Structural Particularities of Gall Neoformations Induced by Monarthropalpus flavus in the Leaves of Buxus sempervirens
Abstract
1. Introduction
2. Results
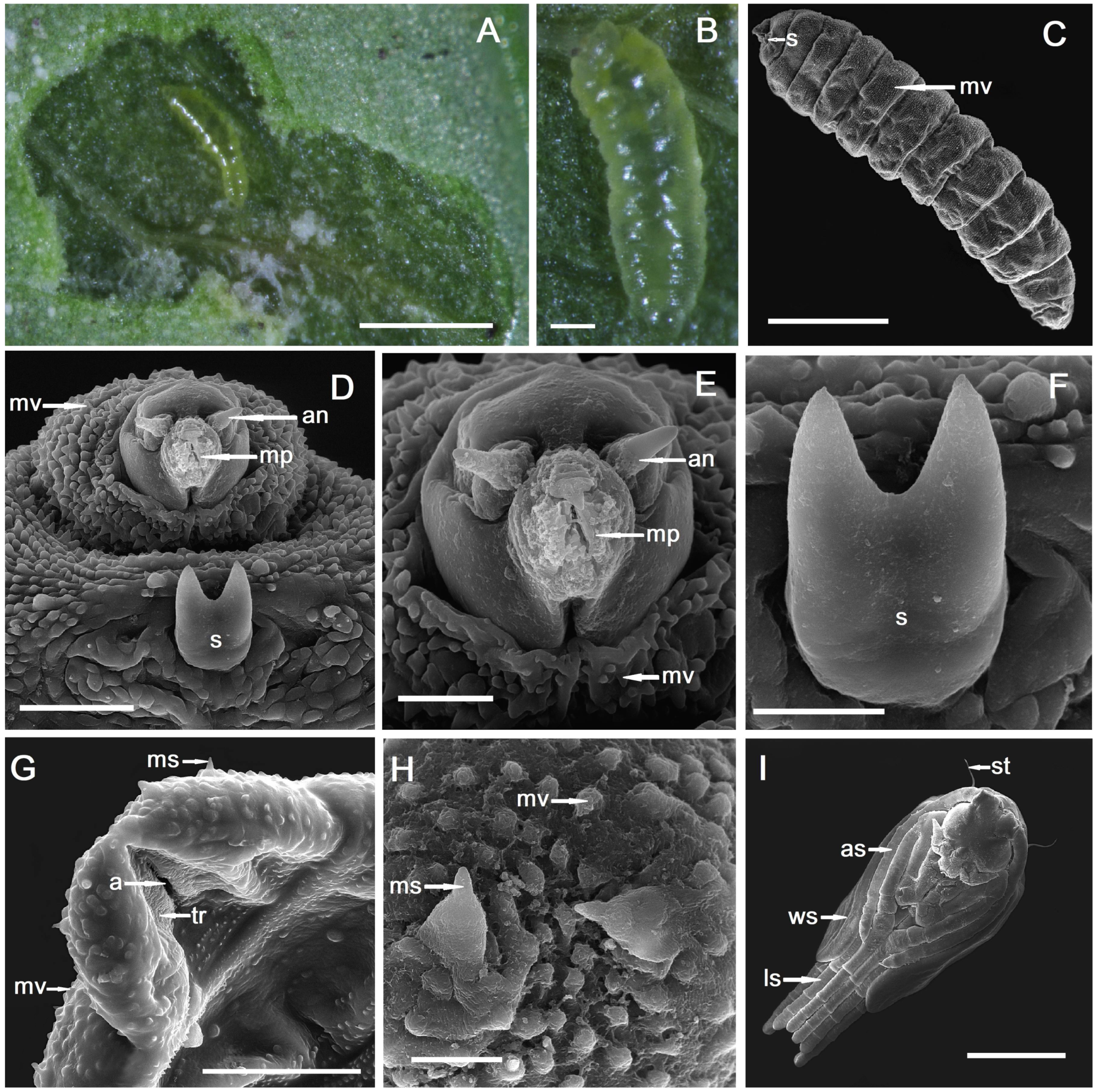
2.1. Gall Inducer—Monarthropalpus flavus
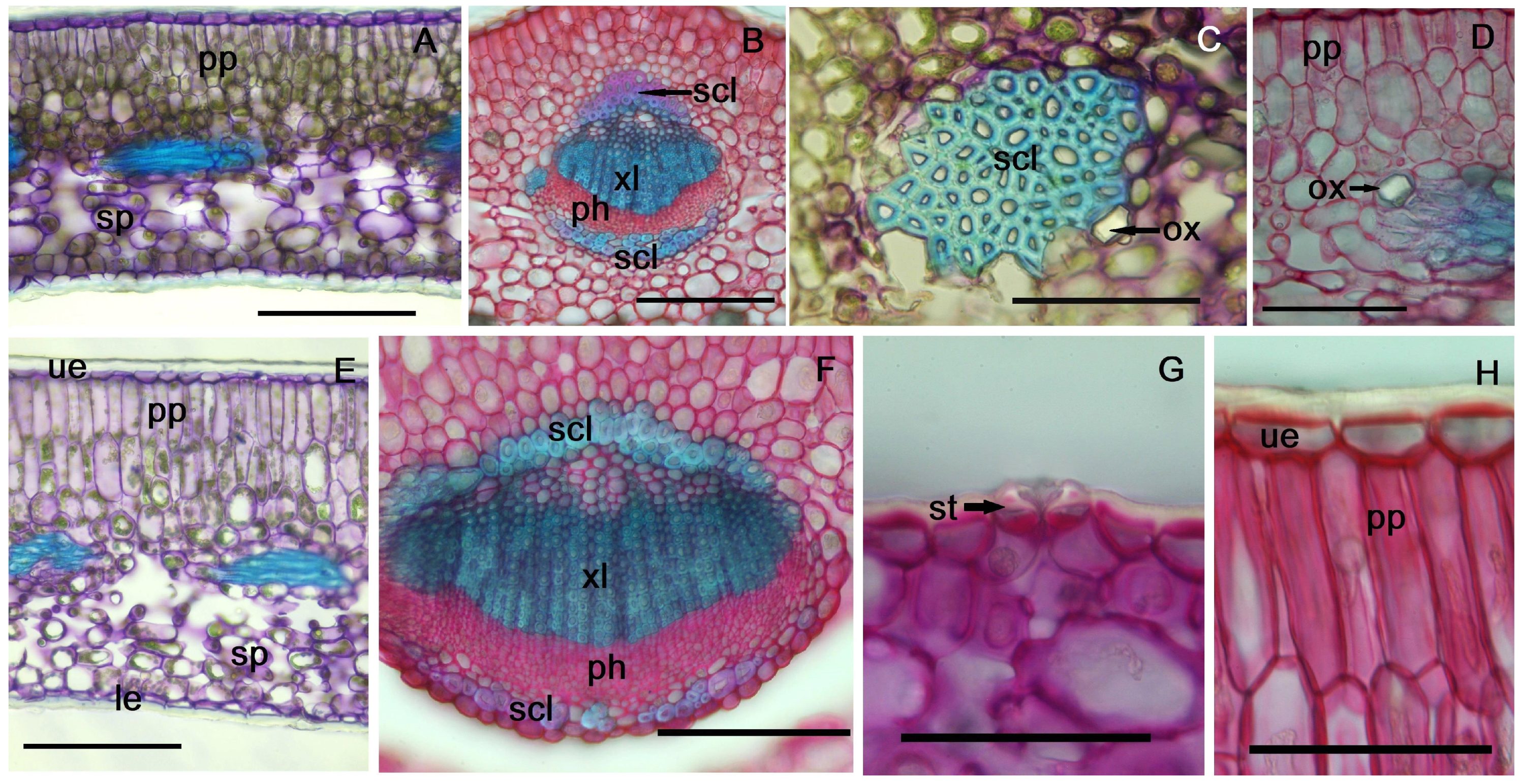
2.2. Non-Galled Leaf Anatomical Features
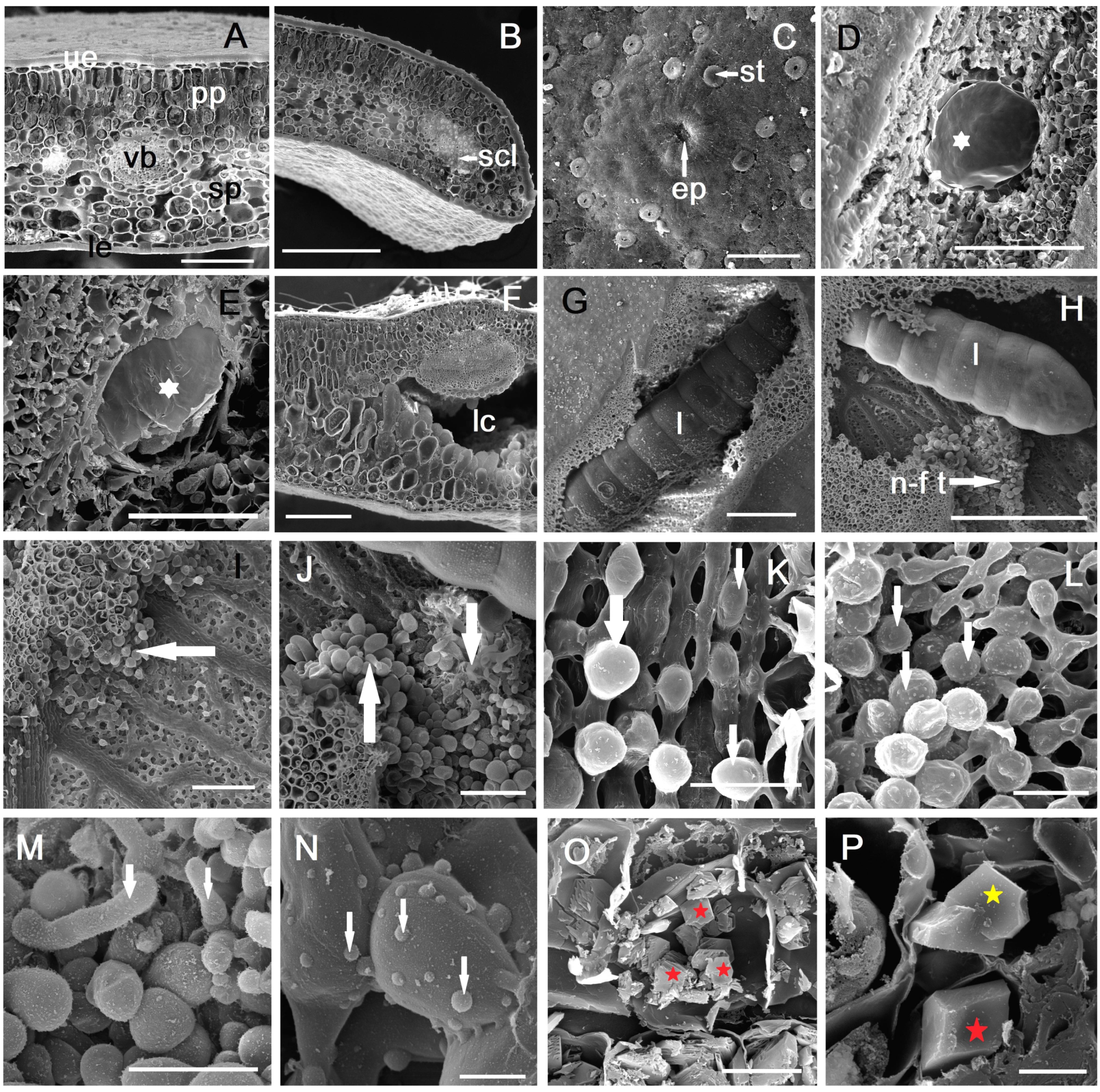
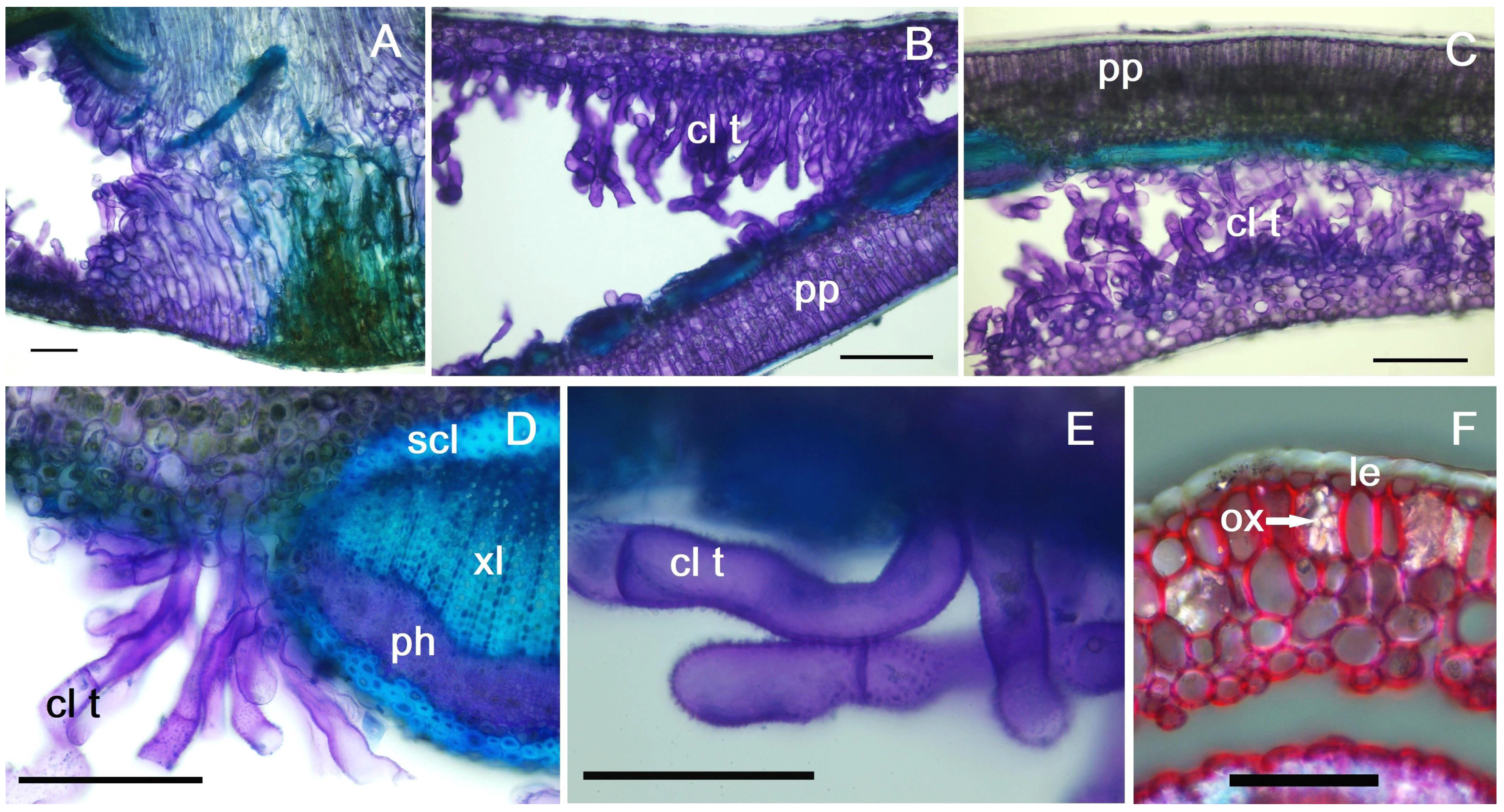
2.3. Anatomy of Galled Leaves
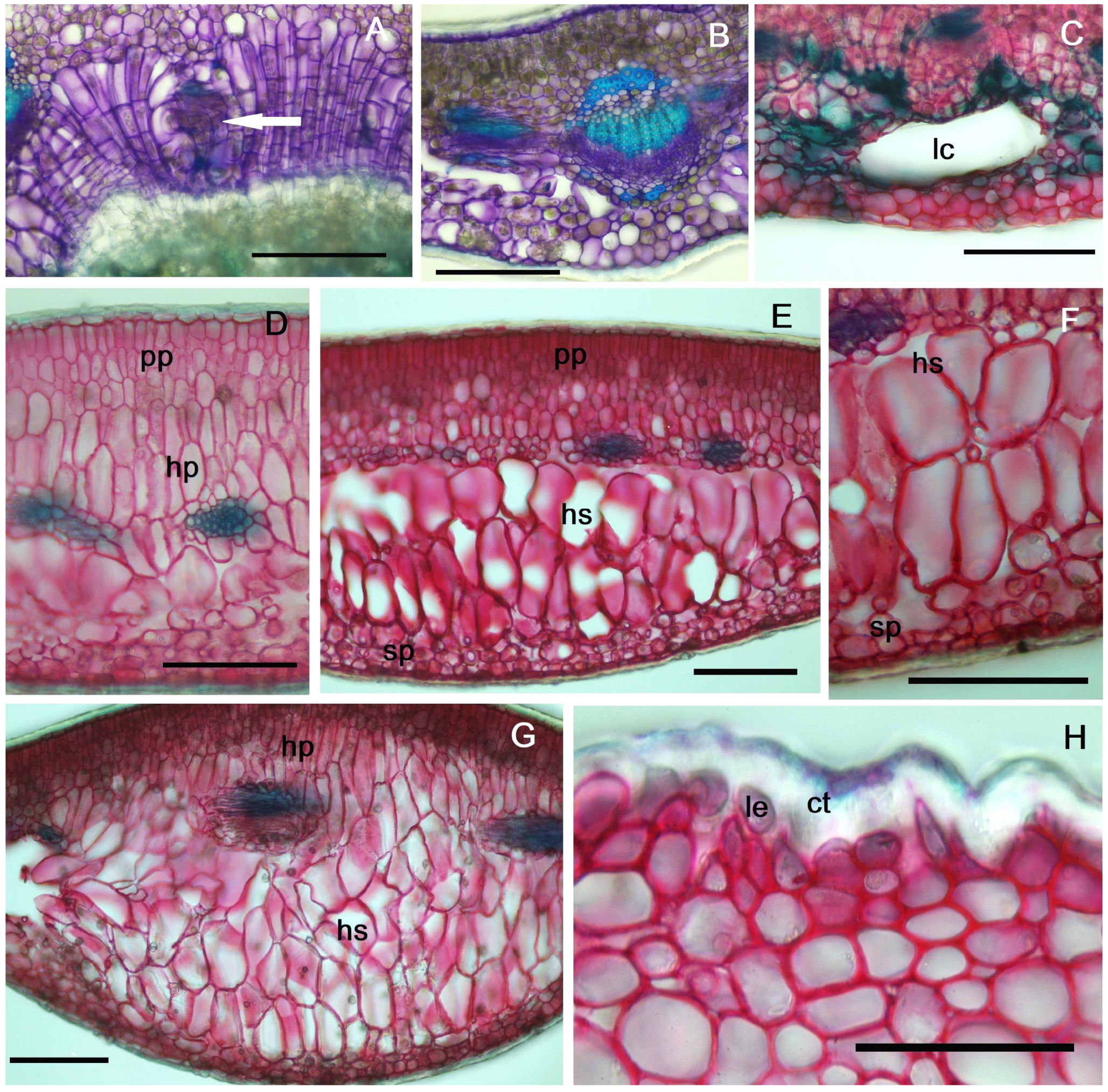
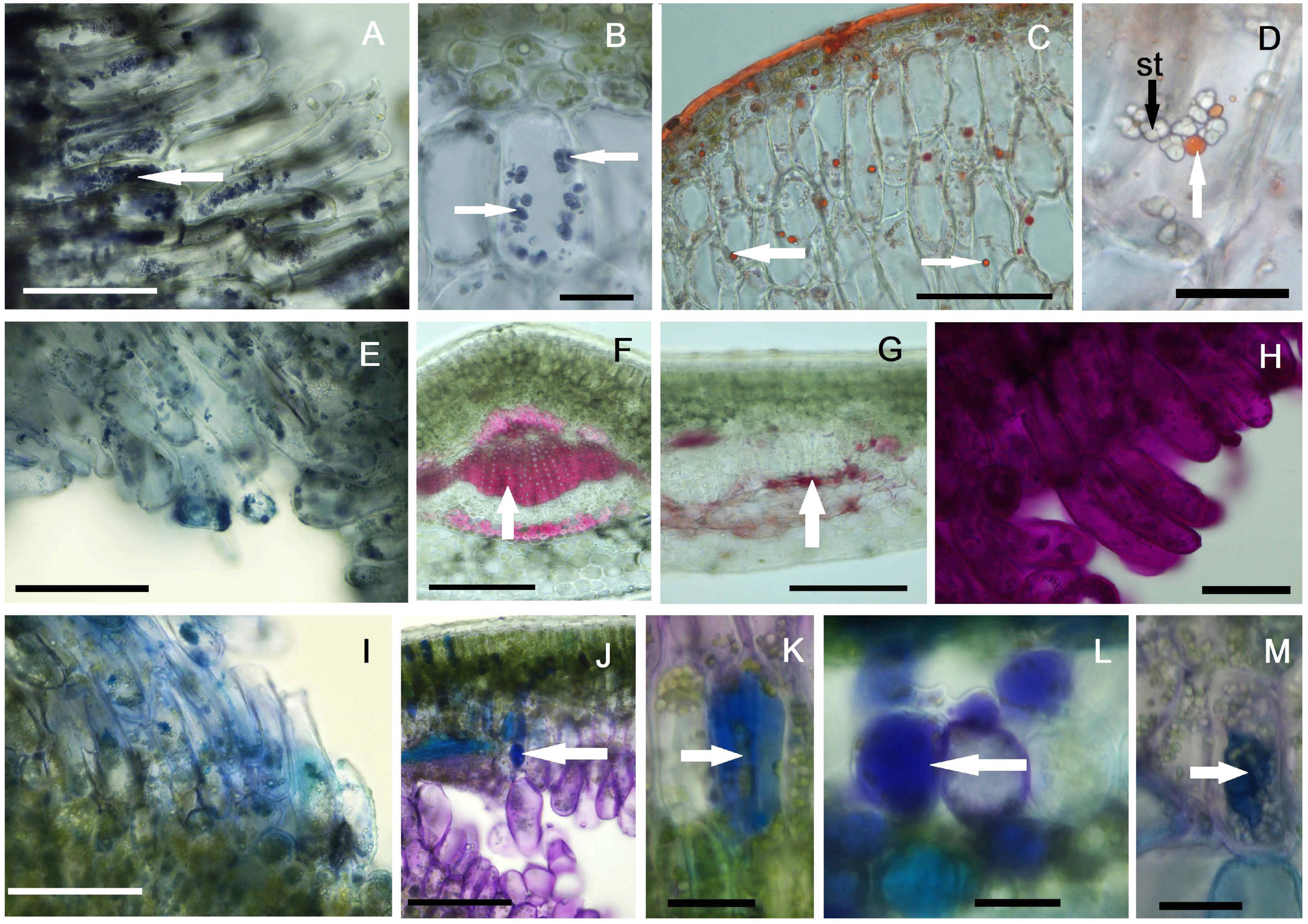
2.4. Histochemical Profiles of the Galls
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Light Microscopy
4.3. Histo-Anatomical Investigations
4.4. Histochemical Investigations
4.5. Scanning Electron Microscopy Investigations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- POWO—Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/ (accessed on 26 November 2024).
- Di Domenico, F.; Lucchese, F.; Magri, D. Late glacial and Holocene history of Buxus sempervirens L. in Italy. Ann. Bot. 2011, 1, 45–58. [Google Scholar]
- Gostin, I.N. Leaf micromorphology in Buxus sempervirens L. during the ontogenesis. Analele Univ. din Oradea Fasc. Biol. 2009, 16, 57–60. [Google Scholar]
- Amtaghri, S.; Eddouks, M. Pharmacological and Phytochemical Properties of the Genus Buxus: A Review. Fitoterapia 2024, 177, 106081. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, P.-A.; Sauvion, N.; Thiéry, D.; Rebaudo, F.; Jacquin-Joly, E. Plant-Insect Interactions. In Ecology; Oxford University Press: Oxford, UK, 2018; ISBN 978-0-19-983006-0. [Google Scholar]
- Barbero, F.; Mannino, G.; Casacci, L.P. The Role of Biogenic Amines in Social Insects: With a Special Focus on Ants. Insects 2023, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.E.; Gostin, I.N. A New Species of Megastigmus and First Record of the Genus and Megastigmidae Family from the Paradise of the Maldives Archipelago. Insects 2023, 14, 677. [Google Scholar] [CrossRef]
- Sharma, H.C.; Dhillon, M.K. Climate Change Effects on Arthropod Diversity and Its Implications for Pest Management and Sustainable Crop Production. In Agronomy Monographs; Hatfield, J.L., Sivakumar, M.V.K., Prueger, J.H., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Inc.: Madison, WI, USA, 2018; pp. 595–619. ISBN 978-0-89118-358-7. [Google Scholar]
- Yactayo-Chang, J.P.; Tang, H.V.; Mendoza, J.; Christensen, S.A.; Block, A.K. Plant Defense Chemicals against Insect Pests. Agronomy 2020, 10, 1156. [Google Scholar] [CrossRef]
- Tooker, J.F.; Giron, D. The Evolution of Endophagy in Herbivorous Insects. Front. Plant Sci. 2020, 11, 581816. [Google Scholar] [CrossRef]
- Nastasi, L.F.; Smith, C.N.; Tooker, J.F.; Deans, A.R. Catalog of Endophytic Insects Associated with Rosinweeds (Silphium L., Heliantheae, Asteraceae) in North America. Psyche A J. Entomol. 2024, 2024, 8464635. [Google Scholar] [CrossRef]
- Giron, D.; Huguet, E.; Stone, G.N.; Body, M. Insect-Induced Effects on Plants and Possible Effectors Used by Galling and Leaf-Mining Insects to Manipulate Their Host-Plant. J. Insect Physiol. 2016, 84, 70–89. [Google Scholar] [CrossRef]
- Hering, E.M. Biology of the Leaf Miners; Junk: s’Gravenhage, The Netherlands, 1951. [Google Scholar]
- Isaias, R.M.D.S.; Jorge, N.D.C.; Ferreira, B.G.; Fochezato, J.; Moreira, G.R.P. How Cells and Tissues of Daphnopsis fasciculata (Thymelaeaceae) React to the Leaf-mining Habit of Phyllocnistis hemera (Lepidoptera: Gracillariidae). J. Plant Res. 2021, 134, 535–541. [Google Scholar] [CrossRef]
- Soporan, C.R.; Oltean, I.; Florian, T.; Macavei, L. Chemical methods of fight against Monarthropalpus buxi Geoff. species. Bull. UASMV Ser. Agric. 2013, 70, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Raupp, M.J.; Mars, I.H.; d’Eustachio, G. Integrated approaches for managing the boxwood leafminer, Monarthropalpus flavus. In Nursery crops; development, evaluation, production and use. XXVI International Horticultural Congress. ISHS Acta Hortic. 2002, 630, 57–63. [Google Scholar]
- Kaur, B.; Dale, A. Boxwood leafminer Monarthropalpus flavus (Schrank) (Insecta: Diptera: Cecidomyiidae). EENY752/IN1291, 4/2020. EDIS 2020, 5. [Google Scholar] [CrossRef]
- Dobrosavljevic, J.; Markovic, C.; Bojic, S. Overview of leaf miner fauna in Serbia. In Proceedings of the VIII International Agriculture Symposium “AGROSYM 2017”, Jahorina, Bosnia and Herzegovina, 5–8 October 2017; Kovacevic, D., Ed.; University of East Sarajevo, Faculty of Agriculture: Bosnia, Republic of Srpska, 2018; pp. 1490–1498. [Google Scholar]
- Skuhravý, V.; Skuhravá, M.; Brewer, J.W. Some Survival Adaptations of Gall-inducing Midges (Dipt., Cecidomyiidae). J. Appl. Entomol. 1996, 120, 237–239. [Google Scholar] [CrossRef]
- Hamilton, C.C. Notes on the Life History and the Control Methods of the Box Wood Leaf Midge, (Monarthropalpus buxi Labou.). J. Econ. Entomol. 1921, 14, 359–365. [Google Scholar] [CrossRef]
- Mitchell, R.; Chitanava, S.; Dbar, R.; Kramarets, V.; Lehtijärvi, A.; Matchutadze, I.; Mamadashvili, G.; Matsiakh, I.; Nacambo, S.; Papazova-Anakieva, I.; et al. Identifying the Ecological and Societal Consequences of a Decline in Buxus Forests in Europe and the Caucasus. Biol. Invasions 2018, 20, 3605–3620. [Google Scholar] [CrossRef]
- d’Eustachio, G.; Raupp, M. Application of Systemic Insecticides in Relation to Boxwood Leafminer’s Life History. AUF 2001, 27, 255–262. [Google Scholar] [CrossRef]
- Cilbircioğlu, C.; Ünal, S. Gall Midges (Diptera: Cecidomyiidae) in Forest Trees of Turkey. J. Agric. Urban Entomol. 2008, 25, 13–23. [Google Scholar] [CrossRef]
- Eiseman, C.S.; Blyth, J.A. Nantucket’s Neglected Herbivores II: Diptera. Proc. Entomol. Soc. Wash. 2022, 124, 564–605. [Google Scholar] [CrossRef]
- Meyer, J.; Maresquelle, H.-J. Anatomie des Galles, Handb. Pflanzenanat, XIII; Bornträger: Stuttgart, Germany, 1983. [Google Scholar]
- Schread, J.C. Thimet Soil Treatments for the Control of Leaf Miners. J. Econ. Entomol. 1958, 51, 245–246. [Google Scholar] [CrossRef]
- d’Eustachio, G.; Raupp, M. Resistance of Boxwood Varieties to the Boxwood Leafminer, Monarthropalpus flavus (Schrank). J. Environ. Hortic. 2001, 19, 153–157. [Google Scholar] [CrossRef]
- Price, P.W.; Fernandes, G.W.; Waring, G.L. Adaptive Nature of Insect Galls. Environ. Entomol. 1987, 16, 15–24. [Google Scholar] [CrossRef]
- Rohfritsch, O. Patterns in gall development. In Biology of Insect-Induced Galls; Shorthouse, J.D., Rohfritsch, O., Eds.; Oxford University Press: New York, NY, USA, 1992; pp. 60–86. [Google Scholar]
- Schoonhoven, L.M.; van Loon, J.J.A.; Dicke, M. Insect-Plant Biology, 2nd ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Redfern, M. Plant galls: An intimate association between animals and plants. Antenna 1997, 21, 55–63. [Google Scholar]
- Fernandes, G.; Carneiro, M.A.A.; Isaias, R. Gall-Inducing Insects: From Anatomy to Biodiversity. In Insect Bioecology and Nutrition for Integrated Pest Management; Panizzi, A.R., Parra, J.R.P., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 369–395. ISBN 978-1-4398-3708-5. [Google Scholar]
- Melo De Pinna, G.F.A.; Kraus, J.E.; De Menezes, N.L. Morphology and anatomy of leaf mine in Richterago riparia Roque (Asteraceae) in the Campos Rupestres of Serra do Cipó, Brazil. Braz. J. Biol. 2002, 62, 179–185. [Google Scholar] [CrossRef]
- Isaias, R.M.D.S.; Kraus, J.E.; Costa, E.C.; Carneiro, R.G.D.S. The anatomy of neotropical galls and the untold lessons about the morphogenetical potentialities of plants. Rodriguésia 2024, 75, e01542023. [Google Scholar]
- Del Bene, G.; Grossoni, P.; Mori, B. Observations on the Morpho-Anatomical Development of the Boxwood Leaf Gall Induced by Monarthropalpus buxi (Laboulbene) (Diptera Cecidomyiidae). Adv. Hort. Sci. 1996, 10, 99–103. [Google Scholar] [CrossRef]
- Abrahamson, W.G.; Mccrea, K.D.; Whitwell, A.J.; Vernieri, L.A. The role of phenolics in goldenrod ball gall resistance. Biochem. Syst. Ecol. 1991, 19, 615–622. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The Roles of Plant Phenolics in Defence and Communication during Agrobacterium and Rhizobium Infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef]
- Nyman, R.; Julkunen-Titto, R. Manipulation of the phenolic chemistry of willows by gall-inducing sawflies. Proc. Natl. Acad. Sci. USA 2001, 97, 13184–13187. [Google Scholar] [CrossRef]
- Sá, C.E.M.D.; Silveira, F.A.O.; Santos, J.C.; Isaias, R.M.D.S.; Fernandes, G.W. Anatomical and Developmental Aspects of Leaf Galls Induced by Schizomyia macrocapillata Maia (Diptera: Cecidomyiidae) on Bauhinia brevipes Vogel (Fabaceae). Rev. Bras. Bot. 2009, 32, 319–327. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The Multifunctional Roles of Polyphenols in Plant-Herbivore Interactions. IJMS 2021, 22, 1442. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.G.; Isaias, R.M.D.S. Floral-like Destiny Induced by a Galling Cecidomyiidae on the Axillary Buds of Marcetia taxifolia (Melastomataceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 391–400. [Google Scholar] [CrossRef]
- Moura, M.Z.D.; Soares, G.L.G.; Isaias, R.M.D.S. Species-Specific Changes in Tissue Morphogenesis Induced by Two Arthropod Leaf Gallers in Lantana camara L. (Verbenaceae). Aust. J. Bot. 2008, 56, 153. [Google Scholar] [CrossRef]
- Guimarães, A.L.D.A.; Bizarri, C.H.B.; Barbosa, L.S.; Nakamura, M.J.; De Souza Ramos, M.F.; De Macêdo Vieira, A.C. Characterisation of the Effects of Leaf Galls of Clusiamyia nitida (Cecidomyiidae) on Clusia lanceolata Cambess. (Clusiaceae): Anatomical Aspects and Chemical Analysis of Essential Oil. Flora Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 165–173. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Magalhães, T.A.; Carneiro, R.G.S.; Alvim, M.N.; Isaias, R.M.S. Do Cecidomyiidae Galls of Aspidosperma spruceanum (Apocynaceae) Fit the Pre-Established Cytological and Histochemical Patterns? Protoplasma 2010, 242, 81–93. [Google Scholar] [CrossRef]
- Bronner, R. The role of nutritive cells in the nutrition of cynipids and cecidomyiids. In Biology of Insect Induced Galls; Shorthouse, J.D., Rohfritsch, O., Eds.; Oxford University Press: New York, NY, USA, 1992; pp. 118–140. [Google Scholar]
- Bedetti, C.S.; Ferreira, B.G.; de Castro, N.M.; dos Santos Isaias, R.M. The influence of parasitoidism on the anatomical and histochemical profiles of the host leaves in a galling Lepidoptera—Bauhinia ungulata system. Rev. Bras. Biociênc. 2013, 11, 242–249. [Google Scholar]
- Schönrogge, K.; Harper, L.J.; Lichtenstein, C.P. The Protein Content of Tissues in Cynipid Galls (Hymenoptera: Cynipidae): Similarities between Cynipid Galls and Seeds. Plant Cell Environ. 2000, 23, 215–222. [Google Scholar] [CrossRef]
- Takeda, S.; Hirano, T.; Ohshima, I.; Sato, M.H. Recent Progress Regarding the Molecular Aspects of Insect Gall Formation. Int. J. Mol. Sci. 2021, 22, 9424. [Google Scholar] [CrossRef]
- Costa, E.C.; Carneiro, R.G.D.S.; Silva, J.S.; Isaias, R.M.D.S. Biology and Development of Galls Induced by Lopesia sp. (Diptera: Cecidomyiidae) on Leaves of Mimosa gemmulata (Leguminosae: Caesalpinioideae). Aust. J. Bot. 2018, 66, 161. [Google Scholar] [CrossRef]
- Marquesine, R.R.; Canaveze, Y.; Ferreira, B.G. Ontogenetic Differences in Sun and Shade Galls of Clinodiplosis profusa on Eugenia uniflora Leaves and the Cytological Antioxidant Mechanisms in Gall Cells. Protoplasma 2024, 262, 15–34. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and Function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Arduin, M.; Kraus, J.E. Anatomia e Ontogenia de Galhas Foliares de Piptadenia gonoacantha (Fabales, Mimosaceae). Bol. Bot. 1995, 14, 109. [Google Scholar] [CrossRef]
- Rezende, U.C.; Cardoso, J.C.F.; Kuster, V.C.; Gonçalves, L.A.; Oliveira, D.C. How the Activity of Natural Enemies Changes the Structure and Metabolism of the Nutritive Tissue in Galls? Evidence from the Palaeomystella oligophaga (Lepidoptera)-Macairea radula (Metastomataceae) System. Protoplasma 2019, 256, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Gostin, I.; Ivanescu, L. Leaf structure and development in Buxus sempervirens. Nat. Montenegrina 2008, 7, 27–32. [Google Scholar]
- Staszak, A.M.; Ratajczak, E.; Leśniewska, J.; Piotrowska-Niczyporuk, A.; Kostro-Ambroziak, A. A Broad Spectrum of Host Plant Responses to the Actions of the Gall Midge: Case Study of Robinia pseudoacacia L. and Obolodiplosis robiniae (Haldeman). BMC Plant Biol. 2023, 23, 19. [Google Scholar] [CrossRef]
- Popescu, I.E.; Gostin, I.N. Lasioptera rubi, a Pest of Rubus idaeus: Galls Morphology, Anatomy and Histochemistry. Agriculture 2024, 14, 1761. [Google Scholar] [CrossRef]
- Popescu, I.E.; Gostin, I.N.; Blidar, C.F. An Overview of the Mechanisms of Action and Administration Technologies of the Essential Oils Used as Green Insecticides. AgriEngineering 2024, 6, 1195–1217. [Google Scholar] [CrossRef]
- Jensen, W.A. Botanical Histochemistry: Principles and Practice; W. H. Freeman and Co.: San Francisco, CA, USA, 1962. [Google Scholar]
- Şerbănescu-Jitariu, G.; Andrei, M.; Mitroiu-Rădulescu, N.; Petria, E. Practicum de Biologie Vegetală; CERES: Bucureşti, Romania, 1983. [Google Scholar]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGrawHill: New York, NY, USA, 1940. [Google Scholar]
- Brundrett, M.C.; Kendrick, B.; Peterson, C.A. Efficient Lipid Staining in Plant Material with Sudan Red 7B or Fluoral Yellow 088 in Polyethylene Glycol-Glycerol. Biotech. Histochem. 1991, 66, 111–116. [Google Scholar] [CrossRef]
- David, R.; Carde, J.P. Histochimie-Coloration Differentielle Des Inclusions Lipidiques et Terpeniques des Pseudophylles du Pin Maritime au Moyen du Reactif NADI. Comptes Rendus Hebd. Seances Acad. Sci. 1964, 258, 1338. [Google Scholar]
- McManus, J.F.A. Histological and Histochemical Uses of Periodic Acid. Stain Technol. 1948, 23, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Gahan, P.B. Plant Histochemistry and Cytochemistry: An Introduction; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Gutmann, M. Improved Staining Procedures for Photographic Documentation of Phenolic Deposits in Semithin Sections of Plant Tissue. J. Microsc. 1995, 179, 277–281. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, S.; Mariama, K.; Elsen, A.; De Waele, D. Phenols and Lignin Are Involved in the Defence Response of Banana (Musa) Plants to Radopholus similis Infection. Nematology 2014, 16, 565–576. [Google Scholar] [CrossRef]
- Bedetti, C.S.; Modolo, L.V.; Isaias, R.M.D.S. The Role of Phenolics in the Control of Auxin in Galls of Piptadenia gonoacantha (Mart.) MacBr (Fabaceae: Mimosoideae). Biochem. Syst. Ecol. 2014, 55, 53–59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gostin, I.N.; Popescu, I.E.; Blidar, C.F. Structural Particularities of Gall Neoformations Induced by Monarthropalpus flavus in the Leaves of Buxus sempervirens. Plants 2025, 14, 453. https://doi.org/10.3390/plants14030453
Gostin IN, Popescu IE, Blidar CF. Structural Particularities of Gall Neoformations Induced by Monarthropalpus flavus in the Leaves of Buxus sempervirens. Plants. 2025; 14(3):453. https://doi.org/10.3390/plants14030453
Chicago/Turabian StyleGostin, Irina Neta, Irinel Eugen Popescu, and Cristian Felix Blidar. 2025. "Structural Particularities of Gall Neoformations Induced by Monarthropalpus flavus in the Leaves of Buxus sempervirens" Plants 14, no. 3: 453. https://doi.org/10.3390/plants14030453
APA StyleGostin, I. N., Popescu, I. E., & Blidar, C. F. (2025). Structural Particularities of Gall Neoformations Induced by Monarthropalpus flavus in the Leaves of Buxus sempervirens. Plants, 14(3), 453. https://doi.org/10.3390/plants14030453







