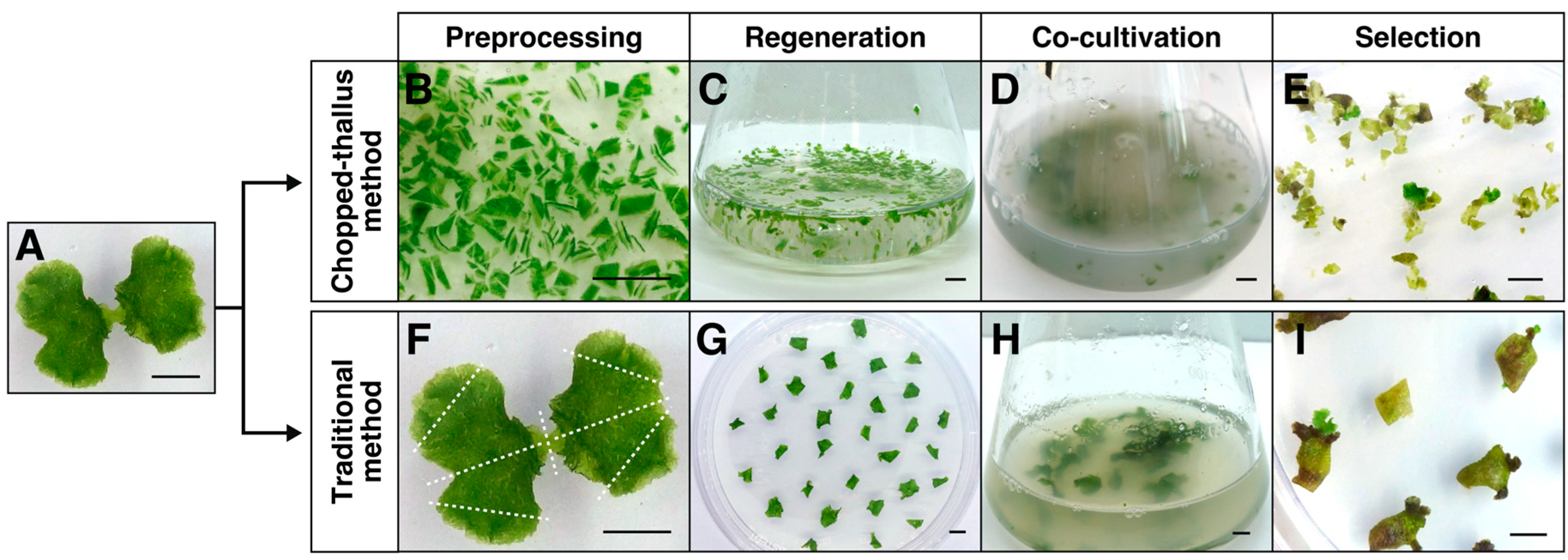
2.1. Plant Material and Preparation
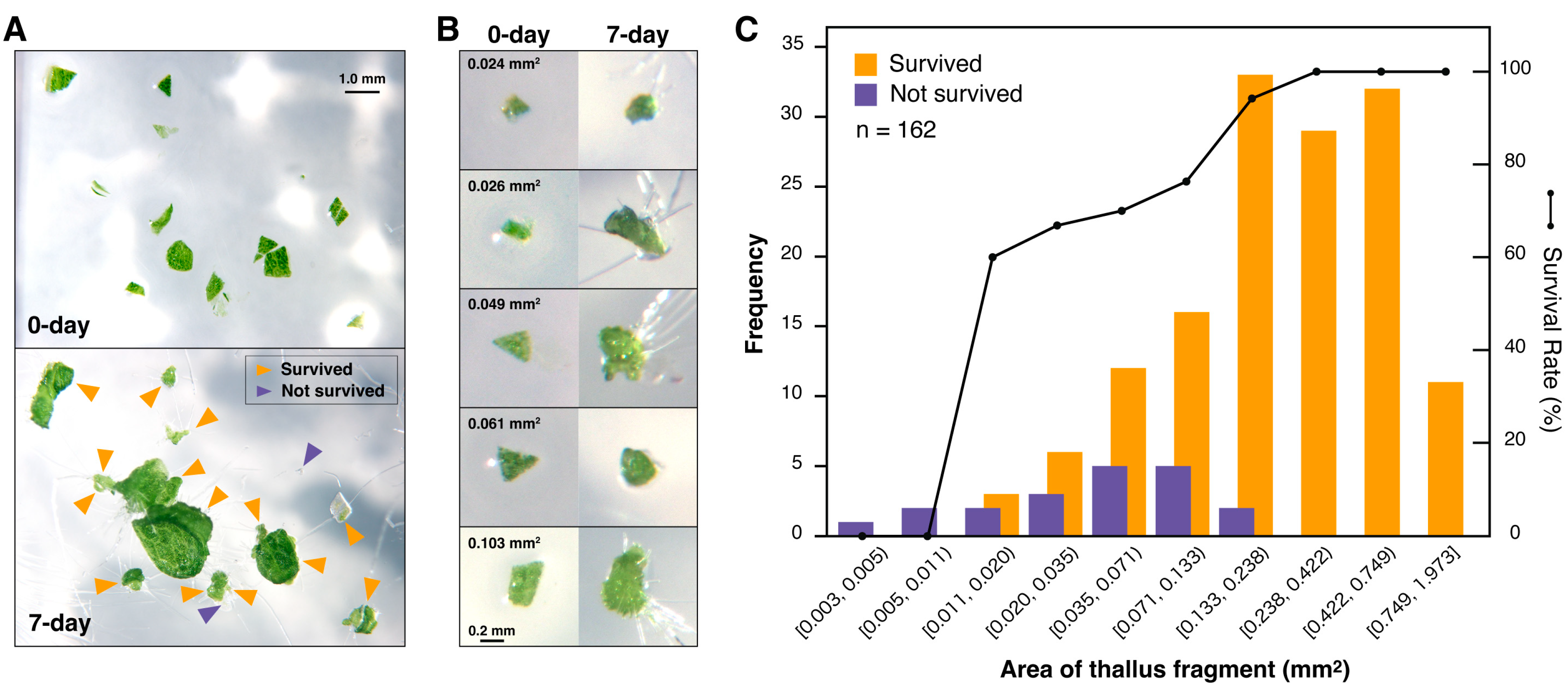
2.2. Analysis of Thallus Size and Regeneration
2.3. Plasmid Construction
2.4. Bacterial Strain and Culturing
2.5. Agrobacterium-Mediated Transformation of Marchantia
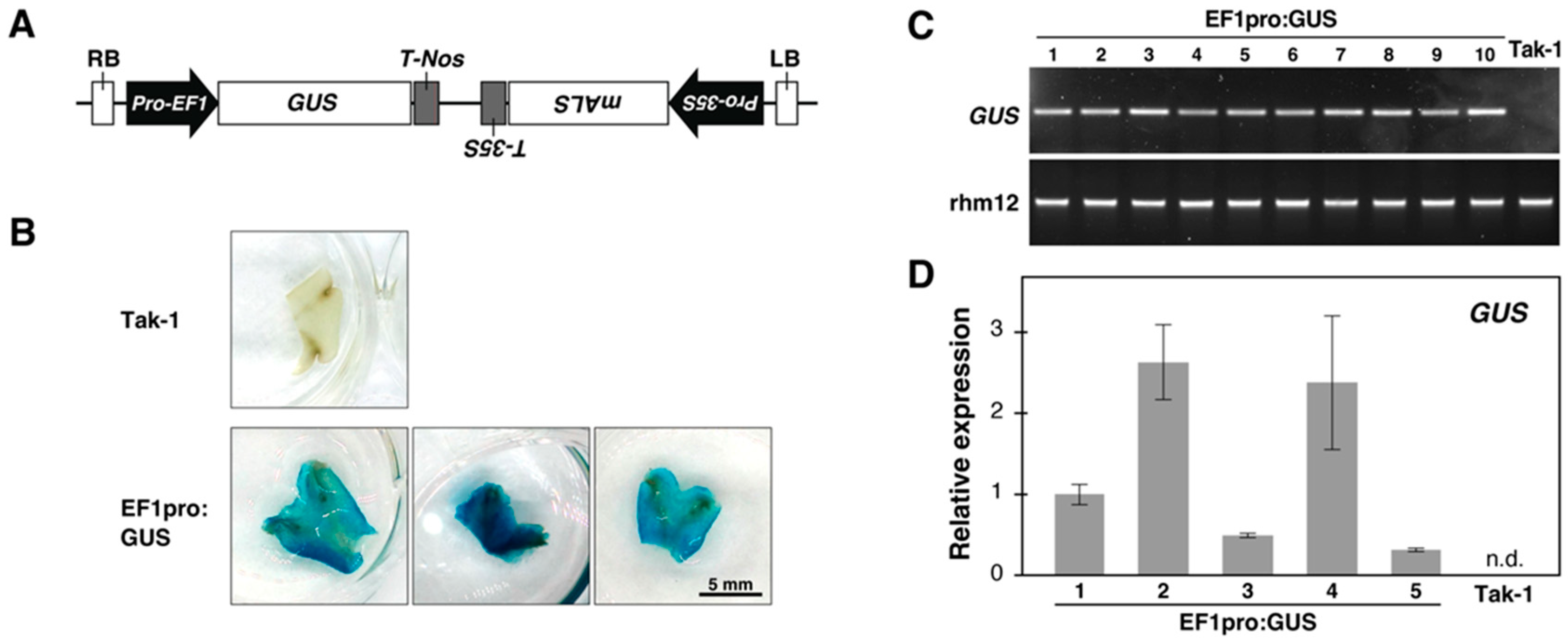
2.6. GUS Enzyme Activity Check via Histochemical Assay
2.7. Genomic DNA Isolation and PCR Analysis
2.8. Quantitative Real-Time PCR (qPCR)
2.9. Statistical Analysis
3. Results
3.1. Thallus Finely Chopped into Micro-Fragments Generates a Large Number of Regenerated Plants
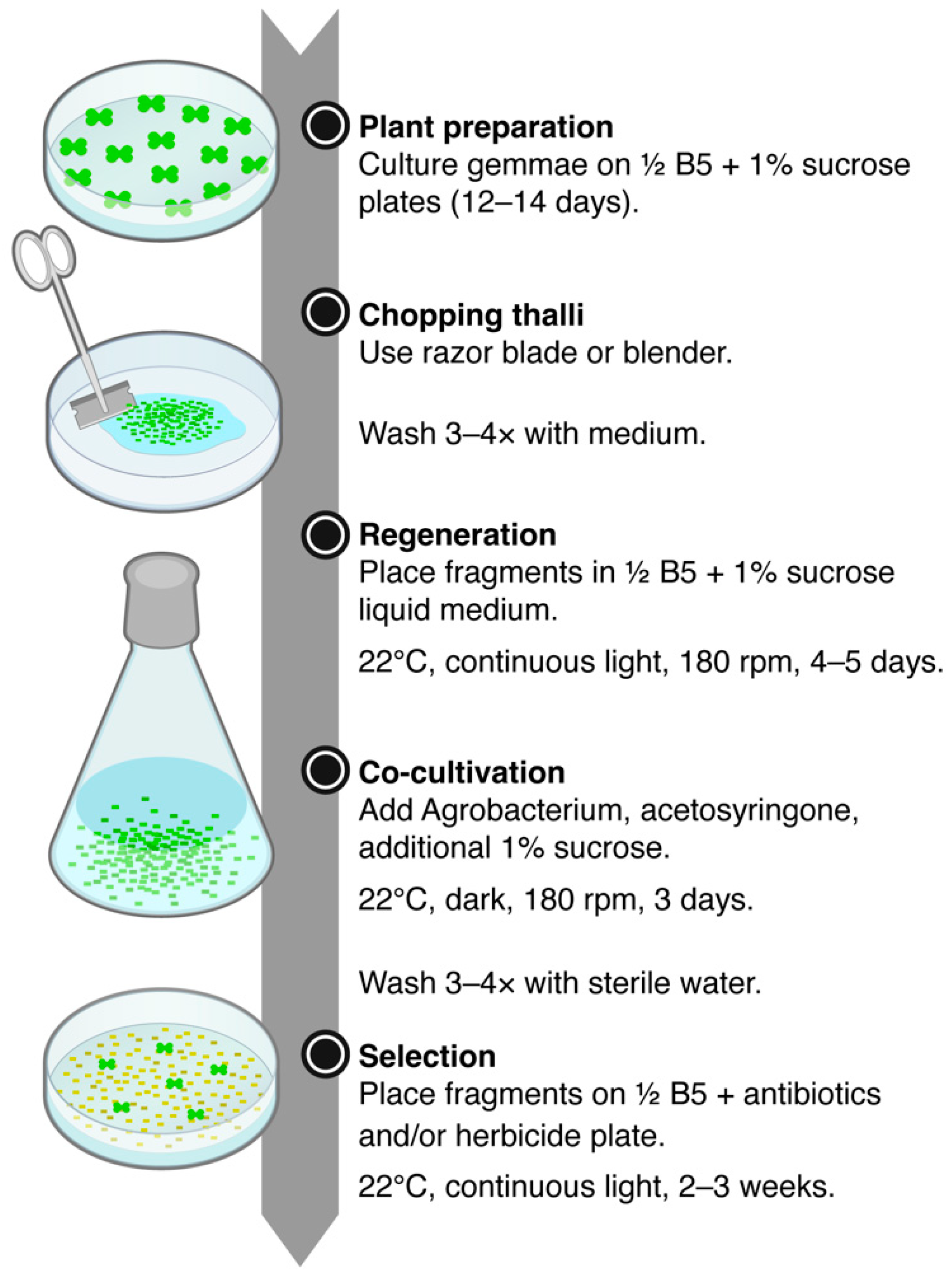
3.2. Establishing the Chopped-Thallus Method for Thallus Transformation
3.3. The Chopped-Thallus Method Simplifies Transformation and Enhances Efficiency
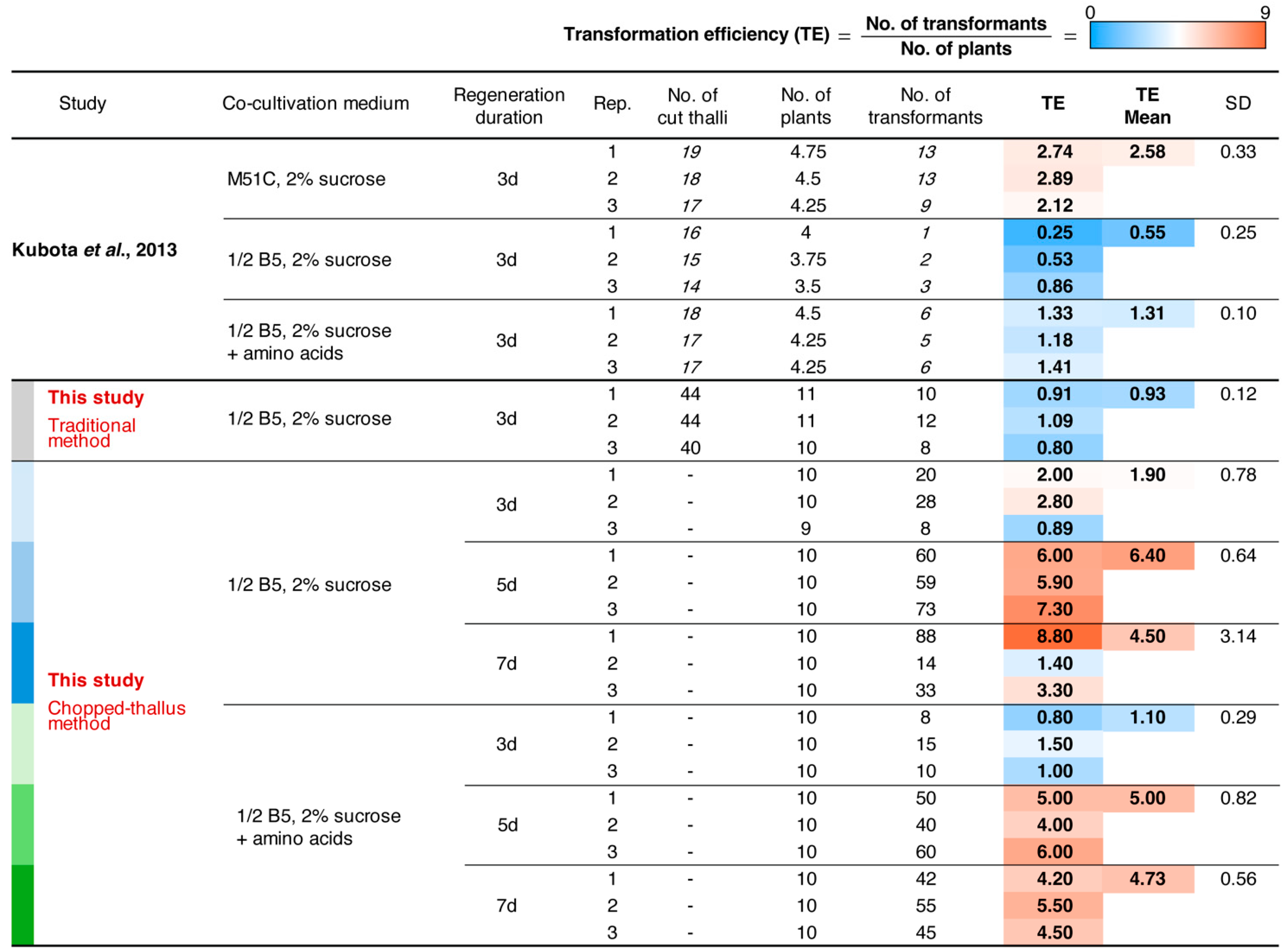
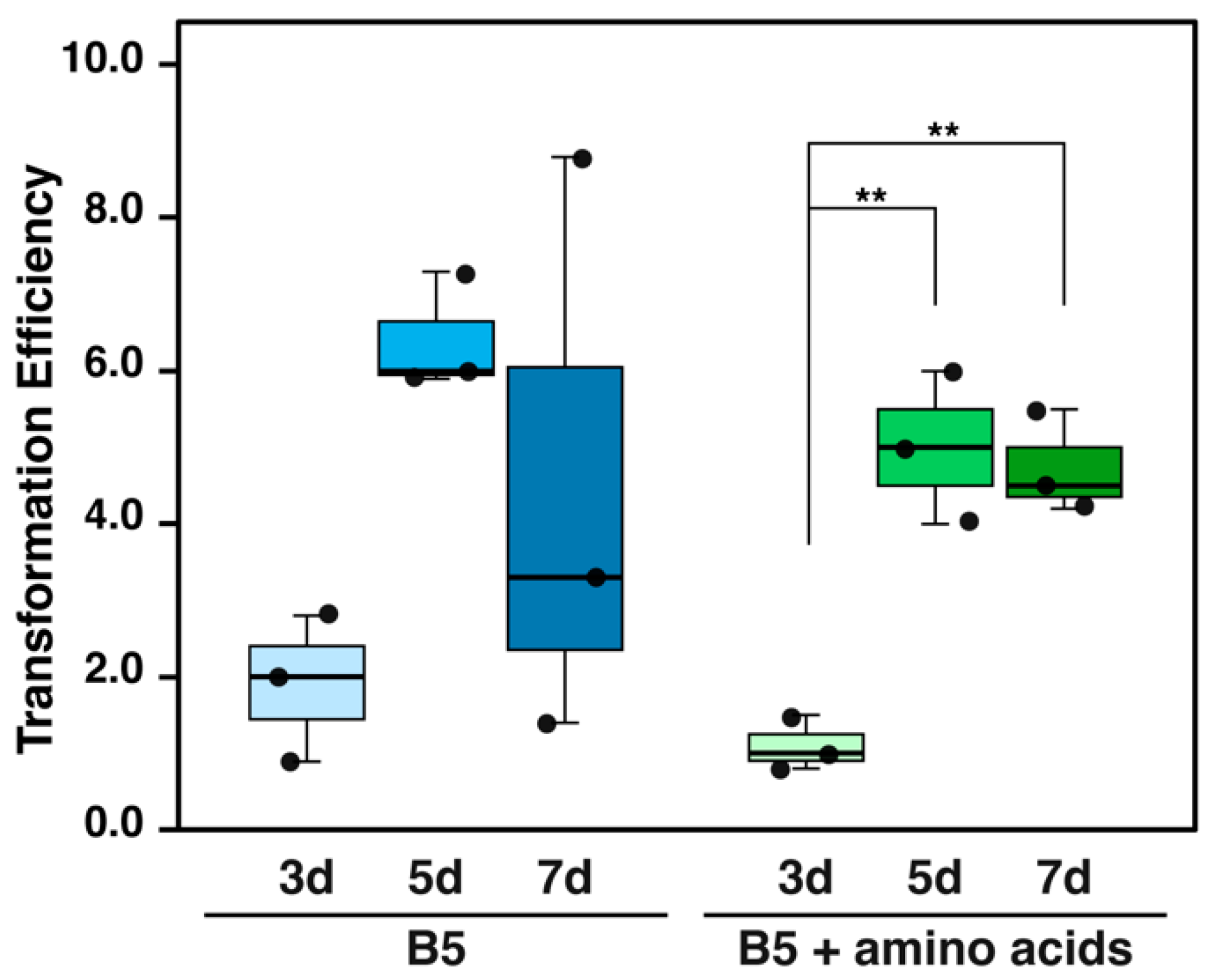
3.4. Optimizing Regeneration Period and Medium Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| EF1 | Elongation factor 1-α |
| GUS | β-glucuronidase |
| HSD | Honestly significant difference |
| LB | Left border |
| mALS | mutated acetolactate synthase |
| Marchantia | Marchantia polymorpha |
| RB | Right border |
| Tak | Takaragaike |
| TE | Transformation efficiency |
References
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.Q.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 2017, 171, 287–304.e15. [Google Scholar] [CrossRef]
- Li, F.W.; Nishiyama, T.; Waller, M.; Frangedakis, E.; Keller, J.; Li, Z.; Fernandez-Pozo, N.; Barker, M.S.; Bennett, T.; Blazquez, M.A.; et al. Anthoceros genomes illuminate the origin of land plants and the unique biology of hornworts. Nat. Plants 2020, 6, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, K.; Nishihama, R.; Yamato, K.T.; Kohchi, T. Molecular genetic tools and techniques for Marchantia polymorpha Research. Plant Cell Physiol. 2016, 57, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Kohchi, T.; Yamato, K.T.; Ishizaki, K.; Yamaoka, S.; Nishihama, R. Development and Molecular Genetics of Marchantia polymorpha. Annu. Rev. Plant Biol. 2021, 72, 677–702. [Google Scholar] [CrossRef]
- Ishizaki, K.; Chiyoda, S.; Yamato, K.T.; Kohchi, T. Agrobacterium-mediated transformation of the haploid liverwort Marchantia polymorpha L., an emerging model for plant biology. Plant Cell Physiol. 2008, 49, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Ishizaki, K.; Hosaka, M.; Kohchi, T. Efficient Agrobacterium-mediated transformation of the liverwort Marchantia polymorpha using regenerating thalli. Biosci. Biotechnol. Biochem. 2013, 77, 167–172. [Google Scholar] [CrossRef]
- Tsuboyama, S.; Nonaka, S.; Ezura, H.; Kodama, Y. Improved G-AgarTrap: A highly efficient transformation method for intact gemmalings of the liverwort Marchantia polymorpha. Sci. Rep. 2018, 8, 10800. [Google Scholar] [CrossRef]
- Iwakawa, H.; Melkonian, K.; Schlüter, T.; Jeon, H.-W.; Nishihama, R.; Motose, H.; Nakagami, H. Agrobacterium-mediated transient transformation of Marchantia liverworts. Plant Cell Physiol. 2021, 62, 1718–1727. [Google Scholar] [CrossRef]
- Takenaka, M.; Yamaoka, S.; Hanajiri, T.; Shimizu-Ueda, Y.; Yamato, K.T.; Fukuzawa, H.; Ohyama, K. Direct transformation and plant regeneration of the haploid liverwort Marchantia polymorpha L. Transgenic Res. 2000, 9, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Tsuboyama, S.; Kodama, Y. AgarTrap: A simplified Agrobacterium-mediated transformation method for sporelings of the liverwort Marchantia polymorpha L. Plant Cell Physiol. 2014, 55, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, Y.; Minamino, N.; Shimokawa, E.; Kawamura, S.; Komatsu, A.; Hiwatashi, T.; Nishihama, R.; Ueda, T.; Kohchi, T.; Kondo, Y. Harnessing deep learning to analyze cryptic morphological variability of Marchantia polymorpha. Plant Cell Physiol. 2023, 64, 1343–1355. [Google Scholar] [CrossRef]
- Guerrero, S.; Roces, V.; Garcia-Campa, L.; Valledor, L.; Meijon, M. Proteomic dynamics revealed sex-biased responses to combined heat-drought stress in Marchantia. J. Integr. Plant Biol. 2024, 66, 2226–2241. [Google Scholar] [CrossRef] [PubMed]
- Tsuboyama-Tanaka, S.; Kodama, Y. AgarTrap-mediated genetic transformation using intact gemmae/gemmalings of the liverwort Marchantia polymorpha L. J. Plant Res. 2015, 128, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Norizuki, T.; Minamino, N.; Sato, M.; Ueda, T. Autophagy regulates plastid reorganization during spermatogenesis in the liverwort Marchantia polymorpha. Front. Plant Sci. 2023, 14, 1101983. [Google Scholar] [CrossRef]
- de Necker, N.J. Physiologia Museorum; G.F. Schwan: Mannheim, Germany, 1774. [Google Scholar]
- Cavers, F. On asexual reproduction and regeneration in Hepaticae. New Phytol. 1903, 2, 121–133. [Google Scholar] [CrossRef]
- Ono, K.; Ohyama, K.; Gamborg, O.L. Regeneration of the liverwort Marchantia polymorpha L. From protoplasts isolated from cell suspension culture. Plant Sci. Lett. 1979, 14, 225–229. [Google Scholar] [CrossRef]
- Mori, K.; Matsushima, H.; Takeuchi, M. A study of cell wall regeneration of protoplasts in Marchantia polymorpha L. J. Plant Res. 1983, 96, 281–289. [Google Scholar] [CrossRef]
- Seo, D.H.; Kim, S.; Seo, H.J.; Lee, S.E.; Lim, C.J.; Yang, H.W.; Cui, L.H.; Ahn, M.Y.; Yu, S.G.; Cho, N.H.; et al. A simple protocol for thallus culture-based genetic transformation of the liverwort Marchantia polymorpha. J. Plant Biol. 2022, 65, 11–19. [Google Scholar] [CrossRef]
- Althoff, F.; Zachgo, S. Transformation of Riccia fluitans, an amphibious liverwort dynamically responding to environmental changes. Int. J. Mol. Sci. 2020, 21, 5410. [Google Scholar] [CrossRef] [PubMed]
- Frangedakis, E.; Waller, M.; Nishiyama, T.; Tsukaya, H.; Xu, X.; Yue, Y.; Tjahjadi, M.; Gunadi, A.; Van Eck, J.; Li, F.-W.; et al. An Agrobacterium-mediated stable transformation technique for the hornwort model Anthoceros agrestis. New Phytol. 2021, 232, 1488–1505. [Google Scholar] [CrossRef] [PubMed]
- Waller, M.; Frangedakis, E.; Marron, A.O.; Sauret-Gueto, S.; Rever, J.; Sabbagh, C.R.R.; Hibberd, J.M.; Haseloff, J.; Renzaglia, K.S.; Szovenyi, P. An optimized transformation protocol for Anthoceros agrestis and three more hornwort species. Plant J. 2023, 114, 699–718. [Google Scholar] [CrossRef]
- Okada, S.; Fujisawa, M.; Sone, T.; Nakayama, S.; Nishiyama, R.; Takenaka, M.; Yamaoka, S.; Sakaida, M.; Kono, K.; Takahama, M.; et al. Construction of male and female PAC genomic libraries suitable for identification of Y-chromosome-specific clones from the liverwort, Marchantia polymorpha. Plant J. 2000, 24, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, K.; Nishihama, R.; Ueda, M.; Inoue, K.; Ishida, S.; Nishimura, Y.; Shikanai, T.; Kohchi, T. Development of Gateway binary vector series with four different selection markers for the liverwort Marchantia polymorpha. PLoS ONE 2015, 10, e0138876. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Hayashi, K.; Nishio, T.; Bando, T.; Okada, S.; Yamato, K.T.; Fukuzawa, H.; Ohyama, K. Isolation of X and Y chromosome-specific DNA markers from a liverwort, Marchantia polymorpha, by representational difference analysis. Genetics 2001, 159, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Saint-Marcoux, D.; Proust, H.; Dolan, L.; Langdale, J.A. Identification of reference genes for real-time quantitative PCR experiments in the liverwort Marchantia polymorpha. PLoS ONE 2015, 10, e0118678. [Google Scholar] [CrossRef] [PubMed]
- Nishihama, R.; Ishizaki, K.; Hosaka, M.; Matsuda, Y.; Kubota, A.; Kohchi, T. Phytochrome-mediated regulation of cell division and growth during regeneration and sporeling development in the liverwort Marchantia polymorpha. J. Plant Res. 2015, 128, 407–421. [Google Scholar] [CrossRef]
- Tsuboyama-Tanaka, S.; Nonaka, S.; Kodama, Y. A highly efficient AgarTrap method for genetic transformation of mature thalli of the liverwort Marchantia polymorpha L. Plant Biotechnol. 2015, 32, 333–336. [Google Scholar] [CrossRef]
- Sugano, S.S.; Nishihama, R.; Shirakawa, M.; Takagi, J.; Matsuda, Y.; Ishida, S.; Shimada, T.; Hara-Nishimura, I.; Osakabe, K.; Kohchi, T. Efficient CRISPR/Cas9-based genome editing and its application to conditional genetic analysis in Marchantia polymorpha. PLoS ONE 2018, 13, e0205117. [Google Scholar] [CrossRef] [PubMed]

| Media and Durations 1,2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Starting Material | Plant Development (Duration) | Pre-Culture/ Regeneration (Duration) | Co-Culture (Duration) | Selection | Transformation Efficiency 3 | Estimated Number of Transformants per Experiment | Uniform Genetic Background | Advantage | Disadvantage | |
| Traditional | Ishizaki et al. 2008 [5] | Spore | - | M51C 4 liquid (5 d) | ------> (7 d) | M51C 4 solid | N.D. 5 (600–800 transformants per sporangium) | - | no | - | - |
| Kubota et al. 2013 [6] | Thallus | 1/2 B5 solid (2 w) | 1/2 B5 + sucrose solid (3 d) | M51C 4 liquid (3 d) | 1/2 B5 solid | 2.58 ± 0.33 | 26 transformants (when 10 plants are used with 1 flask and 1 selection plate) | yes | One of the most common and reliable methods; low residual Agrobacterium | Meristem removal and quartering; transferring at each stage; achieving high transformation efficiency requires M51C medium; requires a shaker. | |
| AgarTrap | Tsuboyama et al. 2014 [10] | Spore | - | M51C 4 solid (3 d) | ------> (3 d) | ------> | 0.16 ± 0.09 | - | no | - | - |
| Tsuboyama-Tanaka et al. 2015 [30] | Thallus | 1/2 B5 solid (1 m) | M51C 4 solid (0 d) | ------> (3 d) | ------> | 0.55 ± 0.05 6 | - | yes | - | - | |
| Tsuboyama et al. 2018 [7] | Gemma | - | 1/2 B5 + sucrose solid (2 d) | ------> (2 d) | ------> | 0.97 ± 0.05 7 | 49 transformants (when 50 gemmae in 1 plate are used) | yes | All steps completed on a B5 plate; shorter duration due to the use of gemmae. | Agrobacterium overgrowth; affected by humidity conditions; incompatible with gentamycin selection. | |
| Chopped-thallus | This study | Thallus | 1/2 B5 + sucrose solid (2 w) | 1/2 B5 + sucrose liquid (4 d) | ------> (3 d) | 1/2 B5 solid | 6.40 ± 0.64 | 64 transformants (when 10 plants are used with 1 flask and 3 selection plates) | yes | A single plant generates hundreds of fragments; no precise dissection required; scalable; B5 medium is sufficient; low residual Agrobacterium. | Requires a longer duration and additional chopping effort compared to the AgarTrap method; requires a shaker. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batth, R.; Poormassalehgoo, A.; Bhardwaj, K.; Kaniecka, E.; Goto-Yamada, S. A Simple and Scalable Chopped-Thallus Transformation Method for Marchantia polymorpha. Plants 2025, 14, 582. https://doi.org/10.3390/plants14040582
Batth R, Poormassalehgoo A, Bhardwaj K, Kaniecka E, Goto-Yamada S. A Simple and Scalable Chopped-Thallus Transformation Method for Marchantia polymorpha. Plants. 2025; 14(4):582. https://doi.org/10.3390/plants14040582
Chicago/Turabian StyleBatth, Rituraj, Andisheh Poormassalehgoo, Kritika Bhardwaj, Elżbieta Kaniecka, and Shino Goto-Yamada. 2025. "A Simple and Scalable Chopped-Thallus Transformation Method for Marchantia polymorpha" Plants 14, no. 4: 582. https://doi.org/10.3390/plants14040582
APA StyleBatth, R., Poormassalehgoo, A., Bhardwaj, K., Kaniecka, E., & Goto-Yamada, S. (2025). A Simple and Scalable Chopped-Thallus Transformation Method for Marchantia polymorpha. Plants, 14(4), 582. https://doi.org/10.3390/plants14040582






