Interclonal Variation in Heavy Metal Accumulation Among Poplar and Willow Clones: Implications for Phytoremediation of Contaminated Landfill Soils
Abstract
1. Introduction
2. Results
2.1. Substrate Characteristics
2.2. Analysis of Plant Material
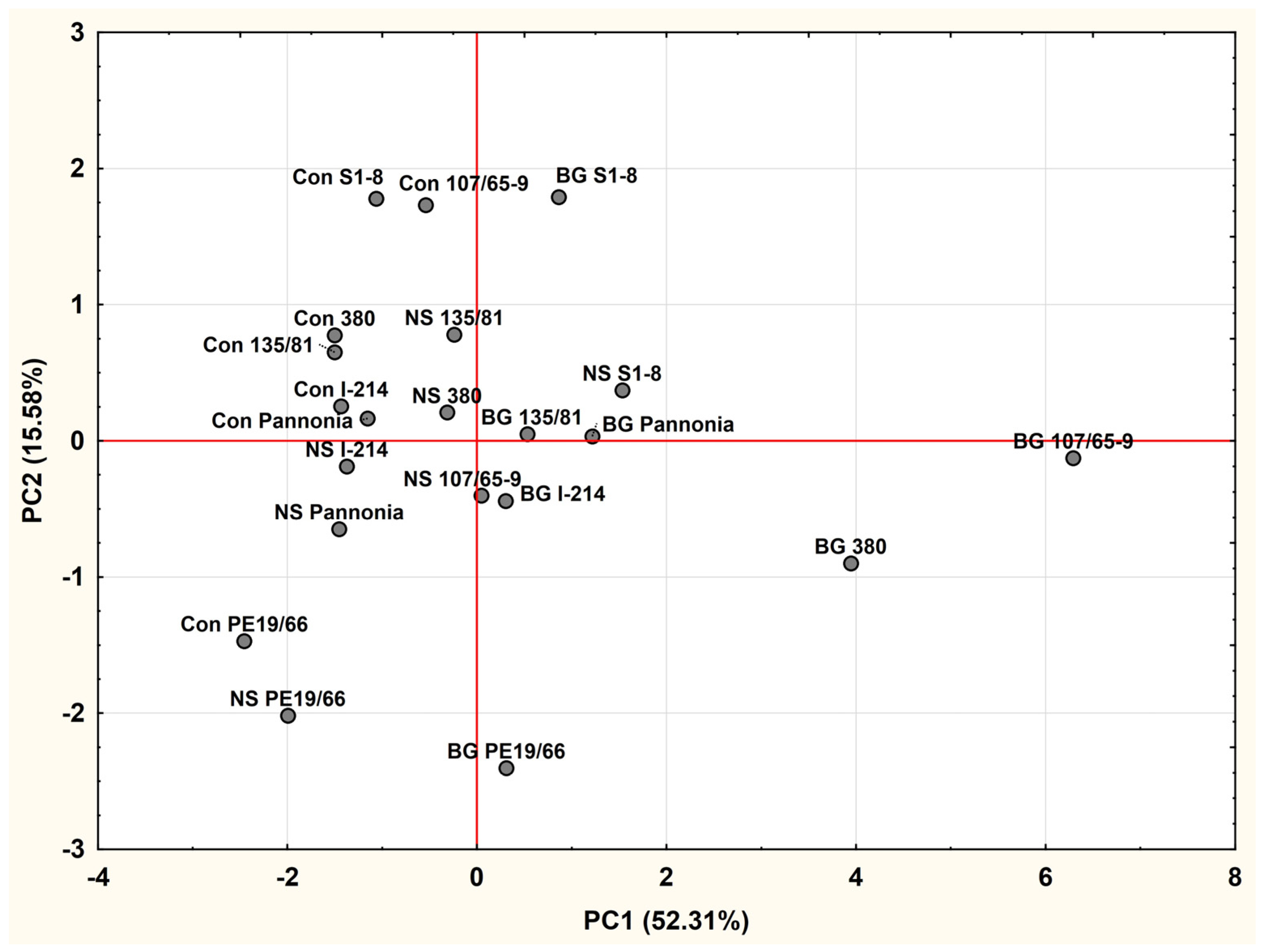
2.3. Principal Component Analysis
2.4. Bioavailability
3. Discussion
4. Materials and Methods
4.1. Location of Soil Material
4.2. Granulometric Composition and Chemical Properties of Substrates
4.3. Heavy Metal Content Analysis
4.4. Plant Material
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.K.; Tripathi, D.K.; et al. Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front. Environ. Sci. 2016, 4, 69. [Google Scholar] [CrossRef]
- Mehes-Smith, M.; Nkongolo, K.; Cholew, E. Coping mechanisms of plants to metal contaminated soil. In Environmental Change and Sustainability; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum Springer: Basel, Switzerland, 2012; Volume 101. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Vaverková, M.D. Landfill impacts on the environment—Review. Geosciences 2019, 9, 431. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Z. A comprehensive review of landfill leachate treatment technologies. Front. Environ. Sci. 2024, 12, 1439128. [Google Scholar] [CrossRef]
- Zalesny, R.S.; Bauer, E.O. Selecting and utilizing Populus and Salix for landfill covers: Implications for leachate irrigation. Int. J. Phytoremediat. 2007, 9, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Kesić, L.; Kovačević, B.; Milović, M.; Poljaković-Pajnik, L.; Pekeč, S.; Orlović, S. Physiological responses of poplar and willow clones grown in pot trails on soil s from landfills. Topola/Poplar 2024, 213, 55–63. [Google Scholar] [CrossRef]
- Guidi Nissim, W.; Castiglione, S.; Guarino, F.; Pastore, M.C.; Labra, M. Beyond cleansing: Ecosystem services related to phytoremediation. Plants 2023, 12, 1031. [Google Scholar] [CrossRef]
- Pajević, S.; Borišev, M.; Nikolić, N.; Arsenov, D.D.; Orlović, S.; Župunski, M. Phytoextraction of heavy metals by fast-growing trees: A review. In Phytoremediation: Management of Environmental Contaminants; Ansari, A., Gill, S., Gill, R., Lanza, G., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 3, pp. 29–64. [Google Scholar] [CrossRef]
- Pilipović, A.; Orlović, S.; Trudić, B.; Katanić, M.; Vasić, V.; Kebert, M. Testing of poplar (Populus sp.) and willow (Salix sp.) for herbicide phytoremediation through investigation on the effect on their physiological parameters. Topola/Poplar 2016, 197/198, 35. [Google Scholar]
- Štrbac, M.; Manojlović, M.; Čabilovski, R.; Petković, K.; Kovačević, D.; Pilipović, A. Woody plants in phytoremediation of pollution of agricultural land with nitrates and pesticides. Topola/Poplar 2022, 210, 73–87. [Google Scholar] [CrossRef]
- Pilipović, A.; Zalesny, R.S., Jr.; Rončević, S.; Nikolić, N.; Orlović, S.; Beljin, J.; Katanić, M. Growth, physiology, and phytoextraction potential of poplar and willow established in soils amended with heavy-metal contaminated, dredged river sediments. J. Environ. Manag. 2019, 239, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Pilipović, A.; Zalesny, R.S., Jr.; Orlović, S.; Drekić, M.; Pekeč, S.; Katanić, M.; Poljakovic-Pajnik, L. Growth and physiological responses of three poplar clones grown on soils artificially contaminated with heavy metals, diesel fuel, and herbicides. Int. J. Phytoremediat. 2020, 22, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.A.; Sofi, Z.M.; Malik, J.A.; Wani, J.A. Phytoremediation of heavy metals using Salix (willows). In Bioremediation and Biotechnology; Bhat, R., Hakeem, K., Dervash, M., Eds.; Springer: Cham, Swizerland, 2020; Volume 2, pp. 257–268. [Google Scholar]
- Isebrands, J.G.; Karnosky, D.F. Environmental benefits of poplar culture. In Poplar Culture in North America; Part A; Dickmann, D.I., Isebrands, J.G., Eckenwalder, J.G., Richardson, J., Eds.; NRC Research Press: Ottawa, ON, Canada, 2001; pp. 207–218. [Google Scholar]
- Kuzovkina, Y.; Volk, T. The characterization of willow (Salix L.) varieties for use in ecological engineering applications: Co-ordination of structure, function and autoecology. Ecol. Eng. 2009, 35, 1178–1189. [Google Scholar] [CrossRef]
- Urošević, J.; Stanković, D.; Jokanović, D.; Trivan, G.; Rodzkin, A.; Jović, Đ.; Jovanović, F. Phytoremediation potential of different genotypes of Salix alba and S. viminalis. Plants 2024, 13, 735. [Google Scholar] [CrossRef]
- Mleczek, M.; Rissmann, I.; Rutkowski, P.; Kaczmarek, Z.; Golinski, P. Accumulation of selected heavy metals by different genotypes of Salix. Environ. Exp. Bot. 2009, 66, 289–296. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Tőzsér, D.; Horváth, R.; Simon, E.; Magura, T. Heavy metal uptake by plant parts of Populus species: A meta-analysis. Environ. Sci. Pollut. Res. 2023, 30, 69416–69430. [Google Scholar] [CrossRef] [PubMed]
- Biró, I.; Takács, T. Study of heavy metal uptake of Populus nigra in relation to phytoremediation. Cereal Res. Commun. 2007, 35, 265–268. [Google Scholar] [CrossRef]
- Chandra, R.; Cho, W.; Kang, H. Phytoextraction potential of four poplar hybrids under greenhouse conditions. For. Sci. Technol. 2016, 12, 199–206. [Google Scholar] [CrossRef]
- Suo, Y.; Tang, N.; Li, H.; Corti, G.; Jiang, L.; Huang, Z.; Zhang, Z.; Huang, J.; Wu, Z.; Feng, C.; et al. Long-term effects of phytoextraction by a poplar clone on the concentration, fractionation, and transportation of heavy metals in mine tailings. Environ. Sci. Pollut. Res. 2021, 28, 47528–47539. [Google Scholar] [CrossRef] [PubMed]
- Landberg, T.; Greger, M. Differences in uptake and tolerance to heavy metals in Salix from unpolluted and polluted areas. J. Appl. Geochem. 1996, 11, 175–180. [Google Scholar] [CrossRef]
- Borišev, M.; Pajević, S.; Nikolić, N.; Pilipović, A.; Krstić, B.; Orlović, S. Phytoextraction of Cd, Ni, and Pb using four willow clones (Salix spp.). Pol. J. Environ. Stud. 2009, 18, 553–561. [Google Scholar]
- Kacálková, L.; Tlustoš, P.; Száková, J. Phytoextraction of risk elements by willow and poplar trees. Int. J. Phytoremediat. 2014, 17, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Zalesny, J.A.; Zalesny, R.S.; Wiese, A.H.; Hall, R.B. Choosing tree genotypes for phytoremediation of landfill leachate using phyto-recurrent selection. Int. J. Phytoremediat. 2007, 9, 513–530. [Google Scholar] [CrossRef]
- The Ministry of Environmental Protection of the Republic of Serbia. Regulation on limit values for pollutants, harmful and hazardous substances in soil. Official Gazette of Republic of Serbia, Official Gazette: Belgrade, Serbia, Nos 30/2018 and 64/2019. (In Serbian)
- Orlović, S.; Guzina, V.; Krstić, B.; Merkulov, L. Genetic variability in anatomical, physiological and growth characteristics of hybrid poplar (Populus × euramericana Dode (Guinier)) and eastern cottonwood (Populus deltoides Bartr.) Clones. Silvae Genet. 1998, 47, 183–190. [Google Scholar]
- Kassen, R. The experimental evolution of specialists, generalists, and the maintenance of diversity. J. Evol. Biol. 2002, 15, 173–190. [Google Scholar] [CrossRef]
- Thijs, S.; Witters, N.; Janssen, J.; Ruttens, A.; Weyens, N.; Herzig, R.; Mench, M.; Van Slycken, S.; Meers, E.; Meiresonne, L.; et al. Tobacco, sunflower and high biomass SRC clones show potential for trace metal phytoextraction on a moderately contaminated field site in Belgium. Front. Plant Sci. 2018, 9, 1879. [Google Scholar] [CrossRef] [PubMed]
- Pajević, S.; Borišev, M.; Nikolić, N.; Krstić, B.; Pilipović, A.; Orlović, S. Phytoremediation capacity of poplar (Populus spp.) and willow (Salix spp.) clones in relation to photosynthesis. Arch. Biol. Sci. 2009, 61, 239–247. [Google Scholar] [CrossRef]
- Trudić, B.; Kebert, M.; Popovic, B.; Stajner, D.; Orlović, S.; Galović, V.; Pilipovic, A. The effect of heavy metal pollution in soil on Serbian poplar clones. Šumarski List 2013, 137, 287–296. [Google Scholar]
- Jiang, C.; Wang, Y.; Chen, Y.; Wang, S.; Mu, C.; Shi, X. The phytoremediation potential of 14 Salix clones grown in Pb/Zn and Cu mine tailings. Forests 2024, 15, 257. [Google Scholar] [CrossRef]
- Kebert, M.; Rapparini, F.; Neri, L.; Bertazza, G.; Orlović, S.; Biondi, S. Copper-induced responses in poplar clones are associated with genotype-and organ-specific changes in peroxidase activity and proline, polyamine, ABA, and IAA levels. J. Plant Growth Regul. 2017, 36, 131–147. [Google Scholar] [CrossRef]
- Di Baccio, D.; Galla, G.; Bracci, T.; Andreucci, A.; Barcaccia, G.; Tognetti, R.; Sebastiani, L. Transcriptome analyses of Populus× euramericana clone I-214 leaves exposed to excess zinc. Tree Physiol. 2011, 31, 1293–1308. [Google Scholar] [CrossRef]
- Cao, Y.; Tanac, Q.; Zhang, F.; Maa, C.; Xiao, J.; Chen, G. Phytoremediation potential evaluation of multiple Salix clones for heavy metals (Cd, Zn and Pb) in flooded soils. Sci. Total Environ. 2022, 813, 152482. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Sources of heavy metals and metalloids in soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Springer Science+Business Media: Dordrecht, Germany, 2013; pp. 11–50. [Google Scholar]
- Huang, B.; Yuan, Z.; Li, D.; Zheng, M.; Nie, X.; Liao, Y. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal (loid) s in soil: A review. Environ. Sci. Process Impacts 2020, 22, 1596–1615. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [PubMed]
- Wang, S.; Shi, X.; Sun, H.; Chen, Y.; Pan, H.; Yang, X.; Rafiq, T. Variations in metal tolerance and accumulation in three hydroponically cultivated varieties of Salix integra treated with lead. PLoS ONE 2014, 9, e108568. [Google Scholar] [CrossRef] [PubMed]
- Pekeč, S.; Marković, M.; Milović, M.; Galović, V.; Karaklić, V.; Radojević, S. Physical and chemical soil properties for poplar production in nursery “Žarkovac” in Kovin Forest Administration. Topola/Poplar 2019, 204, 79–84. (In Serbian) [Google Scholar]
- Cools, N.; De Vos, B. Part X. Sampling and Analysis of Soil. Version 2020-1. In Annex II-VII to Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Co-ordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2020; 29p, Available online: https://www.icp-forests.org/pdf/manual/2016/ICP_Manual_2016_01_part10Annex.pdf (accessed on 15 November 2024).
- Beinabaj, S.M.H.; Heydariyan, H.; Aleii, H.M.; Hosseinzadeh, A. Concentration of heavy metals in leachate, soil, and plants in Tehran’s landfill: Investigation of the effect of landfill age on the intensity of pollution. Heliyon 2023, 9, e13017. [Google Scholar] [CrossRef] [PubMed]
- TIBCO Software Inc. Data Science WorkBench 14.0.0; 2020. Available online: https://docs.tibco.com/products/tibco-data-science-workbench-14-0-0 (accessed on 2 September 2024).
| Granulometric Composition | |||||||
|---|---|---|---|---|---|---|---|
| Substrate | Coarse Sand (%) | Fine Sand (%) | Silt (%) | Clay (%) | Total Sand (%) | Total Clay (%) | Soil Texture |
| Control (Kać) | 42.09 | 47.03 | 7.68 | 3.2 | 89.12 | 10.88 | Sand |
| Belgrade landfill | 4.28 | 35.68 | 28.76 | 31.28 | 39.96 | 60.04 | Clay loam |
| Novi Sad landfill | 22.08 | 41.76 | 19.24 | 16.92 | 63.84 | 36.16 | Sandy loam |
| Chemical Properties | |||||||
| CaCO3 (%) | pH (in H2O) | Humus (%) | N (%) | P2O5 (mg/100 g) | K2O (mg/100 g) | ||
| Control (Kać) | 11.12 | 8.09 | 1.65 | 0.016 | 3.85 | 2.92 | |
| Belgrade landfill | 6.82 | 7.95 | 2.14 | 0.133 | 12.66 | 10.28 | |
| Novi Sad landfill | 13.06 | 8.62 | 2.71 | 0.076 | 8.38 | 6.70 | |
| Substrate | Heavy Metal Content (mg/kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Ba | Cd | Cr | Cu | Fe | Hg | Mn | Ni | Pb | Zn | |
| Control (Kać) | n.d. (1) | 16.30 | n.d. | 50.77 | 25.61 | 8343.87 | n.d. | 163.20 | 15.91 | 14.50 | 28.03 |
| Novi Sad landfill | n.d. | 21.10 | 1.20 * (2) | 64.60 | 33.10 | 10,777.50 | 1.50 | 210.80 | 21.60 | 23.80 | 48.00 |
| Belgrade landfill | 3.69 | 109.00 | 1.83 * | 90.98 | 37.42 * | 33,425.00 | n.d. | 685.20 | 77.68 * | 46.60 | 65.70 |
| MLV | 29 | 160 | 0.8 | 100 | 36 | / | 0.3 | / | 35 | 85 | 140 |
| RV | 55 | 625 | 12 | 380 | 190 | / | 10 | / | 210 | 530 | 720 |
| Source of Variation | Cadmium | Crome | Cupper | Iron | Manganese | Nickel | Lead | Zinc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F (*) | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| Substrate (A) | 75.90 | 0.000 | 77.53 | 0.000 | 4.77 | 0.014 | 60.17 | 0.000 | 132.36 | 0.000 | 64.64 | 0.000 | 76.24 | 0.000 | 12.13 | 0.000 |
| Clone (B) | 99.80 | 0.000 | 21.71 | 0.000 | 13.79 | 0.000 | 22.25 | 0.000 | 13.20 | 0.000 | 25.85 | 0.000 | 9.30 | 0.000 | 51.63 | 0.000 |
| Interaction A × B | 9.91 | 0.000 | 12.86 | 0.000 | 1.18 | 0.326 | 10.10 | 0.000 | 3.75 | 0.001 | 3.84 | 0.001 | 9.19 | 0.000 | 2.61 | 0.010 |
| Treatments | Content in Shoot Tissue (mg/kg DW) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate (a) | Clone (c) | Cadmium | Crome | Copper | Iron | Manganese | Nickel | Lead | Zinc | ||||||||
| Means of Substrates | |||||||||||||||||
| BG | 1.93 | a (b) | 5.63 | a | 52.14 | a | 666.37 | a | 85.28 | a | 7.22 | a | 3.26 | a | 129.17 | a | |
| NS | 1.51 | b | 3.90 | b | 48.39 | b | 425.79 | b | 57.47 | b | 4.75 | b | 2.76 | b | 110.90 | b | |
| Control | 1.50 | b | 3.68 | b | 47.78 | b | 232.73 | c | 47.01 | c | 4.81 | b | 1.91 | c | 122.49 | a | |
| Means of Clones | |||||||||||||||||
| 107/65-9 | 2.40 | a | 6.03 | a | 52.59 | ab | 827.33 | a | 44.25 | c | 6.58 | a | 3.24 | a | 115.75 | cd | |
| 380 | 1.98 | b | 4.88 | b | 43.40 | cd | 574.61 | b | 62.48 | b | 4.29 | bc | 2.94 | ab | 138.55 | b | |
| I-214 | 1.44 | d | 4.05 | c | 56.05 | a | 363.55 | c | 62.26 | b | 3.99 | c | 2.28 | c | 111.84 | d | |
| Pannonia | 1.35 | d | 4.16 | bc | 56.60 | a | 326.70 | c | 71.19 | ab | 5.26 | b | 2.41 | bc | 115.28 | cd | |
| PE19/66 | 1.14 | e | 4.08 | bc | 41.12 | d | 332.59 | c | 74.43 | a | 4.76 | bc | 2.32 | c | 69.11 | e | |
| S1-8 | 1.68 | c | 4.10 | bc | 48.62 | bc | 350.40 | c | 62.27 | b | 7.36 | a | 2.69 | bc | 163.00 | a | |
| 135/81 | 1.49 | d | 3.40 | c | 48.18 | bcd | 278.24 | c | 64.18 | ab | 6.96 | a | 2.52 | bc | 130.64 | bc | |
| Means at the Level of Interaction Substrate × Clone | |||||||||||||||||
| BG | 107/65-9 | 3.04 | a | 9.16 | a | 54.89 | abc | 1498.81 | a | 75.79 | abcde | 9.51 | a | 4.79 | a | 138.90 | abcd |
| 380 | 2.25 | bc | 7.56 | a | 44.65 | bcd | 1110.83 | a | 90.95 | ab | 6.79 | bc | 4.42 | a | 145.23 | abc | |
| I-214 | 1.65 | defghi | 4.69 | bcd | 59.36 | ab | 427.72 | bc | 83.20 | abc | 4.90 | cdefgh | 2.64 | bcdef | 112.30 | cdef | |
| Pannonia | 1.75 | defg | 4.71 | bcd | 57.19 | ab | 428.11 | bc | 99.75 | a | 6.91 | bc | 2.82 | bcde | 140.20 | abcd | |
| PE19/66 | 1.39 | fghijkl | 5.06 | b | 46.10 | abcd | 458.17 | bc | 100.05 | a | 6.47 | bcd | 2.88 | bcde | 67.29 | hi | |
| S1-8 | 1.95 | cde | 3.94 | bcde | 53.17 | abc | 324.44 | bc | 65.56 | cdef | 7.94 | ab | 2.71 | bcdef | 168.92 | a | |
| 135/81 | 1.47 | fghijk | 4.32 | bcde | 49.65 | abcd | 416.54 | bc | 81.67 | abcd | 8.00 | ab | 2.59 | cdef | 131.35 | abcde | |
| NS | 107/65-9 | 1.76 | defg | 4.69 | bcd | 53.57 | abc | 698.02 | b | 34.23 | gh | 4.30 | defgh | 3.14 | bc | 87.78 | fghi |
| 380 | 1.98 | cd | 4.36 | bcde | 40.76 | cd | 517.08 | bc | 54.94 | efg | 3.28 | h | 2.55 | cdef | 136.31 | abcde | |
| I-214 | 1.37 | ghijkl | 3.32 | cde | 57.33 | ab | 342.46 | bc | 57.83 | defg | 3.20 | h | 2.28 | cdef | 104.35 | defgh | |
| Pannonia | 1.19 | jkl | 3.16 | de | 52.10 | abcd | 298.94 | bc | 64.82 | cdef | 3.93 | efgh | 2.44 | cdef | 99.17 | efghi | |
| PE19/66 | 1.03 | l | 3.63 | bcde | 37.46 | d | 337.23 | bc | 66.56 | bcdef | 4.18 | defgh | 2.12 | cdef | 65.70 | i | |
| S1-8 | 1.57 | efghij | 4.99 | bc | 48.41 | abcd | 530.50 | bc | 69.64 | bcdef | 7.78 | ab | 3.77 | ab | 151.53 | ab | |
| 135/81 | 1.70 | defgh | 3.14 | de | 49.12 | abcd | 256.29 | c | 54.29 | efg | 6.57 | bcd | 3.03 | bcd | 131.43 | abcde | |
| Control | 107/65-9 | 2.41 | b | 4.23 | bcde | 49.30 | abcd | 285.15 | c | 22.72 | h | 5.92 | bcdefg | 1.80 | ef | 120.57 | bcdef |
| 380 | 1.78 | def | 3.25 | de | 44.43 | bcd | 215.60 | c | 46.79 | fgh | 3.17 | h | 2.12 | cdef | 135.21 | abcde | |
| I-214 | 1.28 | ijkl | 4.15 | bcde | 51.47 | abcd | 320.47 | bc | 45.77 | fgh | 3.88 | fgh | 1.93 | def | 118.86 | bcdef | |
| Pannonia | 1.12 | kl | 4.61 | bcd | 60.51 | a | 253.07 | c | 49.00 | fg | 4.95 | cdefgh | 1.97 | def | 106.46 | defg | |
| PE19/66 | 0.99 | l | 3.53 | bcde | 39.81 | cd | 202.35 | c | 56.66 | efg | 3.64 | gh | 1.95 | def | 74.34 | ghi | |
| S1-8 | 1.53 | fghij | 3.37 | bcde | 44.28 | bcd | 196.27 | c | 51.60 | efg | 6.34 | bcde | 1.61 | f | 168.56 | a | |
| 135/81 | 1.30 | hijkl | 2.75 | e | 45.76 | abcd | 161.89 | c | 56.59 | efg | 6.31 | bcdef | 1.93 | def | 129.15 | bcde | |
| Shoot Content | Rotated Principal Component (*) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Cadmium | 0.785 | 0.489 | −0.200 | 0.056 |
| Chromium | 0.927 | 0.093 | 0.237 | 0.104 |
| Copper | 0.090 | 0.076 | 0.037 | 0.992 |
| Iron | 0.969 | 0.059 | 0.167 | 0.040 |
| Manganese | 0.207 | 0.005 | 0.942 | 0.028 |
| Nickel | 0.383 | 0.654 | 0.479 | 0.093 |
| Lead | 0.850 | 0.161 | 0.365 | 0.051 |
| Zinc | 0.079 | 0.948 | −0.041 | 0.052 |
| Eigenvalue Contribution to the total variance | 3.342 | 1.610 | 1.376 | 1.015 |
| 0.418 | 0.201 | 0.172 | 0.127 | |
| Substrate (a) | Clone (b) | Cadmium | Chromium | Copper | Iron | Manganese | Nickel | Lead | Zinc |
|---|---|---|---|---|---|---|---|---|---|
| BG | 107/65-9 | 1.660 | 0.101 | 1.467 | 0.045 | 0.111 | 0.122 | 0.103 | 2.114 |
| 380 | 1.232 | 0.083 | 1.193 | 0.033 | 0.133 | 0.087 | 0.095 | 2.211 | |
| I-214 | 0.904 | 0.052 | 1.586 | 0.013 | 0.121 | 0.063 | 0.057 | 1.709 | |
| Pannonia | 0.954 | 0.052 | 1.528 | 0.013 | 0.146 | 0.089 | 0.061 | 2.134 | |
| PE19/66 | 0.760 | 0.056 | 1.232 | 0.014 | 0.146 | 0.083 | 0.062 | 1.024 | |
| S1-8 | 1.065 | 0.043 | 1.421 | 0.010 | 0.096 | 0.102 | 0.058 | 2.571 | |
| 135/81 | 0.801 | 0.047 | 1.327 | 0.012 | 0.119 | 0.103 | 0.056 | 1.999 | |
| NS | 107/65-9 | 1.466 | 0.073 | 1.618 | 0.065 | 0.162 | 0.199 | 0.132 | 1.829 |
| 380 | 1.647 | 0.067 | 1.231 | 0.048 | 0.261 | 0.152 | 0.107 | 2.840 | |
| I-214 | 1.144 | 0.051 | 1.732 | 0.032 | 0.274 | 0.148 | 0.096 | 2.174 | |
| Pannonia | 0.990 | 0.049 | 1.574 | 0.028 | 0.307 | 0.182 | 0.102 | 2.066 | |
| PE19/66 | 0.862 | 0.056 | 1.132 | 0.031 | 0.316 | 0.193 | 0.089 | 1.369 | |
| S1-8 | 1.309 | 0.077 | 1.462 | 0.049 | 0.330 | 0.360 | 0.158 | 3.157 | |
| 135/81 | 1.416 | 0.049 | 1.484 | 0.024 | 0.258 | 0.304 | 0.127 | 2.738 | |
| Control | 107/65-9 | - | 0.083 | 1.925 | 0.034 | 0.139 | 0.372 | 0.124 | 4.302 |
| 380 | - | 0.064 | 1.735 | 0.026 | 0.287 | 0.200 | 0.146 | 4.824 | |
| I-214 | - | 0.082 | 2.010 | 0.038 | 0.280 | 0.244 | 0.133 | 4.240 | |
| Pannonia | - | 0.091 | 2.363 | 0.030 | 0.300 | 0.311 | 0.136 | 3.798 | |
| PE19/66 | - | 0.070 | 1.555 | 0.024 | 0.347 | 0.229 | 0.135 | 2.652 | |
| S1-8 | - | 0.066 | 1.729 | 0.024 | 0.316 | 0.399 | 0.111 | 6.014 | |
| 135/81 | - | 0.054 | 1.787 | 0.019 | 0.347 | 0.397 | 0.133 | 4.607 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, B.; Milović, M.; Kesić, L.; Pajnik, L.P.; Pekeč, S.; Stanković, D.; Orlović, S. Interclonal Variation in Heavy Metal Accumulation Among Poplar and Willow Clones: Implications for Phytoremediation of Contaminated Landfill Soils. Plants 2025, 14, 567. https://doi.org/10.3390/plants14040567
Kovačević B, Milović M, Kesić L, Pajnik LP, Pekeč S, Stanković D, Orlović S. Interclonal Variation in Heavy Metal Accumulation Among Poplar and Willow Clones: Implications for Phytoremediation of Contaminated Landfill Soils. Plants. 2025; 14(4):567. https://doi.org/10.3390/plants14040567
Chicago/Turabian StyleKovačević, Branislav, Marina Milović, Lazar Kesić, Leopold Poljaković Pajnik, Saša Pekeč, Dragica Stanković, and Saša Orlović. 2025. "Interclonal Variation in Heavy Metal Accumulation Among Poplar and Willow Clones: Implications for Phytoremediation of Contaminated Landfill Soils" Plants 14, no. 4: 567. https://doi.org/10.3390/plants14040567
APA StyleKovačević, B., Milović, M., Kesić, L., Pajnik, L. P., Pekeč, S., Stanković, D., & Orlović, S. (2025). Interclonal Variation in Heavy Metal Accumulation Among Poplar and Willow Clones: Implications for Phytoremediation of Contaminated Landfill Soils. Plants, 14(4), 567. https://doi.org/10.3390/plants14040567








