Tetraploidization Altered Phenotypic Traits and Metabolite Profile of Java Ginseng (Talinum paniculatum (Jacq.) Gaertn.)
Abstract
1. Introduction
2. Results
2.1. Germination of Axillary Buds from Single Node Segments

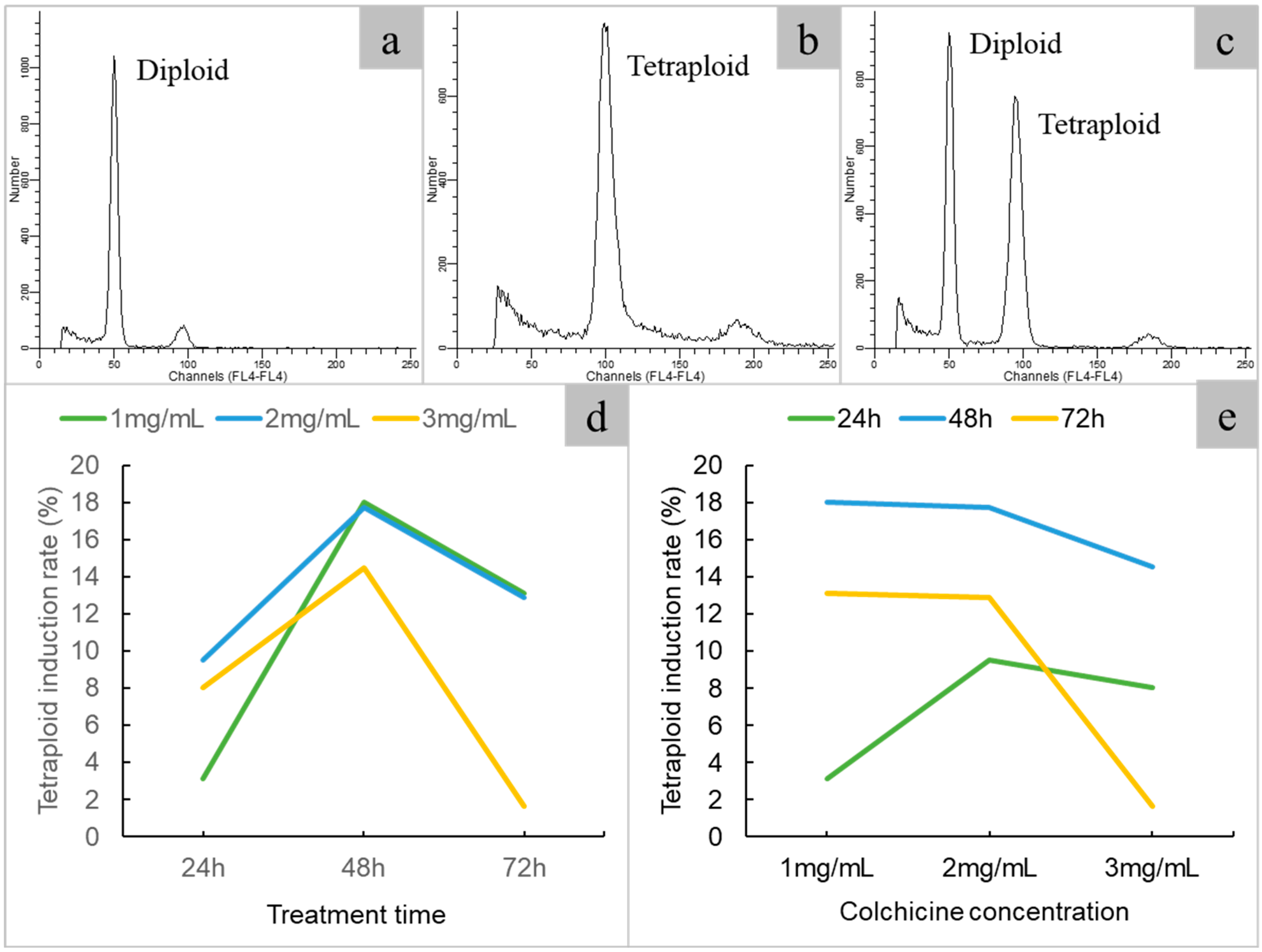
2.2. Tetraploid Induction and Ploidy Identification
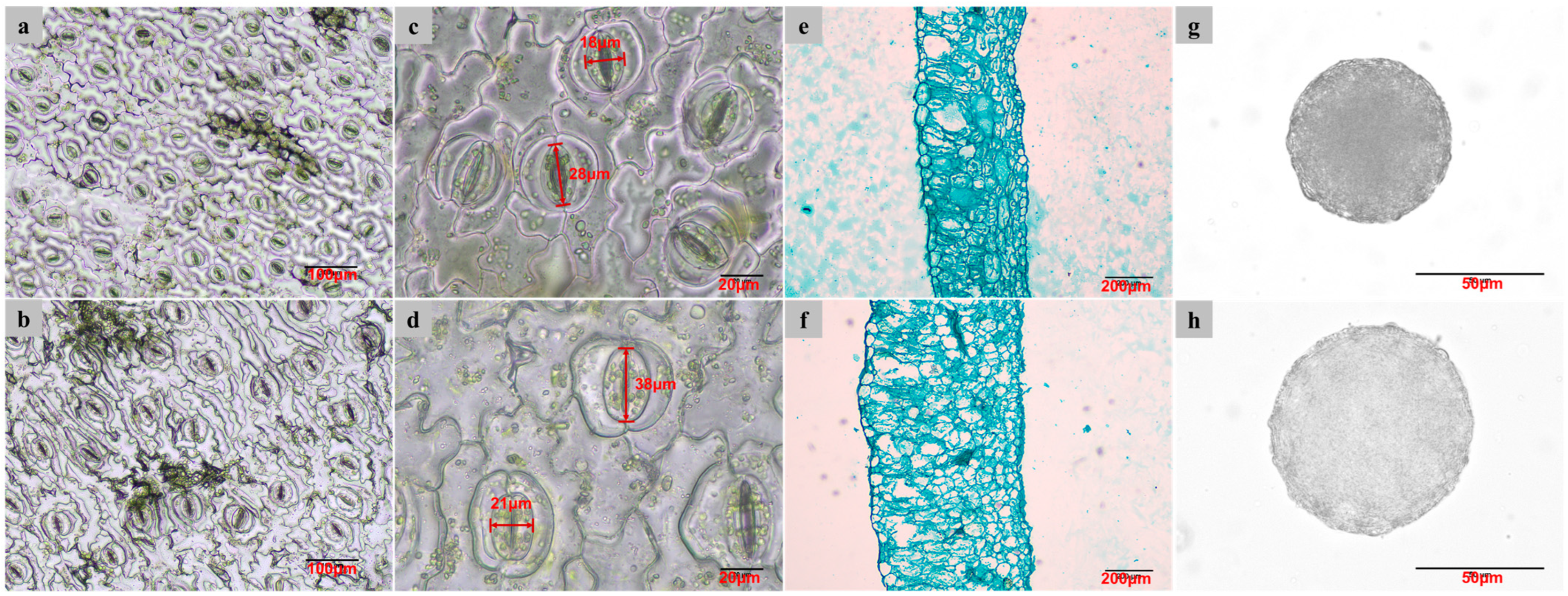
2.3. Phenotypical Changes in Tetraploids
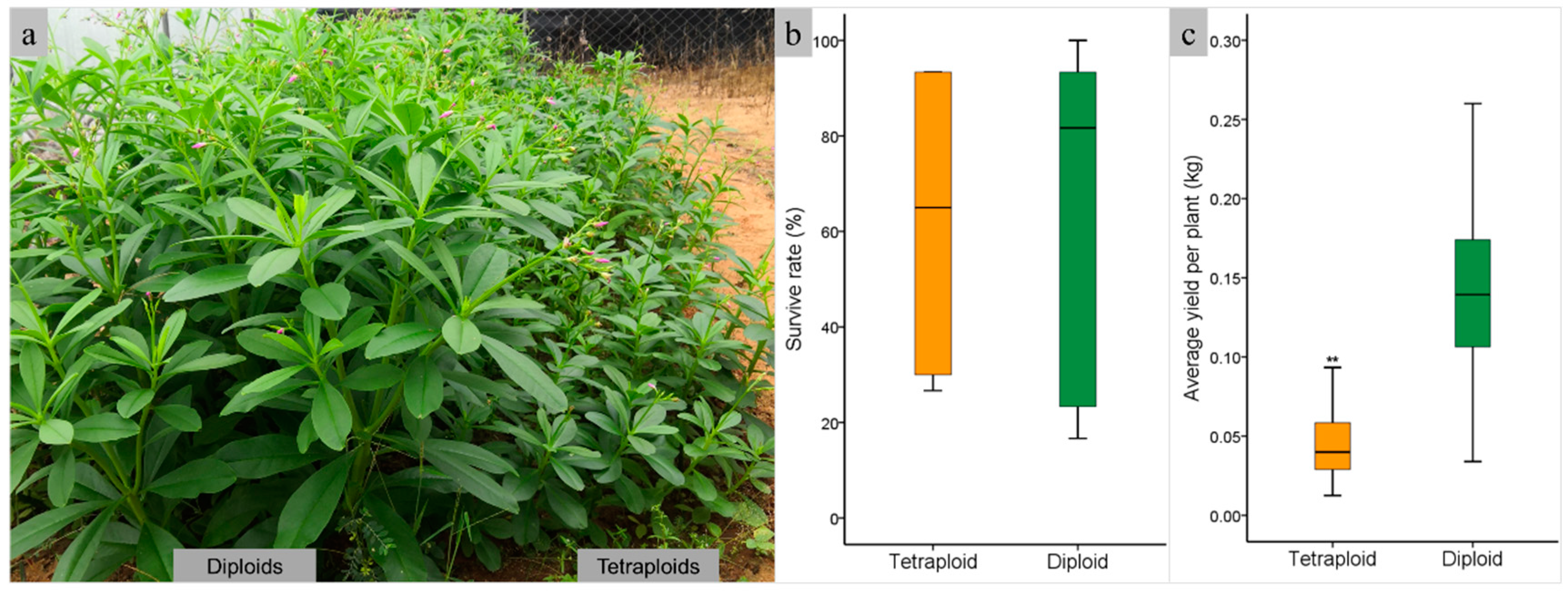
2.4. Comparison of Aboveground Biomass Between Diploid and Tetraploid
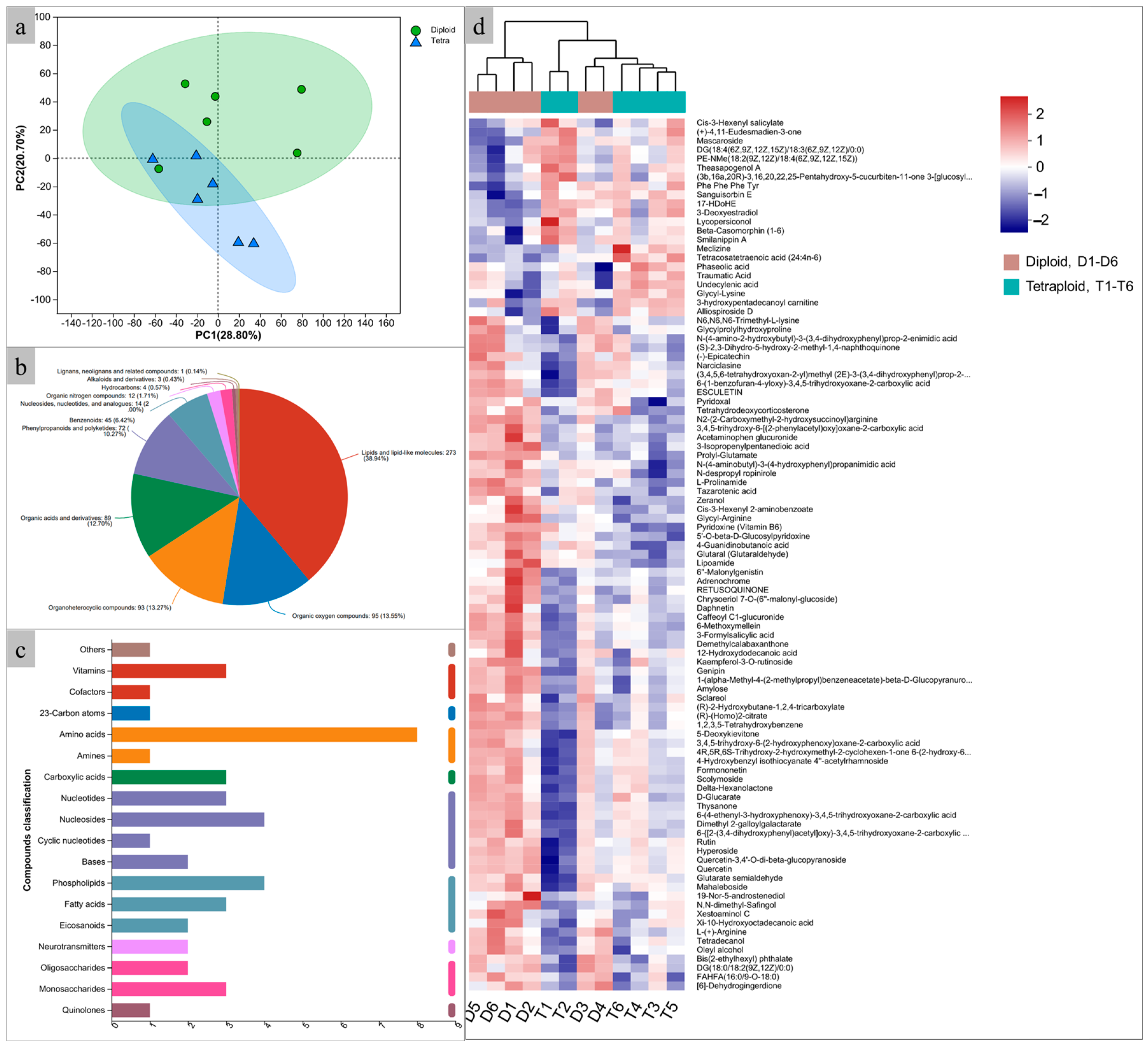
2.5. Metabolite Profile Changes in Tetraploids
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Culture Medium Selection and Polyploid Induction
4.3. Flow Cytometry for Ploidy Identification
4.4. Leaf Stomatal Analysis
4.5. Leaf Thickness Measurement
4.6. Pollen Size Measurement
4.7. Aboveground Biomass of Plantings
4.8. Liquid Chromatography-Mass Spectrometry Analysis
4.9. Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Menezes, F.D.A.B.; Ishizawa, T.A.; Souto, L.R.F.; Oliveira, T.F. Talinum paniculatum (Jacq.) Gaertn. Leaves—Source of nutrients, antioxidant and antibacterial potentials. Acta Sci. Pol. Technol. Aliment. 2021, 20, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Moura, I.O.; Santana, C.C.; Lourenço, Y.R.F.; Souza, M.F.; Silva, A.R.S.T.; Dolabella, S.S.; Silva, A.M.d.O.e.; Oliveira, T.B.; Duarte, M.C.; Faraoni, A.S. Chemical characterization, antioxidant activity and cytotoxicity of the unconventional food plants: Sweet potato (Ipomoea batatas (L.) Lam.) leaf, major gomes (Talinum paniculatum (Jacq.) Gaertn.) and caruru (Amaranthus deflexus L.). Waste Biomass Valor 2021, 12, 2407–2431. [Google Scholar] [CrossRef]
- Sukwan, C.; Wray, S.; Kupittayanant, S. The effects of Ginseng Java root extract on uterine contractility in nonpregnant rats. Physiol. Rep. 2014, 2, e12230. [Google Scholar] [CrossRef] [PubMed]
- Manuhara, Y.W.; Kristanti, A.N.; Utami, E.S.W.; Yachya, A. Effect of sucrose and potassium nitrate on biomass and saponin content of Talinum paniculatum Gaertn. hairy root in balloon-type bubble bioreactor. Asian Pac. J. Trop. Biomed. 2015, 5, 1027–1032. [Google Scholar] [CrossRef]
- Valdés-González, J.A.; Sánchez, M.; Moratilla-Rivera, I.; Iglesias, I.; Gómez-Serranillos, M.P. Immunomodulatory, anti-Inflammatory, and anti-cancer properties of ginseng: A pharmacological update. Molecules 2023, 28, 3863. [Google Scholar] [CrossRef] [PubMed]
- Arman, A. Immunomodulator activity and anthirheumatoid arthritis extract of ethyl acetae ginseng bugis Talinum paniculatum (jactq.Gaertn). J. Glob. Pharma Tecnol. 2020, 12, 246–251. [Google Scholar]
- Cerdeira, C.D.; Da Silva, J.J.; Netto, M.F.R.; Boriollo, M.F.G.; Moraes, G.O.I.; Santos, G.B.; Dos Reis, L.F.C.; Brigagão, M.R.P.L. Talinum paniculatum: A plant with antifungal potential mitigates fluconazole-induced oxidative damage-mediated growth inhibition of Candida albicans. Rev. Colomb. Cienc. Quím. Farm. 2020, 49, 401–431. [Google Scholar] [CrossRef]
- Tolouei, S.E.L.; Palozi, R.A.C.; Tirloni, C.A.S.; Marques, A.A.M.; Schaedler, M.I.; Guarnier, L.P.; Silva, A.O.; de Almeida, V.P.; Manfron Budel, J.; Souza, R.I.C.; et al. Ethnopharmacological approaches to Talinum paniculatum (Jacq.) Gaertn.-Exploring cardiorenal effects from the Brazilian Cerrado. J. Ethnopharmacol. 2019, 238, 111873. [Google Scholar] [CrossRef]
- Souto, C.G.R.G.; Lorençone, B.R.; Marques, A.A.M.; Palozi, R.A.C.; Romão, P.V.M.; Guarnier, L.P.; Tirloni, C.A.S.; dos Santos, A.C.; Souza, R.I.C.; Zago, P.M.J.J.; et al. Cardioprotective effects of Talinum paniculatum (Jacq.) Gaertn. in doxorubicin-induced cardiotoxicity in hypertensive rats. J. Ethnopharmacol. 2021, 281, 114568. [Google Scholar] [CrossRef]
- Dhawan, O.P.; Lavania, U.C. Enhancing the productivity of secondary metabolites via induced polyploidy: A review. Euphytica 1996, 87, 81–89. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Ashman, T.-L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An evolutionary and ecological force in stressful times. Plant Cell 2021, 33, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.L.; Martin, S.L.; James, T.; Golenia, G.; Boudko, E.A.; Hepworth, S.R. Polyploidization for the genetic improvement of Cannabis sativa. Front. Plant Sci. 2019, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-H.; Shi, S.-K.; Huang, B.; Chen, J.-T. Enhanced agronomic traits and medicinal constituents of autotetraploids in Anoectochilus formosanus Hayata, a top-grade medicinal orchid. Molecules 2017, 22, 1907. [Google Scholar] [CrossRef]
- Fernando, S.C.; Goodger, J.Q.D.; Chew, B.L.; Cohen, T.J.; Woodrow, I.E. Induction and characterisation of tetraploidy in Eucalyptus polybractea R.T. Baker. Ind. Crops Prod. 2019, 140, 111633. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Qiu, B.; Ma, Z.; Lu, T.; Kang, X.; Yang, J. Induction and characterization of tetraploid through zygotic chromosome doubling in Eucalyptus urophylla. Front. Plant Sci. 2022, 13, 870698. [Google Scholar] [CrossRef]
- Blakeslee, A.F.; Avery, A.G. Methods of inducing doubling of chromosome in plants: By treatment with colchicine. J. Hered. 1937, 28, 393–411. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Hafiz, I.A.; Silvestri, C. Studies on colchicine induced chromosome doubling for enhancement of quality traits in ornamental plants. Plants 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Huang, X.; Li, W. Induction of tetraploid via somatic embryos treated with colchicine in Hevea brasiliensis (Willd. ex A.Juss.) Müll. Arg. Plant Cell Tissue Organ Cult. 2022, 150, 487–491. [Google Scholar] [CrossRef]
- Eng, W.-H.; Ho, W.-S. Polyploidization using colchicine in horticultural plants: A review. Sci. Hortic. 2019, 246, 604–617. [Google Scholar] [CrossRef]
- Tavan, M.; Mirjalili, M.H.; Karimzadeh, G. In vitro polyploidy induction: Changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult. 2015, 122, 573–583. [Google Scholar] [CrossRef]
- Zhou, H.; Zeng, W.; Yan, H. In vitro induction of tetraploids in cassava variety ‘Xinxuan 048’ using colchicine. Plant Cell Tissue Organ Cult. 2017, 128, 723–729. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef]
- Wu, J.; Sang, Y.; Zhou, Q.; Zhang, P. Colchicine in vitro tetraploid induction of Populus hopeiensis from leaf blades. Plant Cell Tissue Organ Cult. 2020, 141, 339–349. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, S.; Xu, T.; Kang, X. Morphological, transcriptome, and hormone analysis of dwarfism in tetraploids of Populus alba × P. glandulosa. Int. J. Mol. Sci. 2022, 23, 9762. [Google Scholar] [CrossRef] [PubMed]
- Beranová, K.; Bharati, R.; Žiarovská, J.; Bilčíková, J.; Hamouzová, K.; Klíma, M.; Fernández-Cusimamani, E. Morphological, cytological, and molecular comparison between diploid and induced autotetraploids of Callisia fragrans (Lindl.) Woodson. Agronomy 2022, 12, 2520. [Google Scholar] [CrossRef]
- Marangelli, F.; Pavese, V.; Vaia, G.; Lupo, M.; Bashir, M.A.; Cristofori, V.; Silvestri, C. In vitro polyploid induction of highbush blueberry through De Novo shoot organogenesis. Plants 2022, 11, 2349. [Google Scholar] [CrossRef] [PubMed]
- Fakhrzad, F.; Jowkar, A.; Shekafandeh, A.; Kermani, M.J.; Moghadam, A. Tetraploidy induction enhances morphological, physiological and biochemical characteristics of wallflower (Erysimum cheiri (L.) Crantz). Sci. Hortic. 2023, 308, 111596. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, H.; Meng, H.; Qiao, L.; Zhang, G.; Cheng, Z. In vitro induction and phenotypic variations of autotetraploid garlic (Allium sativum L.) with dwarfism. Front. Plant Sci. 2022, 13, 917910. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xue, H.; Zhang, L.; Zhang, F.; Ou, C.; Wang, F.; Zhang, Z. Involvement of auxin and brassinosteroid in dwarfism of autotetraploid apple (Malus × domestica). Sci. Rep. 2016, 6, 26719. [Google Scholar] [CrossRef]
- Bhusare, B.P.; John, C.K.; Bhatt, V.P.; Nikam, T.D. Induction of somatic embryogenesis in leaf and root explants of Digitalis lanata Ehrh.: Direct and indirect method. South Afr. J. Bot. 2020, 130, 356–365. [Google Scholar] [CrossRef]
- Mionić Ebersold, M.; Petrović, M.; Fong, W.-K.; Bonvin, D.; Hofmann, H.; Milošević, I. Hexosomes with undecylenic acid efficient against Candida albicans. Nanomaterials 2018, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Zhao, Y.; Yan, H.; Fu, H.; Shen, Y.; Lu, G.; Mei, H.; Qiu, Y.; Li, D.; Liu, W. Antifungal effects of undecylenic acid on the biofilm formation of Candida albicans. Int. J. Clin. Pharmacol. Ther. 2016, 54, 343–353. [Google Scholar] [CrossRef]
- Day, Z.I.; Mayfosh, A.J.; Giel, M.-C.; Hong, Y.; Williams, S.A.; Santavanond, J.P.; Rau, T.F.; Poon, I.K.; Hulett, M.D. Novel formulation of undecylenic acid induces tumor cell apoptosis. Int. J. Mol. Sci. 2022, 23, 14170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qi, L.; Chen, C.; Li, X.; Song, W.; Chen, R.; Han, S. A report of triploid Populus of the section Aigeiros. Silvae Genet. 2004, 53, 69–75. [Google Scholar] [CrossRef]
- Ao, S.; He, L.; Xiao, G.; Chen, J.; He, C. Selection of rubber cold fast and high-yield strains, Yunyan77–2 and Yunyan77–4. J. Yunnan Trop. Crops Sci. Technol. 1998, 21, 3–8, (In Chinese with English abstract). [Google Scholar]
- Li, H.B.; Zhou, T.Y.; Ning, L.Y.; Li, G.H. Cytological identification and breeding course of Hevea Yunyan77-2 and Yunyan77-4. J. Trop. Subtrop. Bot. 2009, 17, 602–605, (in Chinese with English abstract). [Google Scholar]
- Serapiglia, M.J.; Gouker, F.E.; Smart, L.B. Early selection of novel triploid hybrids of shrub willow with improved biomass yield relative to diploids. BMC Plant Biol. 2014, 14, 74. [Google Scholar] [CrossRef][Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hamill, S.D.; Smith, M.K.; Dodd, W.A. In vitro induction of banana autotetraploids by colchicine treatment of micropropagated diploids. Aust. J. Bot. 1992, 40, 887–896. [Google Scholar] [CrossRef]

| Medium | Hormone Concentrations (ppm) | ||
|---|---|---|---|
| 6-BA | NAA | KT | |
| 1 | 3 | 1 | 0 |
| 2 | 0 | 0.5 | 0.5 |
| 3 | 3 | 0 | 0.5 |
| 4 | 0 | 1 | 0.25 |
| 5 | 1.5 | 1 | 0.5 |
| 6 | 3 | 0.5 | 0.25 |
| 7 | 1.5 | 0.5 | 0 |
| 8 | 1.5 | 0 | 0.25 |
| 9 | 0 | 0 | 0 |
| Treatment Time (h) | Colchicine Concentration (mg/mL) | No. of Explants | No. of Plantlets | No. of Tetraploids | No. of Chimeras | Tetraploid Induction Rates ± SE (%) |
|---|---|---|---|---|---|---|
| 24 | 1 | 64 | 60 | 2 | 8 | 3.13 ± 0.10 |
| 24 | 2 | 63 | 59 | 6 | 9 | 9.52 ± 4.62 |
| 24 | 3 | 62 | 44 | 5 | 11 | 8.06 ± 12.90 |
| 48 | 1 | 61 | 56 | 11 | 7 | 18.03 ± 6.82 |
| 48 | 2 | 62 | 52 | 11 | 9 | 17.74 ± 1.04 |
| 48 | 3 | 62 | 31 | 9 | 8 | 14.52 ± 3.65 |
| 72 | 1 | 61 | 45 | 8 | 7 | 13.11 ± 1.88 |
| 72 | 2 | 62 | 34 | 8 | 10 | 12.9 ± 5.31 |
| 72 | 3 | 61 | 17 | 1 | 4 | 1.64 ± 1.72 |
| Traits | Diploids | Tetraploids |
|---|---|---|
| Stomatal length (µm) | 30.01 ± 3.45 b | 40.78 ± 4.76 a |
| Stomatal width (µm) | 18.59 ± 2.33 b | 22.82 ± 3.65 a |
| Stomatal density (mm−2) | 149.93 ± 31.79 a | 53.68 ± 10.36 b |
| Leaf thickness (mm) | 0.33 ± 0.04 b | 0.62 ± 0.07 a |
| Pollen diameter (μm) | 65.36 ± 1.92 b | 84.95 ± 5.69 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Huang, X.; Gao, X.; Zhang, X.; Huang, H.; Li, W.; Zhang, Y. Tetraploidization Altered Phenotypic Traits and Metabolite Profile of Java Ginseng (Talinum paniculatum (Jacq.) Gaertn.). Plants 2025, 14, 480. https://doi.org/10.3390/plants14030480
Liu Y, Huang X, Gao X, Zhang X, Huang H, Li W, Zhang Y. Tetraploidization Altered Phenotypic Traits and Metabolite Profile of Java Ginseng (Talinum paniculatum (Jacq.) Gaertn.). Plants. 2025; 14(3):480. https://doi.org/10.3390/plants14030480
Chicago/Turabian StyleLiu, Yingying, Xiao Huang, Xinsheng Gao, Xiaofei Zhang, Huasun Huang, Weiguo Li, and Yuanyuan Zhang. 2025. "Tetraploidization Altered Phenotypic Traits and Metabolite Profile of Java Ginseng (Talinum paniculatum (Jacq.) Gaertn.)" Plants 14, no. 3: 480. https://doi.org/10.3390/plants14030480
APA StyleLiu, Y., Huang, X., Gao, X., Zhang, X., Huang, H., Li, W., & Zhang, Y. (2025). Tetraploidization Altered Phenotypic Traits and Metabolite Profile of Java Ginseng (Talinum paniculatum (Jacq.) Gaertn.). Plants, 14(3), 480. https://doi.org/10.3390/plants14030480





