Effects of Boron on the Emergence and Allocation of Metabolic Compounds in Two Herbaceous Cotton Cultivars
Abstract
1. Introduction
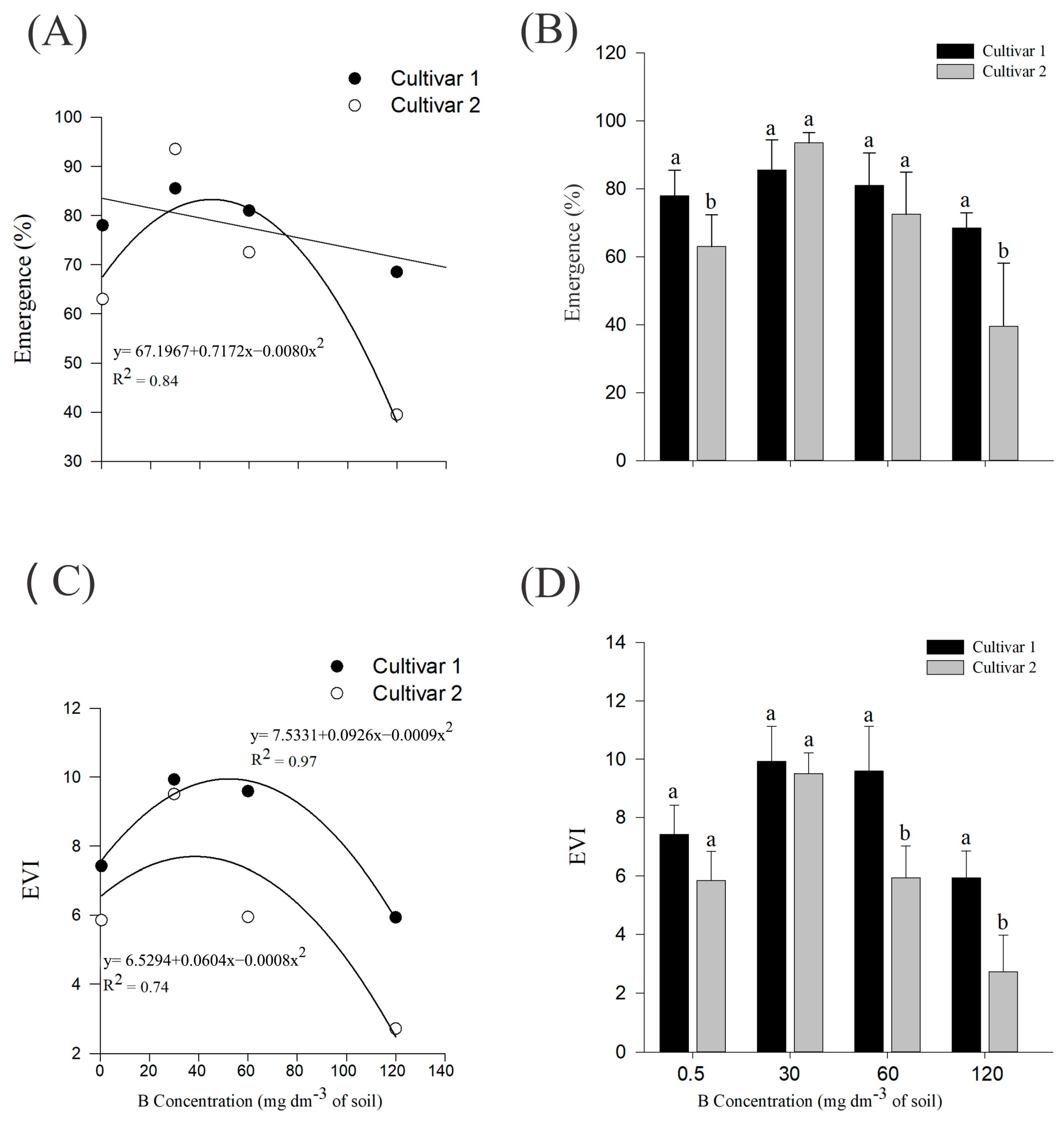
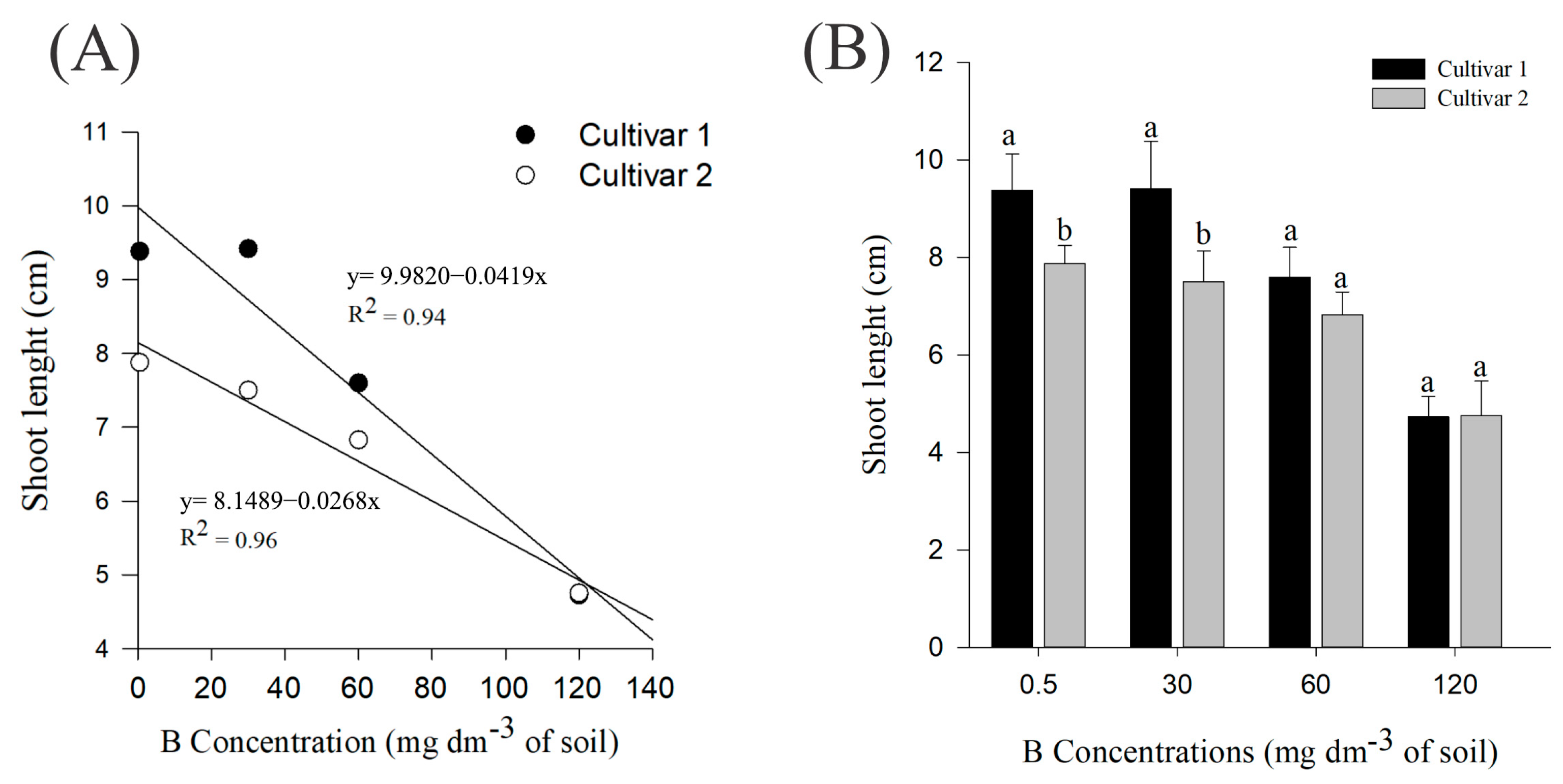
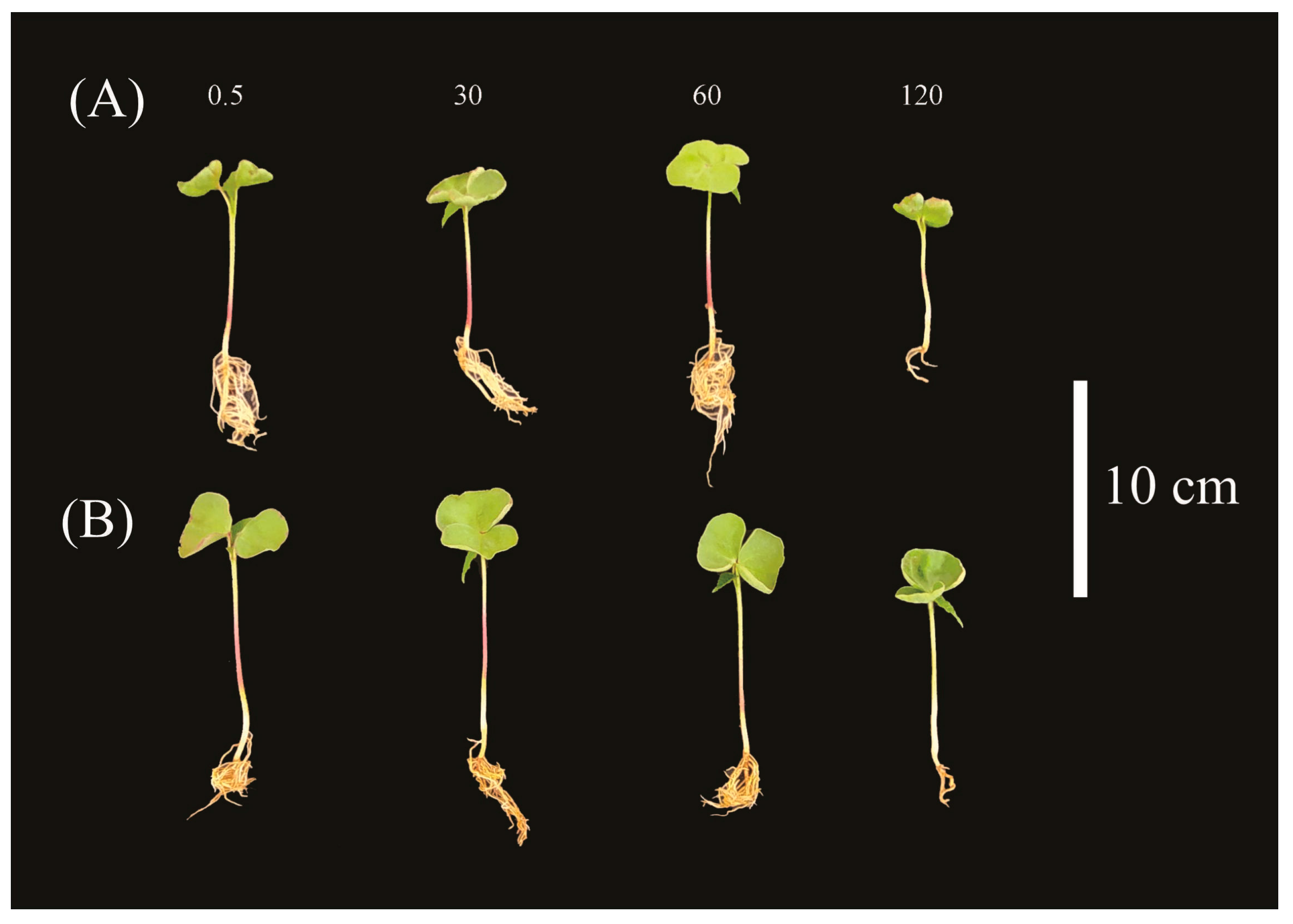
2. Results
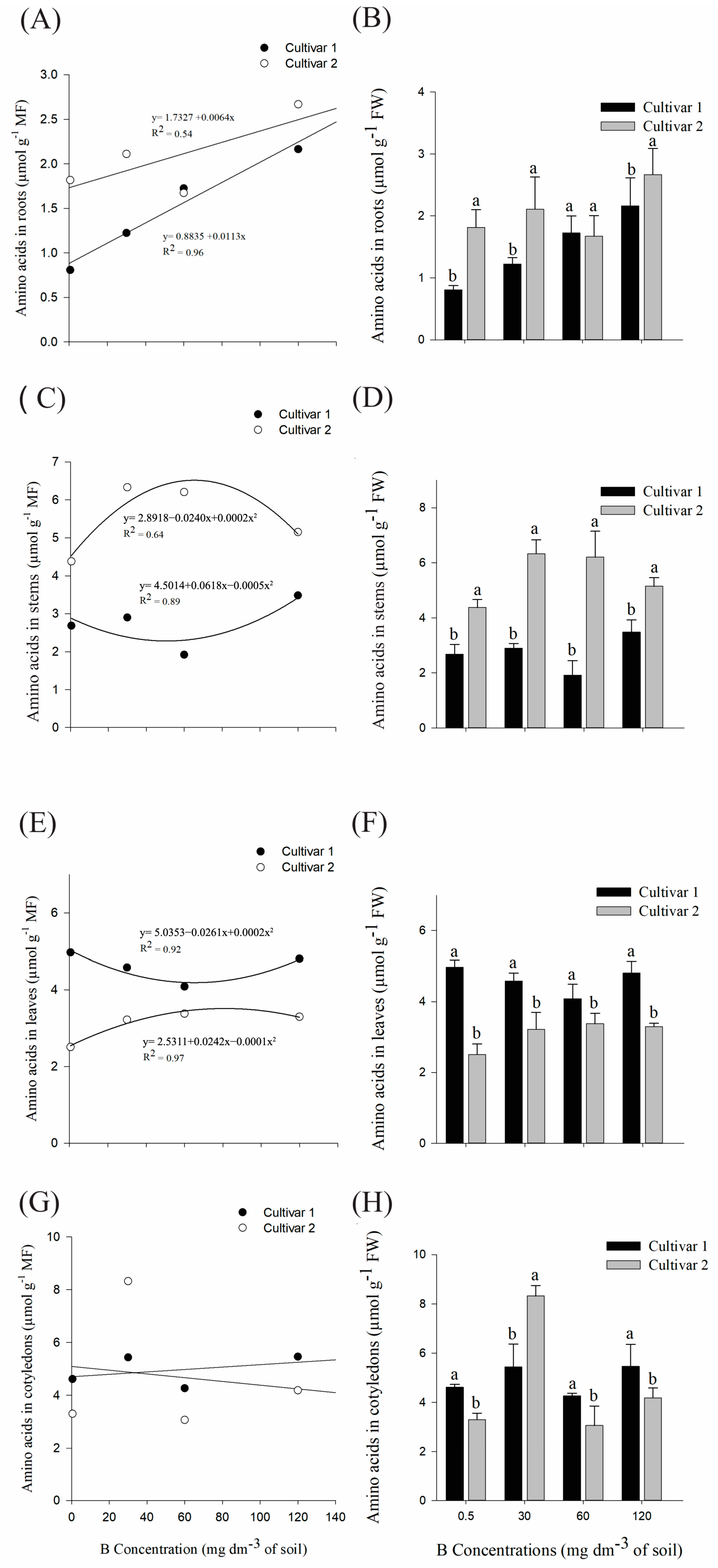
2.1. Physiological Analysis: Quantification of Amino Acids
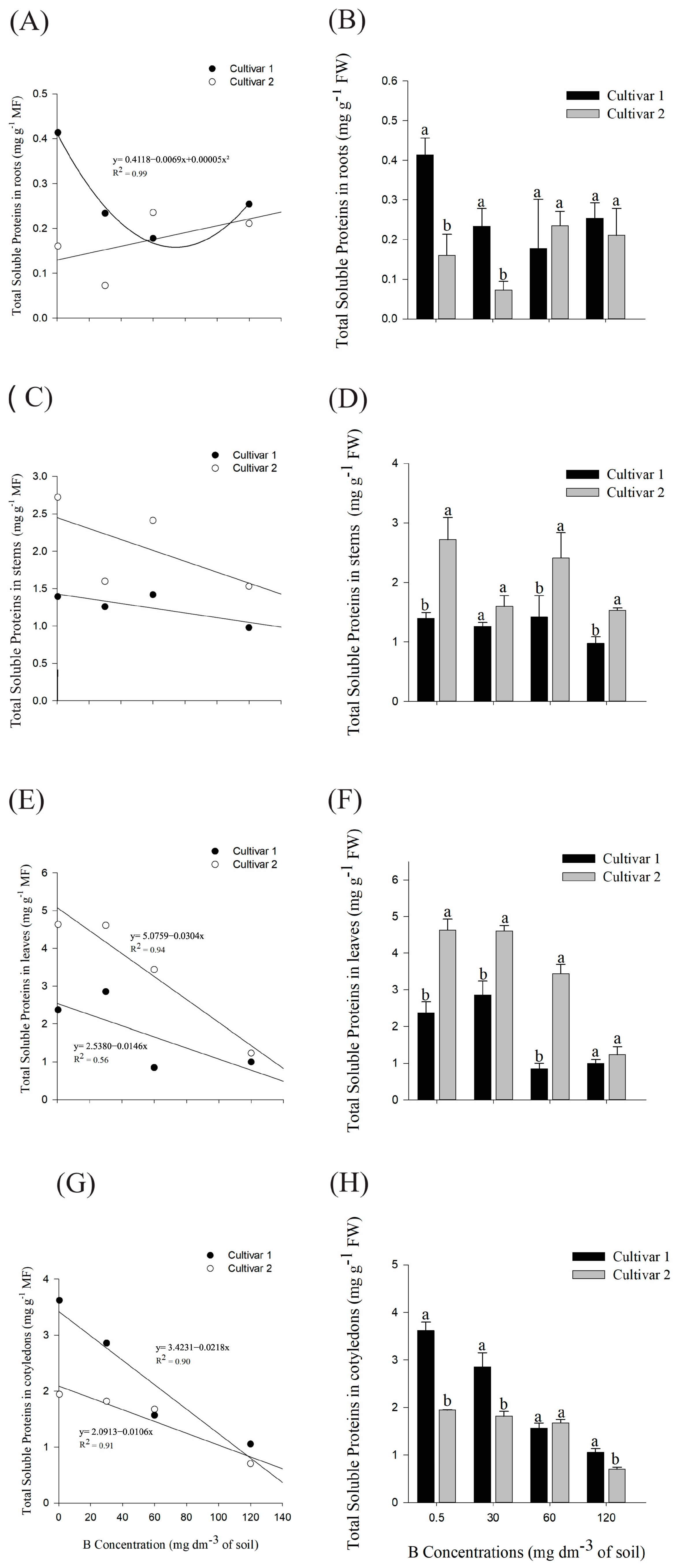
2.1.1. Physiological Analysis: Quantification of Total Soluble Proteins
2.1.2. Physiological Analysis: Quantification of Phenolic Compounds
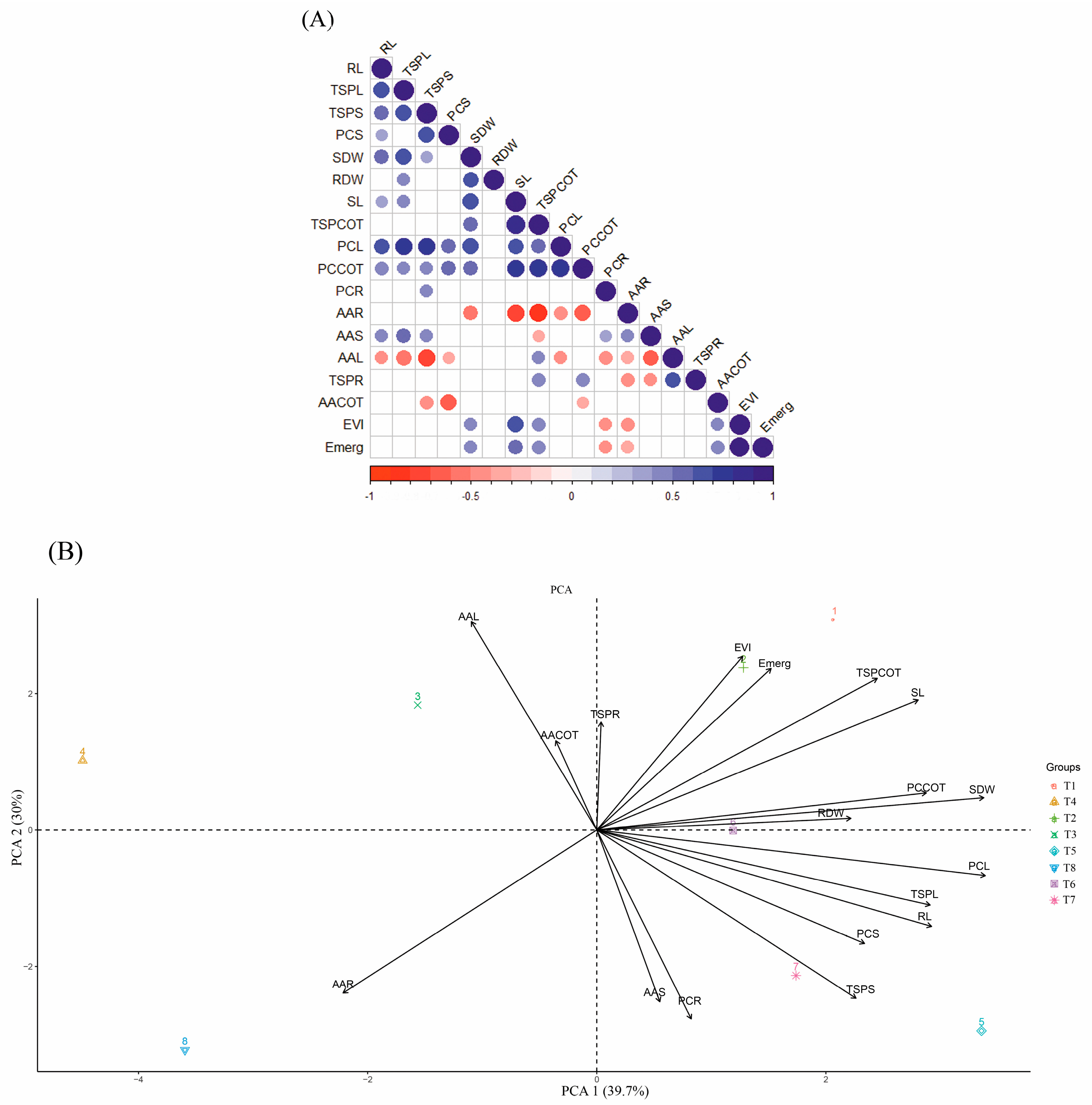
2.2. Pearson Correlation and Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Soil Preparation
4.2. Emergence Evaluations
4.3. Partitioning and Allocation of Metabolic Compounds
4.4. Extraction of Soluble Compounds
4.5. Quantification of Total Soluble Amino Acids
4.6. Quantification of Phenolic Compounds
4.7. Quantification of Total Soluble Proteins
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Şahin, F.I.; Acarali, N. A survey on application areas and advanced materials for boron minerals. In Current Research in Engineering; Bardak, S., Ayata, Ü., Eds.; Gece Publishing: Ankara, Turkey, 2023; pp. 29–44. ISBN 9786254307201. [Google Scholar]
- García-Sánchez, F.; Simón-Grao, S.; Martínez-Nicolás, J.J.; Alfosea-Simón, M.; Liu, C.; Chatzissavvidis, C.; Pérez-Pérez, J.G.; Cámara-Zapata, J.M. Multiple Stresses Occurring with Boron Toxicity and Deficiency in Plants. J. Hazard. Mater. 2020, 397, 122713. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.F.d.S.; Cordeiro, C.F.d.S.; Ferrari, S. Cotton Yield and Boron Dynamics Affected by Cover Crops and Boron Fertilization in a Tropical Sandy Soil. Field Crops Res. 2022, 284, 108575. [Google Scholar] [CrossRef]
- Alwan, A.H.; Al–Rubaee, S.M.; Jassim, A.H. The Influence of Ethephon and Boron on Endogenous Hormones of Two Genotypes of Summer Squash. AIP Conf. Proc. 2023, 2414, 020005. [Google Scholar] [CrossRef]
- Brdar-Jokanović, M. Boron Toxicity and Deficiency in Agricultural Plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef]
- Princi, M.P.; Lupini, A.; Araniti, F.; Longo, C.; Mauceri, A.; Sunseri, F.; Abenavoli, M.R. Chapter 5—Boron Toxicity and Tolerance in Plants: Recent Advances and Future Perspectives. In Plant Metal Interaction; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 115–147. ISBN 978-0-12-803158-2. [Google Scholar]
- Vega, M.; Quintero-Corrales, C.; Mastretta-Yanes, A.; Casas, A.; López-Hilario, V.; Wegier, A. Multiple Domestication Events Explain the Origin of Gossypium Hirsutum Landraces in Mexico. Ecol. Evol. 2023, 13, e9838. [Google Scholar] [CrossRef]
- Alves, F.A.L.; de S. Cavalcante, F.; de Oliveira-Júnior, I.S.; Ferraz, I.; Silva, S.M.S. Competição de variedades de algodão herbáceo para cultivo no agreste pernambucano. Pesqui. Agropecu. Pernambucana 2019, 24, e1971242019. [Google Scholar] [CrossRef]
- Constable, G.A.; Bange, M.P. The Yield Potential of Cotton (Gossypium hirsutum L.). Field Crops Res. 2015, 182, 98–106. [Google Scholar] [CrossRef]
- da Silva, R.P.; Nuñez, D.N.C. Manejos sobre fitopatógenos e doses de Boro na cultura da soja. Braz. J. Sci. 2023, 2, 87–97. [Google Scholar] [CrossRef]
- Corteva. Guia de uso do Produto. 2020. Available online: https://www.corteva.com.br/content/dam/dpagco/corteva/la/br/pt/files/Guia_de_uso_da_tecnologia%20WS3.pdf (accessed on 19 January 2025).
- BASF. Tecnologias Fibermax. Boletim Tecnico. 2018. Available online: https://agriculture.basf.com/dam/jcr:8d32acbf-68f9-4a6e-a9e4-8d683e35fc3f/basf/agriculture/br/assets/guia-variedade-fm-2023/BASF_BOLETIM_GLTP.pdf (accessed on 19 January 2025).
- Marcos Filho, J. Seed Vigor Testing: An Overview of the Past, Present and Future Perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Zini, P.; Fantinel, V.; Poletto, T.; Menegaes, J. Patologia de Sementes Conceitos e Aplicações: Uma Revisão de Literatura. In Sementes: Foco em Pesquisa Sobre Qualidade Fisiológica e Sanitária; Menegaes, J.M., Nunes, U.R., Eds.; Pantanal Editora: Nova Xavantina, Brasil, 2021; pp. 117–133. ISBN 9786588319437. [Google Scholar]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed Germination Test for Toxicity Evaluation of Compost: Its Roles, Problems and Prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Iqbal, S. Boron Biofortification through Seed Coating: A Way to Alleviate Malnutrition and to Improve Grain Yield of Bread Wheat. Pak. J. Agric. Sci. 2021, 58, 1501–1509. [Google Scholar] [CrossRef]
- Day, S.; Aasim, M. Role of Boron in Growth and Development of Plant: Deficiency and Toxicity Perspective. In Plant Micronutrients; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2020; pp. 435–453. ISBN 978-3-030-49855-9. [Google Scholar]
- Kaya, M.D.; Ergin, N. Boron Seed Treatments Induce Germination and Seedling Growth by Reducing Seed-Borne Pathogens in Safflower (Carthamus tinctorius L.). J. Plant Prot. Res. 2023, 63, 481–487. [Google Scholar] [CrossRef]
- Paull, J.G.; Cartwright, B.; Rathjen, A.J. Responses of Wheat and Barley Genotypes to Toxic Concentrations of Soil Boron. Euphytica 1988, 39, 137–144. [Google Scholar] [CrossRef]
- Day, S.; Çikili, Y.; Aasim, M. Screening of Three Safflower (Carthamus tinctorius L.) Cultivars under Boron Stress. Acta Sci. Polonorum. Hortorum Cultus = Ogrod. 2017, 16, 109–116. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; El-Esawi, M.A.; Hussain, S.; Wang, X. Boron-Toxicity Induced Changes in Cell Wall Components, Boron Forms, and Antioxidant Defense System in Rice Seedlings. Ecotoxicol. Environ. Saf. 2021, 216, 112192. [Google Scholar] [CrossRef]
- Martínez-Cuenca, M.-R.; Martínez-Alcántara, B.; Quiñones, A.; Ruiz, M.; Iglesias, D.J.; Primo-Millo, E.; Forner-Giner, M.Á. Physiological and Molecular Responses to Excess Boron in Citrus Macrophylla W. PLoS ONE 2015, 10, e0134372. [Google Scholar] [CrossRef]
- Reid, R.J.; Hayes, J.E.; Post, A.; Stangoulis, J.C.R.; Graham, R.D. A Critical Analysis of the Causes of Boron Toxicity in Plants. Plant Cell Environ. 2004, 27, 1405–1414. [Google Scholar] [CrossRef]
- Long, Y.; Peng, J. Interaction between Boron and Other Elements in Plants. Genes 2023, 14, 130. [Google Scholar] [CrossRef]
- Pratelli, R.; Pilot, G. Regulation of Amino Acid Metabolic Enzymes and Transporters in Plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.-P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Galili, G.; Avin-Wittenberg, T.; Angelovici, R.; Fernie, A.R. The Role of Photosynthesis and Amino Acid Metabolism in the Energy Status during Seed Development. Front. Plant Sci. 2014, 5, 447. [Google Scholar] [CrossRef]
- Moe, L.A. Amino Acids in the Rhizosphere: From Plants to Microbes. Am. J. Bot. 2013, 100, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential Distribution of Amino Acids in Plants. Amino Acids 2017, 49, 821–869. [Google Scholar] [CrossRef]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of Plant Proteins to Heavy Metal Stress—A Review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef]
- Goldberg, A.L. Protein Degradation and Protection against Misfolded or Damaged Proteins. Nature 2003, 426, 895–899. [Google Scholar] [CrossRef]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy Metals and Metalloids As a Cause for Protein Misfolding and Aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef]
- Silva, M.L.C.; Costa, R.S.; Santana, A.D.S.; Koblitz, M.G.B. Compostos fenólicos, carotenóides e atividade antioxidante em produtos vegetais. Semin. Ciênc. Agrár. 2010, 31, 669. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Anzellotti, D.; González-Fontes, A. Changes in Phenolic Metabolism of Tobacco Plants during Short-Term Boron Deficiency. Plant Physiol. Biochem. 2002, 40, 997–1002. [Google Scholar] [CrossRef]
- Heidarabadi, M.D.; Ghanati, F.; Fujiwara, T. Interaction between Boron and Aluminum and Their Effects on Phenolic Metabolism of Linum usitatissimum L. Roots. Plant Physiol. Biochem. 2011, 49, 1377–1383. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Sumbele, S.; Fotelli, M.N.; Nikolopoulos, D.; Tooulakou, G.; Liakoura, V.; Liakapoulos, G.; Bresta, P.; Dotsika, E.; Adams, M.A.; Karabourniotis, G. Photosynthetic capacity is negatively correlated with the concentration of leaf phenolic compounds across a range of different species. AoB Plants 2012, 2012, pls025. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.N.; Rosalem, P.F.; de Araujo, M.A.; Ferreira, T.C.; Martins, A.R.; de Camargos, L.S. Anatomical changes caused by boron in Calopogonium mucunoides Desv. Braz. J. Bot. 2023, 46, 127–137. [Google Scholar] [CrossRef]
- de Mendonça, G.W.; Aguilar, J.V.; Lapaz, A.M.; de Paiva, W.S.; Ferreira, T.C.; Souza, L.A.; Justino, G.C.; de Camargos, L.S. How does boron modify the growth of Crotalaria juncea? A legume with phytostabilizing potential. Int. J. Environ. Stud. 2024, 81, 1918–1932. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Quaggio, J.A.; Cantarella, H.; Silva, N.M.; Carvalho, L.H. Algodão. In Recomendações de Adubação e Calagem para o Estado de São Paulo—B100; Cantarella, H., Quaggio, J.A., Mattos, D., Jr., Boaretto, R.M., Raij, B., Eds.; Instituto Agronômico de Campinas: Campinas, Brasil, 2022; Volume 1, pp. 160–164. [Google Scholar]
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, DF, Brasil, 2018; 356p, ISBN 978-85-7035-800-4. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, DF, Brasil, 2017; 574p, ISBN 978-85-7035-771-7. [Google Scholar]
- Van Raij, B.; de Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brasil, 2001; 285p, ISBN 85-85564-05-9. [Google Scholar]
- Lima, L.R.; Silva, H.F.; Brignoni, A.S.; Silva, F.G.; Camargos, L.S.; Souza, L.A. Characterization of Biomass Sorghum for Copper Phytoremediation: Photosynthetic Response and Possibility as a Bioenergy Feedstock from Contaminated Land. Physiol. Mol. Biol. Plants 2019, 25, 433–441. [Google Scholar] [CrossRef]
- Brasil. Regras Para Análise de Sementes; Mapa: Brasília, DF, Brasil, 2009; 399p, ISBN 978-85-99851-70-8. [Google Scholar]
- Balbinot, A.A., Jr.; Fleck, N.G.; Agostinetto, D.; Rizzardi, M.A.; Merotto, A., Jr.; Vidal, R.A. Velocidade de emergência e crescimento inicial de cultivares de arroz irrigado influenciando a competitividade com as plantas daninhas. Planta Daninha 2001, 19, 305–316. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of Germination—Aid In Selection And Evaluation for Seedling Emergence And Vigor. Crop Sci. 1962, 2. [Google Scholar] [CrossRef]
- Bieleski, R.L.; Turner, N.A. Separation and Estimation of Amino Acids in Crude Plant Extracts by Thin-Layer Electrophoresis and Chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The Determination of Amino-Acids with Ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophane Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| B Concentrations mg dm−3 B | Cultivar 1 | Cultivar 2 | ||
|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |
| 0.5 | 5.390 | 27.157 | 5.612 | 27.157 |
| 30 | 14.615 | 16.020 | 16.722 | 15.930 |
| 60 | 16.364 | 18.525 | 8.072 | 13.939 |
| 120 | 30.563 | 20.421 | 23.069 | 17.155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, R.P.; de Araujo, M.A.; Longhi, L.B.; de Carvalho, I.F.; Cozin, B.B.; de Camargos, L.S. Effects of Boron on the Emergence and Allocation of Metabolic Compounds in Two Herbaceous Cotton Cultivars. Plants 2025, 14, 576. https://doi.org/10.3390/plants14040576
de Souza RP, de Araujo MA, Longhi LB, de Carvalho IF, Cozin BB, de Camargos LS. Effects of Boron on the Emergence and Allocation of Metabolic Compounds in Two Herbaceous Cotton Cultivars. Plants. 2025; 14(4):576. https://doi.org/10.3390/plants14040576
Chicago/Turabian Stylede Souza, Roberta Possas, Maycon Anderson de Araujo, Lucas Baltazar Longhi, Isabella Fiorini de Carvalho, Bruno Bonadio Cozin, and Liliane Santos de Camargos. 2025. "Effects of Boron on the Emergence and Allocation of Metabolic Compounds in Two Herbaceous Cotton Cultivars" Plants 14, no. 4: 576. https://doi.org/10.3390/plants14040576
APA Stylede Souza, R. P., de Araujo, M. A., Longhi, L. B., de Carvalho, I. F., Cozin, B. B., & de Camargos, L. S. (2025). Effects of Boron on the Emergence and Allocation of Metabolic Compounds in Two Herbaceous Cotton Cultivars. Plants, 14(4), 576. https://doi.org/10.3390/plants14040576







