Biosystematic, Essential Oil, and Biological Activity Studies on Medicinal Plant Moluccella L. (Lamiaceae) Species from Turkey
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Morphological Observations
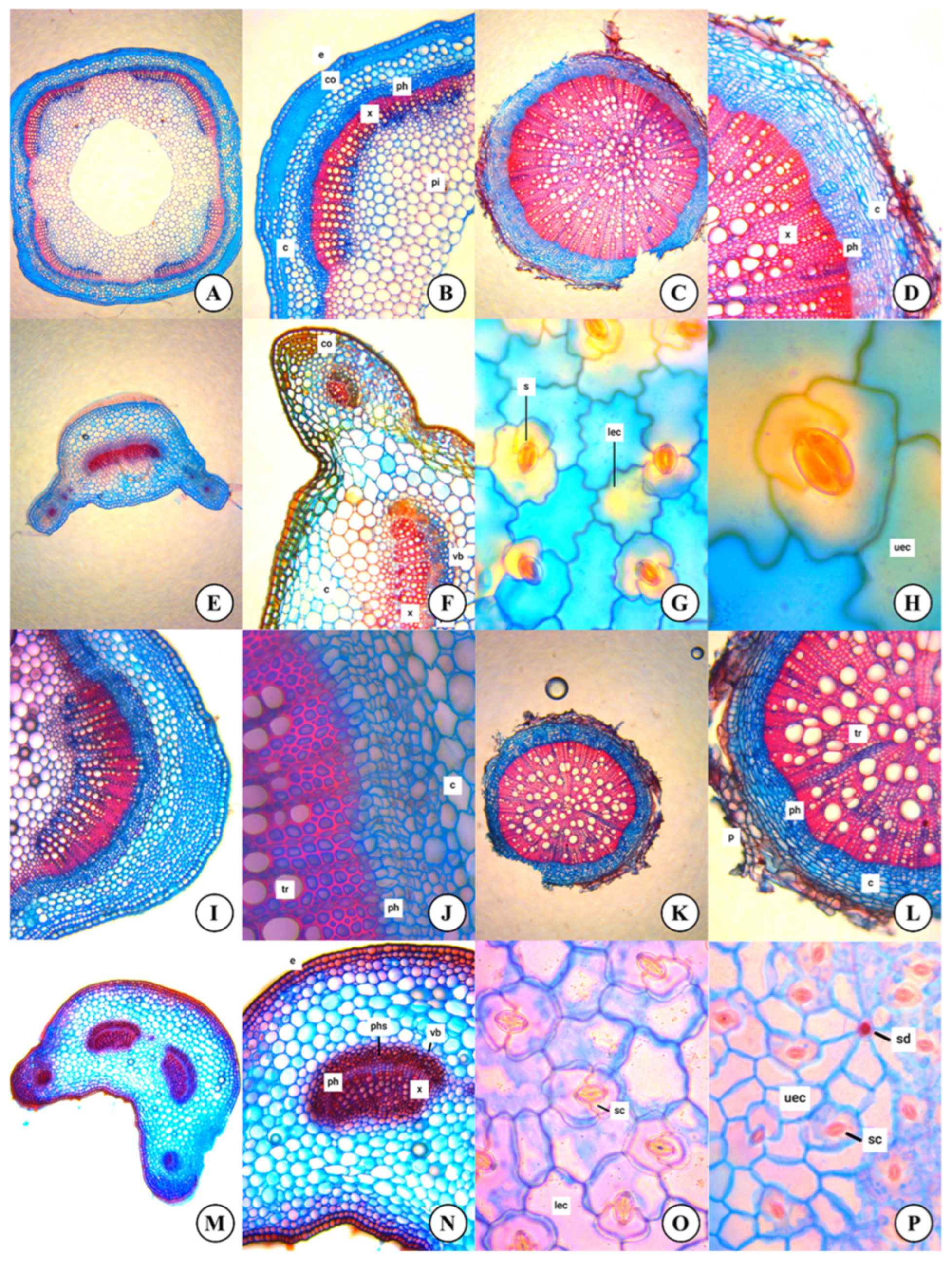
2.3. Anatomical Studies
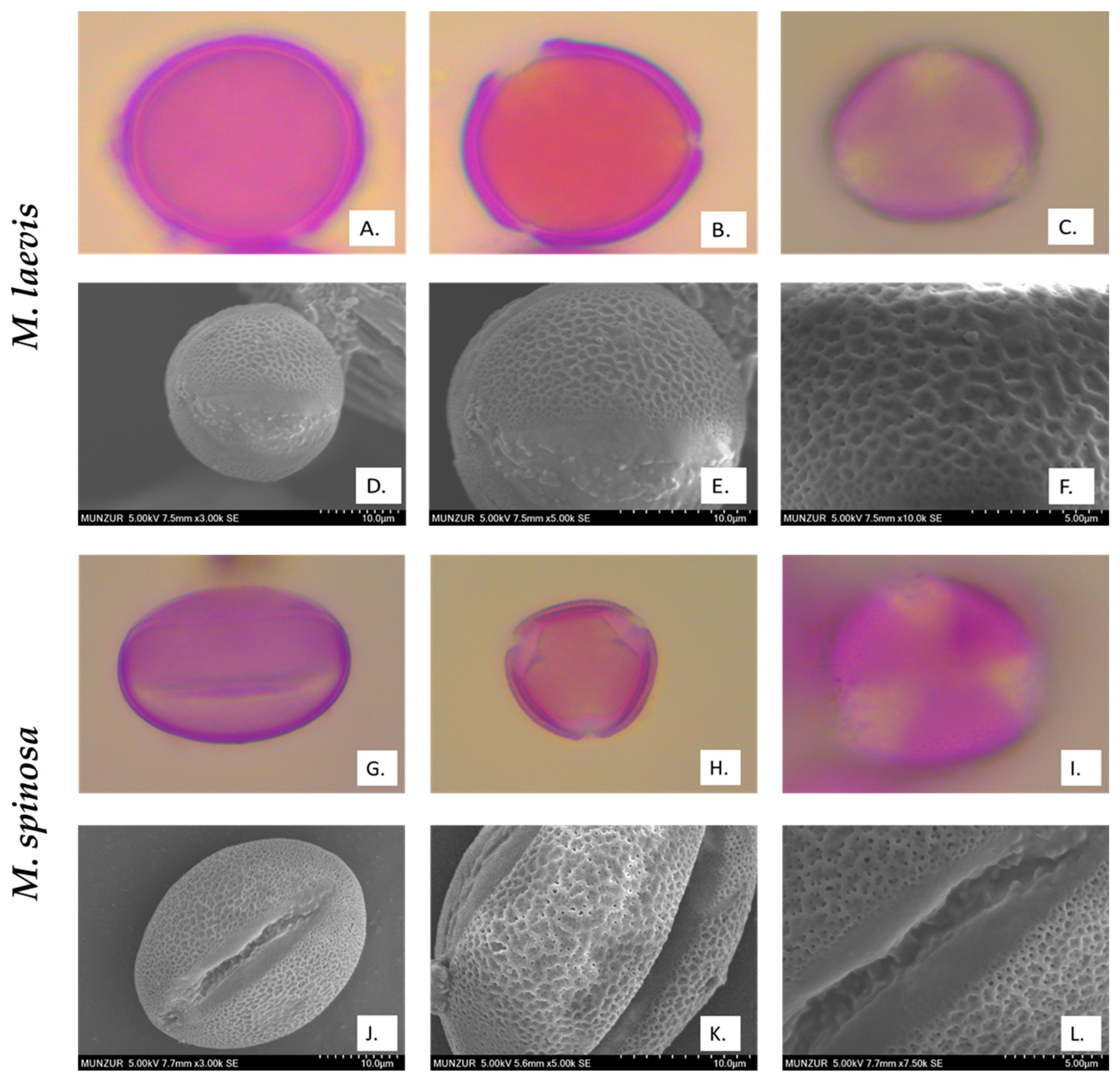
2.4. Palynological Evaluation
2.5. EO Studies
2.6. Antimicrobial Sensitivity
2.6.1. Antimicrobial Activity According to the Agar Disc Diffusion Method Sensitivity Test
2.6.2. Antimicrobial Sensitivity According to the Minimal Inhibition Concentrations (MICs)
2.7. DPPH Radical Scavenging Assay
2.8. Statistical Analysis
3. Results
3.1. Morphological Results
3.2. Anatomical Features
3.3. Palynological Properties
3.4. EOs Results
3.5. Antimicrobial Results
3.6. DPPH Radical Scavenging Results
4. Discussions
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harley, R.M.; Atkins, S.; Budantsev, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; De Kok, R.; Krestovskaja, T.; Morales, R.; et al. Labiatae. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 7, pp. 167–275. [Google Scholar] [CrossRef]
- Hamed, A.; Ahmed, N.; Attia, E.; Desoukey, S. Phytochemical investigation of saponifiable matter & volatile oils and antibacterial activity of Moluccella laevis L., family Lamiaceae (Labiatae). J. Adv. Biomed. Pharm. Sci. 2020, 3, 213–220. [Google Scholar] [CrossRef]
- Abdelaty, N.; Shihata, E.; Hamed, A.; Yehia, S. Biological Potential of Moluccella laevis L. Aerial Parts, Family Lamiaceae (Labiatae). J. Adv. Biomed. Pharm. Sci. 2021, 4, 226–230. [Google Scholar] [CrossRef]
- Doorandishan, M.; Pirhadi, S.; Swilam, M.M.; Gholami, M.; Ebrahimi, P.; El-Seedi, H.R.; Jassbi, A.R. Molecular docking and simulation studies of a novel labdane type diterpene from Moluccella aucheri Scheen (Syn. Otostegia aucheri) as human-AChE inhibitör. J. Mol. Struct. 2021, 1245, 131034. [Google Scholar] [CrossRef]
- Casiglia, S.; Jemia, M.B.; Riccobono, L.; Bruno, M.; Scandolera, E.; Senatore, F. Chemical composition of the essential oil of Moluccella spinosa L. (Lamiaceae) collected wild in Sicily and its activity on microorganisms affecting historical textiles. Nat. Prod. Res. 2015, 29, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Al-Masri, M.; Zaid, A.N.; Hussein, F.; Shadid, K.A.; Al-Rimawi, F.; Shayeb, K.; Sbeih, A.; Eid, A. Assessment of the antimicrobial and free radical scavenging activities of Moluccella spinosa, Helichrysum sanguineum, and Styrax officinalis folkloric medicinal plants from Palestine. Orient. Pharm. Exp. Med. 2018, 18, 107–114. [Google Scholar] [CrossRef]
- Demirpolat, A. Essential Oil Composition Analysis, Antimicrobial Activities, and Biosystematic Studies on Six Species of Salvia. Life 2023, 13, 634. [Google Scholar] [CrossRef]
- Wodehouse, R.P. Pollen Grains, Their Structure, Identification and Significancein Science and Medicine; McGraw-Hill: New York, NY, USA, 1935; Volume 574. [Google Scholar]
- Demirpolat, A. Anatomical and Palynological Characteristics of Endemic Fritillaria gencensis Yıld., Kılıç & A. Demirpolat. Turk. J. Agric. Nat. Sci. 2022, 9, 734–740. [Google Scholar] [CrossRef]
- Demirpolat, A. Anatomical, Palynological and Seed Surface Characteristics of Aethionema sancakense Yıld. & Kılıc (Brassicaceae). Eur. J. Sci. Technol. 2022, 37, 1–7. [Google Scholar] [CrossRef]
- Faegri, K.; Iversen, J. Textbook of Modern Pollen Analysis. Weather 1954, 9, 62. [Google Scholar] [CrossRef]
- Demirpolat, A. Chemical Composition of Essential Oils of Seven Polygonum Species from Turkey: A Chemotaxonomic Approach. Molecules 2022, 27, 9053. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Garg, A.P.; Uniyal, R.C.; Kumari, A. Comparative Analysis of The Antimicrobial Activity of Cinnamon Oil and Cinnamon Extract on somefood-borne microbes. Afr. J. Microbiol. Res. 2008, 2, 247–251. [Google Scholar]
- Torğut, G.; Pıhtılı, G.; Erecevit Sönmez, P.; Erden, Y.; Kırbağ, S. Synthesis and Antimicrobial and Anticancer Activities of Sodium Acrylate Copolymers. J. Bioact. Compat. Polym. 2020, 35, 179–188. [Google Scholar] [CrossRef]
- NCCLS. Methods for Dilution and Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Fifth Edition; NCCLS Document M7-A5; NCCLS: Wayne, PA, USA, 2000. [Google Scholar]
- Liyana-Pathiranan, C.M.; Shahidi, F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J. Agric. Food Chem. 2005, 53, 2433–2440. [Google Scholar] [CrossRef]
- Yılmaz Sancar, P.; İnci, Ş.; Demirpolat, A.; Kırbağ, S.; Civelek, Ş. Antimicrobial, antioxidant and essential oil studies on Veratrum album L. (Melanthiaceae). Int. J. Second. Metab. 2024, 11, 255–265. [Google Scholar] [CrossRef]
- Meng, Z.L.; Qin, R.X.; Wen, R.S.; Li, G.Q.; Liang, Z.Y.; Xie, J.K.; Zhou, Y.H.; Yang, Z.Q. Study on Synthesizing Isobornyl Acetate/Isoborneol from Camphene Using α-Hydroxyl Carboxylic Acid Composite Catalyst. Molecules 2023, 28, 1875. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar] [PubMed] [PubMed Central]
- Davis, P.H. Flora of Turkey and the East Aegean Islands; Edinburgh University Press: Edinburgh, UK, 1982; Volume 7, pp. 155–156. [Google Scholar]
- Abdelaty, N.A.; Attia, E.Z.; Hamed, A.; Desoukey, S.Y. Morphoanatomy studies of the leaves of of Moluccella laevis L. Family: Lamiaceae (Labiatae), cultivated in Egypt. J. Pharm. Phytochem. 2019, 8, 332–337. [Google Scholar] [CrossRef]
- Shehata, I. A pharmacognostical study of Moluccella laevis L. Bull. Fac. Pharm. Cairo Univ. 2001, 39, 239–251. [Google Scholar]
- Bakhshi, O.; Bagherzade, G.; Ghamari Kargar, P. Biosynthesis of Organic Nanocomposite Using Pistacia vera L. Hull: An Efficient Antimicrobial Agent. Bioinorg. Chem. Appl. 2021, 2021, 4105853. [Google Scholar] [CrossRef] [PubMed]
- Tschugaeff, L. Ueber das Thujen, ein neues bicyclisches Terpen. Berichte Dtsch. Chem. Ges. 1900, 33, 3118–3126. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Startseva, V.A.; Vakulenko, I.A.; Khismatulina, I.M.; Lisovskaya, S.A.; Glushko, N.P.; Fassakhov, R.S. Synthesis and antifungal activity of compounds of the pinane series. Pharm. Chem. J. 2009, 43, 251–254. [Google Scholar] [CrossRef]
- Silva, A.C.R.; Lopes, P.M.; Azevedo, M.M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of α-Pinene and β-Pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Felipe, C.F.B.; Albuquerque, A.M.S.; de Pontes, J.L.X.; de Melo, J.V.; Rodrigues, T.C.M.L.; de Sousa, A.M.P.; Monteiro, A.B.; da Silva Ribeiro, A.E.; Lopes, J.P.; Menezes, I.R.A.; et al. Comparative study of α and β-Pinene effect on PTZ-induced convulsions in mice. Fund. Clin. Pharmacol. 2019, 33, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Anneken, D.J.; Both, S.; Christoph, R.; Fieg, G.; Steinberner, U.; Westfechtel, A. Fatty Acids. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Chang, P.; Terbach, N.; Plant, N.; Chen, P.E.; Walker, M.C.; Williams, R.S. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology 2013, 69, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Aneja, M.; Gianfagna, T.J.; Hebbar, P.K. Trichoderma harzianum produces nonanoic acid, an inhibitor of spore germination and mycelial growth of two cacao pathogens. Physiol. Mol. Plant Pathol. 2005, 67, 304–307. [Google Scholar] [CrossRef]
- Miller, T.W. Natural herbicides and amendments for organic weed control. In Crop Protection Products for Organic Agriculture; American Chemical Society: Washington, DC, USA, 2006; Volume 12, pp. 174–185. [Google Scholar] [CrossRef]
- Yannai, S. Dictionary of Food Compounds with CD-ROM: Additives, Flavors, and Ingredients, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2003; p. 1784. [Google Scholar] [CrossRef]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Met. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Faydaoğlu, E.; Sürücüoğlu, M.S. History of the Use of Medical and Aromatic Plants and their Economic Importance. Kastamonu Üniversitesi Orman Fakültesi Derg. 2011, 11, 1,52–67. [Google Scholar]
- Dar, R.A.; Shahnawaz, M.; Qazi, P.H. General overview of medicinal plants: A review. J. Phytophar. 2017, 6, 349–351. [Google Scholar] [CrossRef]
- Rawat, D.; Shrivastava, S.; Naik, R.A.; Chhonker, S.K.; Mehrotra, A.; Koiri, R.K. An overview of natural plant products in the treatment of hepatocellular carcinoma. Anti-Cancer Agents Med. Chem. 2018, 18, 1838–1859. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 5, 35. [Google Scholar] [CrossRef]
| Species | Locality | Collection Date and Collector Number |
|---|---|---|
| M. laevis | B7, Elazig; Isikyolu village, roadsides and gardens, 1182 m. Long: 39° 2′37.312″ E Lat: 38° 30′55.825″ N | 25 July 2023 P. Yılmaz Sancar 3501 |
| M. spinosa | C5, Mersin; Silifke, Atakent neighbourhood, Merdivensay locality, under Ceratonia siliqua, 82 m. Long: 34° 4′41.515″ E Lat: 36° 25′52.426″ N | 7 May 2023 P. Yılmaz Sancar 3521 |
| M. laevis | M. spinosa | |
|---|---|---|
| Life cycle | Annual | Annual or short-lived perennial |
| Stems size (cm) and colour | 10–70 cm, initially green and straw-coloured when mature | 20–110 cm, initially green and purple when mature |
| Hairs of stem | Glabrous | Glabrous |
| Leaf shapes | Orbicular–ovate, palmate–crenate or incised, longly petiolate | Orbicular–ovate, coarsely palmate lobed or serrate, shortly petiolate |
| Width of the leaf (mm) | 18–40 | 23–43 |
| Length of leaf (mm) | 15–35 | 20–38 |
| Bracteoles (mm) | Spiny, 5–11 | Spiny, 7–15 |
| Petiole (mm) | 5–30 | 10–19 |
| Inflorescens | Verticillate | Verticillate |
| Flowers | 6–12 flowers | 8–10 flowers |
| Calyx | Pale green with whitish reticulate veins. Limb greaty expanded, membranous, 20–40 mm across, with 5 mucros, not spiny, abscissing in fruit, with nutlets. | Green-burgundy, reticulate veins. Narrow limb structure, rigid, 15–30 mm across, with 8 mucros, rigid and spiny, ersisting in fruit, with nutlets |
| Corolla | c. 20–25 mm; upper lip entire, shortly hairy. Purplish-pink or mauvish-white | c. 20–40 mm, upper lip broadly emarginate or bifid, villous. Pale pinkish-violet or white. |
| Fl. | 6–8. Fallow and cornfields, waste ground, vineyards, eroded bank. | 4–6. Stony ground (on lime-stone). |
| Alt. | c. 100–1300 m | c. 0–600 m |
| Seed | 4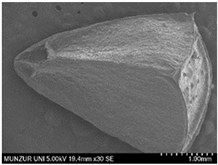 | 4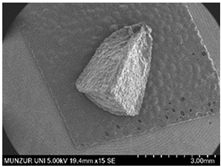 |
| M. laevis | M. spinosa | |
|---|---|---|
| Stem | ||
| Collenchyma layers | 4–5 | 6–7 |
| Phloem layers (inner) | 4–5 | 5–7 |
| Xylem layers | 6–12 | 6–12 |
| Phloem layers | 3–5 | 2–4 |
| Root | ||
| Periderm layers | 1–3 | 2–5 |
| Cortex layers | 6–8 | 3–6 |
| Sclerenchyma layers | 3–5 | 3–5 |
| Pith region | 10–18 | 12–20 |
| Petiole | ||
| Petiole shape | sulcat | sulcat |
| Collenchyma cell layers | 1–3 | 1–2 |
| Parenchyma cell layers | 4–7 | 4–5 |
| Taxa | Shape | Type and Number of Apertures | Exine Ornamentation | P | E | P/E Ratio | Clg | Clt |
|---|---|---|---|---|---|---|---|---|
| M. laevis | Spheroidal | 3-colporate | Reticulate | 2.80 | 2.82 | 0.99 | 2.47 | 0.58 |
| M. spinosa | Prolate | 3-colporate | Reticulate | 3.91 | 2.57 | 1.57 | 3.08 | 0.3 |
| No | Components | RT | IM | M. laevis % | M. spinosa % |
|---|---|---|---|---|---|
| 1. | α-Thujene | 9.89 | RI, MS | 5.93 | - |
| 2. | α-Pinene | 11.49 | RI, MS | 2.85 | - |
| 3. | Sabinone | 12.45 | RI, MS | 2.87 | - |
| 4. | β-Pinene | 13.52 | RI, MS | 5.64 | - |
| 5. | p-Cymene | 14.00 | RI, MS | 1.55 | - |
| 6. | α-Phellandrene | 14.92 | RI, MS | 1.13 | - |
| 7. | Limonene | 16.18 | RI, MS | 2.00 | - |
| 8. | 2-Pentyl furan | 20.01 | RI, MS | 0.87 | - |
| 9. | Alloocimene | 20.76 | RI, MS | 1.83 | - |
| 10. | Δ-Carene | 21.54 | RI, MS | 3.21 | - |
| 11. | 1.8-Cineole | 22.01 | RI, MS | 0.74 | - |
| 12. | Trans-Camphor | 24.87 | RI, MS | 0.22 | - |
| 13. | α-Camphoneal | 25.98 | RI, MS | 0.76 | - |
| 14. | Terpineol | 27.04 | RI, MS | 0.68 | - |
| 15. | β-Caryophyllene | 38.01 | RI, MS | 5.65 | - |
| 16. | Isobornyl acetate | 39.83 | RI, MS | 28.01 | - |
| 17. | α-Caryophyllene oxide | 42.00 | RI, MS | 3.62 | 0.11 |
| 18. | Spathulenol | 42.03 | RI, MS | 4.02 | - |
| 19. | Widdrol | 43.01 | RI, MS | 0.81 | - |
| 20. | Nonanol | 45.11 | RI, MS | 0.03 | - |
| 21. | Safranal | 45.76 | RI, MS | 0.32 | - |
| 22. | Geranyl acetate | 46.87 | RI, MS | 0.42 | - |
| 23. | 2-Tridecenal | 46.98 | RI, MS | 0.15 | 0.36 |
| 24. | Pyrrole | 47.04 | RI, MS | 0.92 | - |
| 25. | 2-Butanone | 48.07 | RI, MS | 0.45 | - |
| 26. | Tricosane | 48.15 | RI, MS | 0.43 | - |
| 27. | Tetracosane | 49.00 | RI, MS | 2.31 | - |
| 28. | Pentacosane | 49.85 | RI, MS | 2.54 | 0.19 |
| 29. | Hexacosane | 49.87 | RI, MS | 0.32 | - |
| 30. | Heptacosane | 49.95 | RI, MS | 0.19 | 1.81 |
| 31. | Dibutyl phthalate | 50.01 | RI, MS | 0.41 | 0.77 |
| 32. | 3-Hexanol | 8.00 | RI, MS | 0.80 | 0.20 |
| 33. | 2-Heptanol | 8.33 | RI, MS | 0.76 | 0.29 |
| 34. | Orcinol | 11.02 | RI, MS | - | 0.11 |
| 35. | Linalool Oxide | 12.46 | RI, MS | - | 0.13 |
| 36. | Carvomenthone | 14.44 | RI, MS | - | 0.09 |
| 37. | Acetophenone | 15.74 | RI, MS | - | 0.20 |
| 38. | 1,8-Menthadien-4-ol | 15.97 | RI, MS | - | 0.08 |
| 39. | Cymene | 16.16 | RI, MS | - | 0.17 |
| 40. | Δ-Terpineol | 16.43 | RI, MS | - | 0.10 |
| 41. | Thymol | 19.21 | RI, MS | - | 0.23 |
| 42. | β-Damascenone | 23.14 | RI, MS | - | 0.13 |
| 43. | Indole | 23.36 | RI, MS | - | 0.67 |
| 44. | Pentadecanol | 26.26 | RI, MS | - | 0.53 |
| 45. | Lauric acid | 28.74 | RI, MS | - | 0.28 |
| 46. | α-Benzenemethanol,4-Hydroxy | 29.19 | RI, MS | - | 0.18 |
| 47. | Caryophyllene oxide | 29.35 | RI, MS | - | 0.34 |
| 48. | 4 H-Pyran-4-one, 2,6-dimethyl | 29.98 | RI, MS | - | 0.13 |
| 49. | Octadecanol | 30.13 | RI, MS | - | 0.14 |
| 50. | Heptanol | 30.76 | RI, MS | - | 0.20 |
| 51. | Dimethylamphetamine | 31.22 | RI, MS | - | 0.12 |
| 52. | N-Isopropyl-3-phenylpropanamide | 31.29 | RI, MS | - | 0.20 |
| 53. | γ-Dehydro-ar-himachalene | 31.38 | RI, MS | - | 0.80 |
| 54. | 1,7-Octadiene | 31.48 | RI, MS | - | 0.14 |
| 55. | Palmitic aldehyde | 32.10 | RI, MS | - | 0.31 |
| 56. | Naphthalene | 32.23 | RI, MS | - | 0.61 |
| 57. | Hexadecanol | 32.61 | RI, MS | - | 0.59 |
| 58. | Myristyl aldehyde | 32.90 | RI, MS | - | 0.14 |
| 59. | 3-Tetradecene | 33.03 | RI, MS | - | 0.33 |
| 60. | Cinnamaldehyde | 33.30 | RI, MS | - | 0.50 |
| 61. | Valeric acid | 33.67 | RI, MS | - | 1.69 |
| 62. | Menthyl lactate | 34.04 | RI, MS | - | 0.43 |
| 63. | 2-Methylundecanal | 34.53 | RI, MS | - | 1.69 |
| 64. | Salicylate | 34.59 | RI, MS | - | 1.50 |
| 65. | 2-Bromoethanol | 34.76 | RI, MS | - | 0.33 |
| 66. | β-Citronellol | 34.92 | RI, MS | - | 0.59 |
| 67. | Decanol | 35.20 | RI, MS | - | 0.58 |
| 68. | 2-Pentadecanone | 35.43 | RI, MS | - | 8.65 |
| 69. | Diethyl phthalate | 35.79 | RI, MS | - | 2.47 |
| 70. | n-Hexadecanol | 36.27 | RI, MS | - | 1.91 |
| 71. | 2-Amino-5-(4-chlorophenyl)-1,3,4-thiadiazole | 37.28 | RI, MS | - | 1.92 |
| 72. | Nonanoic acid | 38.40 | RI, MS | - | 34.41 |
| 73. | 3,7-Dimethyldibenzothiophene | 39.19 | RI, MS | - | 2.44 |
| 74. | Octadecene | 39.31 | RI, MS | - | 1.35 |
| 75. | Manool | 41.00 | RI, MS | - | 0.45 |
| 76. | Verbanol | 42.51 | RI, MS | - | 1.47 |
| 77. | I-Menthol | 42.74 | RI, MS | - | 1.58 |
| 78. | Iridolactone | 43.44 | RI, MS | - | 1.81 |
| 79. | Epilongipinanol | 43.61 | RI, MS | - | 5.19 |
| 80. | Stearic acid | 44.30 | RI, MS | - | 1.53 |
| 81. | Nonadecane | 47.74 | RI, MS | - | 1.11 |
| 82. | Δ-Nonalactone | 48.66 | RI, MS | - | 0.60 |
| 83. | Eicosane | 51.79 | RI, MS | - | 3.07 |
| 84. | Docosane | 54.02 | RI, MS | - | 0.73 |
| TOTAL | 85.29 | 88.02 | |||
| Microorganisms | M. laevis | M. spinosa | Methanol | Standard Antibiotics |
|---|---|---|---|---|
| E. coli | 14.33 ± 0.3 d | - | - | 19.33 ± 0.3 cd* |
| P. aeruginosa | 11.6 ± 0.3 d | 11.6 ± 0.3 d | 10.3 ± 0.3 d | 14.33 ± 0.3 d* |
| S. aureus | 12.3 ± 0.3 d | 10.66 ± 0.3 d | 12.6± 0.3 d | 19.33 ± 0.3 cd* |
| E. faecalis | 10.66 ± 0.3 d | 12.66 ± 0.3 d | 13.6± 0.3 d | 19.66 ± 0.3 cd* |
| C. albicans | - | - | - | 28.33 ±0.33 cd** |
| C. glabrata | - | - | - | 28.66 ± 0.33 cd** |
| C. tropicalis | 9.3 ± 0.3 d | 9.3 ± 0.3 d | 10.3 ± 0.3 d | 30.66 ± 0.33 cd** |
| E. floccosum | 28.3 ± 0.3 cd | 28.3 ± 0.3 cd | - | 19.66 ± 0.33 cd** |
| Microorganism | MIC Values of M. laevis | MIC Values of M. spinosa | MIC Values of Methanol |
|---|---|---|---|
| E. coli | - | - | - |
| P. aeruginosa | 62.5 | 62.5 | 62.5 |
| S. aureus | - | 62.5 | 62.5 |
| E. faecalis | 62.5 | - | 62.5 |
| C. albicans | - | - | 62.5 |
| C. glabrata | - | - | 62.5 |
| C. tropicalis | - | - | 125 |
| E. floccosum | - | - | 62.5 |
| 100 mg/mL | 250 mg/mL | 500 mg/mL | 750 mg/mL | 1000 mg/mL | |
|---|---|---|---|---|---|
| M. laevis | 10.93 ± 0.45 | 27.23 ± 0.61 | 56.91 ± 0.06 | 81.47 ± 0.53 | 84.15 ± 0.59 |
| M. spinosa | 1.22 ± 0.09 | 16.74 ± 0.85 | 40.17 ± 1.79 | 51.56 ± 0.93 | 73.66 ± 1.06 |
| Vitamin C | 99.9 ± 0.2 | ||||
| %99.9 MeOH | 2.7 ± 0.3 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sancar, P.Y. Biosystematic, Essential Oil, and Biological Activity Studies on Medicinal Plant Moluccella L. (Lamiaceae) Species from Turkey. Plants 2025, 14, 542. https://doi.org/10.3390/plants14040542
Sancar PY. Biosystematic, Essential Oil, and Biological Activity Studies on Medicinal Plant Moluccella L. (Lamiaceae) Species from Turkey. Plants. 2025; 14(4):542. https://doi.org/10.3390/plants14040542
Chicago/Turabian StyleSancar, Pelin Yilmaz. 2025. "Biosystematic, Essential Oil, and Biological Activity Studies on Medicinal Plant Moluccella L. (Lamiaceae) Species from Turkey" Plants 14, no. 4: 542. https://doi.org/10.3390/plants14040542
APA StyleSancar, P. Y. (2025). Biosystematic, Essential Oil, and Biological Activity Studies on Medicinal Plant Moluccella L. (Lamiaceae) Species from Turkey. Plants, 14(4), 542. https://doi.org/10.3390/plants14040542







