Thinning Intensity Enhances Soil Multifunctionality and Microbial Residue Contributions to Organic Carbon Sequestration in Chinese Fir Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental Design and Soil Sample Collection
2.3. Soil Chemical Property Analysis
2.4. Soil Enzyme Activity Analysis
2.5. Soil Phospholipid Fatty Acid Analysis
2.6. Soil Ecosystem Multifunctionality Analysis
2.7. Soil Amino Sugar Analysis
2.8. Statistical Analysis
3. Results
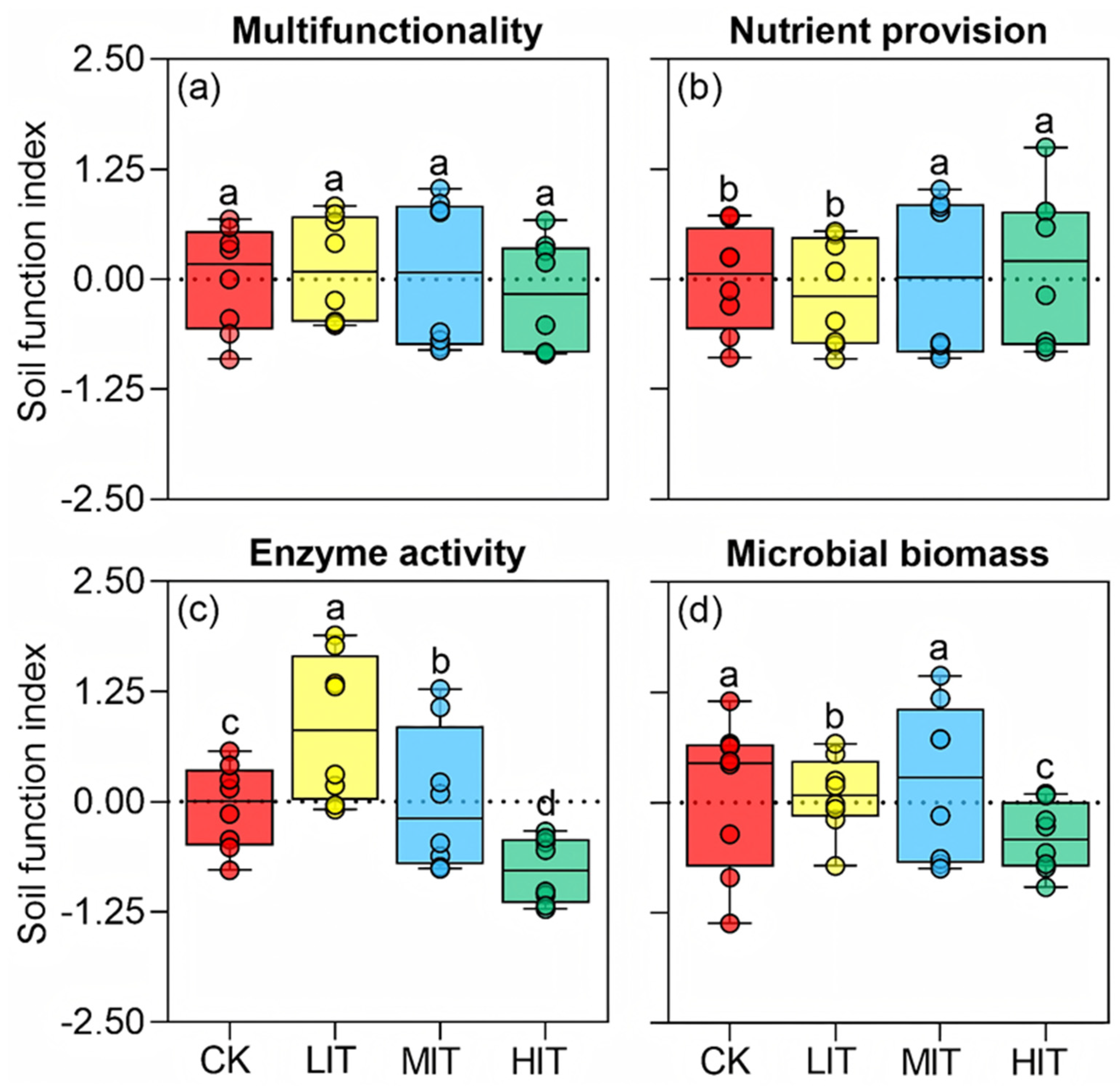
3.1. Soil Ecosystem Multifunctionality
3.2. Soil Phospholipid Fatty Acids (PLFAs)
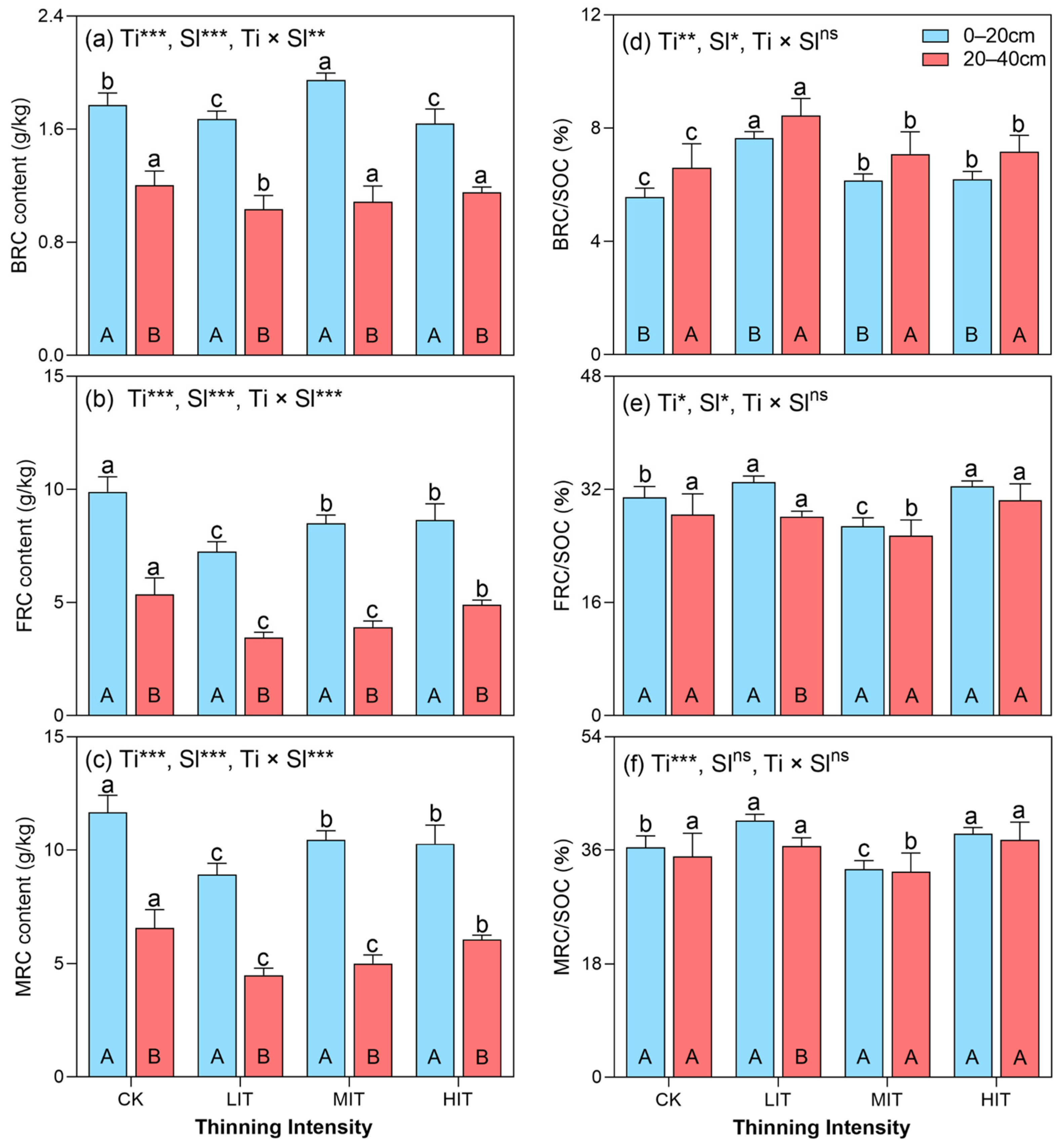
3.3. The Distributions of Soil BRC, FRC, and MRC Contents and Their Contribution to SOC
3.4. Correlation Between Microbial Residue Carbon and Soil Multifunctionality Factors
4. Discussion
4.1. Soil Multifunctionality Factors at Different Thinning Intensities
4.2. Vertical Distributions of Phospholipid Fatty Acid (PLFA) Contents Under Different Thinning Intensities in CFPs
4.3. Contribution of Soil MRC to SOC Accumulation and Its Driving Factors in Different Thinning Intensities
4.4. Correlation Relationships of Soil Biotic and Abiotic Factors with MRC Under Different Thinning Intensities of CFPs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manning, P.; van der Plas, F.; Soliveres, S.; Allan, E.; Maestre, F.T.; Mace, G.; Whittingham, M.J.; Fischer, M. Redefining ecosystem multifunctionality. Nat. Ecol. Evol. 2018, 2, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guan, D.; Li, W.; Sun, D.; Jin, C.; Yuan, F.; Wang, A.; Wu, J. The effects of forest thinning on soil carbon stocks and dynamics: A meta-analysis. For. Ecol. Manag. 2018, 429, 36–43. [Google Scholar] [CrossRef]
- Bravo-Oviedo, A.; Ruiz-Peinado, R.; Modrego, P.; Alonso, R.; Montero, G. Forest thinning impact on carbon stock and soil condition in Southern European populations of P. sylvestris L. For. Ecol. Manag. 2015, 357, 259–267. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, C.; Zhou, Z. Thinning promotes the nitrogen and phosphorous cycling in forest soils. Agric. For. Meteorol. 2021, 311, 108665. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Wang, Y.; Jiang, P.; Hu, Y.; Ouyang, S.; Wu, H.; Lei, P.; Kuzyakov, Y.; Xiang, W. Plantations thinning: A meta-analysis of consequences for soil properties and microbial functions. Sci. Total Environ. 2023, 877, 162894. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Qu, Q.; Xue, S.; Wang, M. A global analysis of the effects of forest thinning on soil N stocks and dynamics. Catena 2024, 246, 108411. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Intergovernmental Panel on Climate Change. 2023. Available online: https://www.ipcc.ch/report/ar6/syr/ (accessed on 1 May 2024).
- Wang, H.; Chen, D.; Wu, C.; Guo, L.; Sun, X.; Zhang, S. Forest thinning alleviates the negative effects of precipitation reduction on soil microbial diversity and multifunctnality. Biol. Fertil. Soils 2023, 59, 423–440. [Google Scholar] [CrossRef]
- Singh, J.S.; Gupta, V.K. Soil microbial biomass: A key soil driver in management of ecosystem functioning. Sci. Total Environ. 2018, 634, 497–500. [Google Scholar] [CrossRef]
- Liang, C.; Cheng, G.; Wixon, D.L.; Balser, T.C. An Absorbing Markov Chain approach to understanding the microbial role in soil carbon stabilization. Biogeochemistry 2011, 106, 303–309. [Google Scholar] [CrossRef]
- Shao, P.; Xie, H.; Bao, X.; Liang, C. Variation of Microbial Residues during Forest Secondary Succession in Topsoil and Subsoi. Acta Pedol. Sin. 2021, 58, 1050–1059. [Google Scholar]
- Mou, Z.J.; Kuang, L.H.; He, L.F.; Zhang, J.; Zhang, X.; Hui, D.; Li, Y.; Wu, W.; Mei, Q.; He, X.; et al. Climatic and edaphic controls over the elevational pattern of microbial necromass in subtropical forests. Catena 2021, 207, 105707. [Google Scholar] [CrossRef]
- Quanyi, H.; Liu, T.; Ding, H.; Li, C.; Tan, W.; Yu, M.; Liu, J.; Cao, C. Effects of Nitrogen Fertilizer on Soil Microbial Residues and Their Contribution to Soil Organic Carbon and Total Nitrogen in a Rice-Wheat System. Appl. Soil Ecol. 2023, 181, 104648. [Google Scholar]
- Bhople, P.; Keiblinger, K.; Djukic, I.; Liu, D.; Zehetner, F.; Zechmeister-Boltenstern, S.; Joergensen, R.G.; Murugan, R. Microbial necromass formation, enzyme activities, and community structure in two alpine elevation gradients with different bedrock types. Geoderma 2021, 386, 114922. [Google Scholar] [CrossRef]
- Tian, J.; Dungait, J.A.J.; Lu, X.; Yang, Y.; Hartley, I.P.; Zhang, W.; Mo, J.; Yu, G.; Zhou, J.; Kuzyakov, Y. Long-term nitrogen addition modifies microbial composition and functions for slow carbon cycling and increased sequestration in tropical forest soil. Glob. Change Biol. 2019, 25, 3267–3281. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, M.; Yu, C.; Zhang, H.; Yan, N.; Wu, Q.; Song, Y.; Li, X. Soil nutrients, enzyme activities, and microbial communities differ among biocrust types and soil layers in a degraded karst ecosystem. Catena 2022, 212, 106057. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kaestner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Shi, K.; Liao, J.; Zou, X.; Chen, H.Y.H.; Delgado-Baquerizo, M.; Yan, Z.; Ren, T.; Ruan, H. Accumulation of soil microbial extracellular and cellular residues during forest rewilding: Implications for soil carbon stabilization in older plantations. Soil Biol. Biochem. 2024, 188, 109250. [Google Scholar] [CrossRef]
- Xu, F.; Li, C.; Chen, Y.; Wu, J.; Bai, H.; Fan, S.; Yang, Y.; Zhang, Y.; Li, S.; Su, J. Soil microbial community structure and soil fertility jointly regulate soil microbial residue carbon during the conversion from subtropical primary forest to plantations. Geoderma 2024, 441, 116767. [Google Scholar] [CrossRef]
- Jing, Y.; Zhao, X.; Liu, S.; Tian, P.; Sun, Z.; Chen, L.; Wang, Q. Influence of tree species on soil microbial residue accumulation and distribution among soil aggregates in subtropical plantations of China. Ecol. Process. 2023, 12, 32. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, M.; Yu, Q.; Dun, X.; Xu, J.; Gao, P. The effect of thinning intensity on the soil carbon pool mediated by soil microbial communities and necromass carbon in coastal zone protected forests. Sci. Total Environ. 2023, 881, 163492. [Google Scholar] [CrossRef]
- Xu, H.; Gan, Q.; Huang, L.; Pan, X.; Liu, T.; Wang, R.; Wang, L.; Zhang, L.; Li, H.; Wang, L.; et al. Effects of forest thinning on soil microbial biomass and enzyme activity. CATENA 2024, 239, 107938. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, Y. Influence of thinning on carbon storage mediated by soil physicochemical properties and microbial community composition in large Chinese fir timber plantation. Carbon. Balance Manag. 2024, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dong, Q.; Yang, J.; Tan, B.; Xu, Z.; Wu, Q.; Xu, H. Effect of Forest Thinning on Soil Phosphorus Stocks and Dynamics on a Global Scale. Forests 2024, 15, 362. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Wang, L. Soil microbial functional diversity and biomass as affected by different thinning intensities in a Chinese fir plantation. Appl. Soil Ecol. 2015, 92, 35–44. [Google Scholar]
- Lull, C.; Bautista, I.; Lidón, A.; del Campo, A.D.; González-Sanchis, M.; García-Prats, A. Temporal effects of thinning on soil organic carbon pools, basal respiration and enzyme activities in a Mediterranean holm oak forest. For. Ecol. Manag. 2020, 464, 118088. [Google Scholar] [CrossRef]
- Cui, Z.W.; Peng, L.H.; Ma, D.X.; Wang, J.Q.; Jiang, X.Q.; Jiang, X.G.; Ma, X.Q.; Lin, K.M. Effects of thinning on soil microbial residue carbon in Chinese fir plantation. Sci. Silvae Sin. 2023, 59, 41–52. [Google Scholar]
- Huang, Y.; Liang, C.; Duan, X.; Chen, H.; Li, D. Variation of microbial residue contribution to soil organic carbon sequestration following land use change in a subtropical karst region. Geoderma 2019, 353, 340–346. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Hobbs, P.J.; Frostegard, A. Changes in soil fungal: Bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol. Fertil. Soils 1996, 22, 261–264. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 1 July 2024).
- Zhang, X.D.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Hu, H.; Qian, C.; Xue, K.; Liang, Y. Reducing the uncertainty in estimating soil microbial-derived carbon storage. Proc. Natl. Acad. Sci. USA 2024, 121, e2401916121. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Yu, J.; Li, J.; Shangguan, Z.; Deng, L. Thinning increases forest ecosystem carbon stocks. For. Ecol. Manag. 2024, 555, 121702. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, C.; Zhou, Z. Impacts of forest thinning on soil microbial community structure and extracellular enzyme activities: A global meta-analysis. Soil Biol. Biochem. 2020, 149, 107915. [Google Scholar] [CrossRef]
- Wu, R.; Cheng, X.; Han, H. The effect of forest thinning on soil microbial community structure and function. Forests 2019, 10, 352. [Google Scholar] [CrossRef]
- Teste, F.P.; Lieffers, V.J.; Strelkov, S.E. Ectomycorrhizal community responses to intensive forest management: Thinning alters impacts of fertilization. Plant Soil 2012, 360, 333–347. [Google Scholar] [CrossRef]
- Fox, J.F. Intermediate-disturbance hypothesis. Science 1979, 204, 1344–1345. [Google Scholar] [CrossRef]
- Williams, N.G.; Powers, M.D. Carbon storage implications of active management in mature Pseudotsuga menziesii forests of western Oregon. For. Ecol. Manag. 2019, 432, 761–775. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Z.; Zhong, Z.; Li, Q.; Bian, F. Forest management alters soil microbial necromass and its contribution to soil organic carbon in Moso bamboo plantations in subtropical China. Appl. Soil Ecol. 2024, 196, 105320. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H.; Chen, X.; Wang, J.; Chen, B.; Wang, D.; Guan, Q. Soil labile organic carbon and carbon-cycle enzyme activities under different thinning intensities in Chinese fir plantations. Appl. Soil Ecol. 2016, 107, 162–169. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, K.; Song, C.; Cui, C.; Peng, L.; Zheng, H.; Zheng, M.; Ren, Z.; Qiu, M. Short-term effects of thinning on carbon storage in Chinese fir plantation. J. Nanjing For. Univ. 2022, 46, 65–73. [Google Scholar]
- Yu, Y.; Zhang, X.; Dai, X.; Lü, S.; Yang, Y.; Shi, L. Carbon distribution and influencing factors of microbial residues in forest soil profile in subtropical red soil region. Acta Ecol. Sin. 2022, 42, 1108–1117. [Google Scholar]
- Gong, C.; Tan, Q.; Liu, G.; Xu, M. Forest thinning increases soil carbon stocks in China. For. Ecol. Manag. 2021, 482, 118812. [Google Scholar] [CrossRef]
- Mazza, G.; Agnelli, A.E.; Cantiani, P.; Chiavetta, U.; Doukalianou, F.; Kitikidou, K.; Milios, E.; Orfanoudakis, M.; Radoglou, K.; Lagomarsino, A. Short-term effects of thinning on soil CO2, N2O and CH4 fluxes in Mediterranean forest ecosystems. Sci. Total Environ. 2019, 651, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, A.D.; Otsuki, K.; Serengil, Y.; Blanco, J.A.; Yousefpour, R.; Wei, X. A global synthesis on the effects of thinning on hydrological processes: Implications for forest management. For. Ecol. Manag. 2022, 519, 120324. [Google Scholar] [CrossRef]
- Yao, L.; Wu, C.; Jiang, B.; Wu, M.; Shao, X.; Li, N. Thinning alters nitrogen transformation processes in subtropical forest soil: Key roles of physicochemical properties. Sci. Total Environ. 2024, 949, 175086. [Google Scholar] [CrossRef] [PubMed]
- Oorthuis, R.; Vaunat, J.; Hürlimann, M.; Lloret, A.; Moya, J.; Puig-Polo, C.; Fraccica, A. Slope Orientation and Vegetation Effects on Soil Thermo-Hydraulic Behavior. An Experimental Study. Sustainability 2021, 13, 14. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Baldocchi, D.D.; Gershenson, A.; Goldstein, A.; Misson, L.; Wong, S. Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Glob. Change Biol. 2007, 13, 2018–2035. [Google Scholar] [CrossRef]
- Liu, K.-L.; Chen, B.-Y.; Zhang, B.; Wang, R.-H.; Wang, C.-S. Understory vegetation diversity, soil properties and microbial community response to different thinning intensities in Cryptomeria japonica var. sinensis plantations. Front. Microbiol. 2023, 14, 1117384. [Google Scholar] [CrossRef] [PubMed]
- Mushinski, R.M.; Gentry, T.J.; Dorosky, R.J.; Boutton, T.W. Forest harvest intensity and soil depth alter inorganic nitrogen pool sizes and ammonia oxidizer community composition. Soil Biol. Biochem. 2017, 112, 216–227. [Google Scholar] [CrossRef]
- Ye, Y.; Sun, X.; Zhao, J.; Chen, X.; Wang, M.; Li, J.; Guan, Q. Thinning alters the network patterns and keystone taxa of rhizosphere soil microbial communities in Chinese fir plantation. Appl. Soil Ecol. 2023, 189, 104956. [Google Scholar] [CrossRef]
- Nath, P.C.; Sileshi, G.W.; Ray, P.; Das, A.K.; Nath, A.J. Variations in soil properties and stoichiometric ratios with stand age under agarwood monoculture and polyculture on smallholder farms. Catena 2022, 213, 106174. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Nierop, K.G.; Simpson, M.J. Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Zarafshar, M.; Vincent, G.; Korboulewsky, N.; Bazot, S. The impact of stand composition and tree density on topsoil characteristics and soil microbial activities. CATENA 2024, 234, 107541. [Google Scholar] [CrossRef]
- Schwab, P.; Grebner, D.; Walters, S. Impact of thinning on forest soil health and productivity. J. For. Sci. 2012, 58, 104–112. [Google Scholar]



| Thinning Intensity | Altitude (m) | Slope Aspect | Gradient (°) | Mean DBH (m) | Average Tree Height (m) | Reserved Density | Canopy Density |
|---|---|---|---|---|---|---|---|
| CK | 204 | W | 25 | 17.4 | 14.3 | 1353 ± 11.0 | 0.9 |
| 186 | S | 23 | 18 | 14.2 | |||
| 188 | SW | 24 | 18.3 | 14.5 | |||
| LIT | 190 | S | 26 | 19.4 | 14.3 | 1082 ± 7.2 | 0.8 |
| 209 | SW | 24 | 18.8 | 14.0 | |||
| 189 | S | 24 | 19.6 | 14.3 | |||
| MIT | 193 | NW | 25 | 20.1 | 14.6 | 947 ± 5.7 | 0.75 |
| 195 | N | 26 | 19.9 | 14.5 | |||
| 190 | S | 24 | 20.3 | 14.4 | |||
| HIT | 231 | SE | 25 | 21.3 | 15.2 | 750 ± 6.2 | 0.68 |
| 201 | W | 24 | 20.9 | 15.1 | |||
| 186 | SW | 24 | 20.2 | 14.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Lei, J.; Peng, Y.; Wang, R.; Chen, X.; Liu, Z.; Gao, X.; Dang, P.; Yan, W. Thinning Intensity Enhances Soil Multifunctionality and Microbial Residue Contributions to Organic Carbon Sequestration in Chinese Fir Plantations. Plants 2025, 14, 579. https://doi.org/10.3390/plants14040579
He T, Lei J, Peng Y, Wang R, Chen X, Liu Z, Gao X, Dang P, Yan W. Thinning Intensity Enhances Soil Multifunctionality and Microbial Residue Contributions to Organic Carbon Sequestration in Chinese Fir Plantations. Plants. 2025; 14(4):579. https://doi.org/10.3390/plants14040579
Chicago/Turabian StyleHe, Ting, Junjie Lei, Yuanying Peng, Ruihui Wang, Xiaoyong Chen, Zongxin Liu, Xiaoqian Gao, Peng Dang, and Wende Yan. 2025. "Thinning Intensity Enhances Soil Multifunctionality and Microbial Residue Contributions to Organic Carbon Sequestration in Chinese Fir Plantations" Plants 14, no. 4: 579. https://doi.org/10.3390/plants14040579
APA StyleHe, T., Lei, J., Peng, Y., Wang, R., Chen, X., Liu, Z., Gao, X., Dang, P., & Yan, W. (2025). Thinning Intensity Enhances Soil Multifunctionality and Microbial Residue Contributions to Organic Carbon Sequestration in Chinese Fir Plantations. Plants, 14(4), 579. https://doi.org/10.3390/plants14040579






