The Establishment of a Highly Efficient In Vitro Regeneration System for Viburnum opulus L. ‘Roseum’
Abstract
1. Introduction
2. Results
2.1. Surface Sterilization of Explants
2.2. Primary Bud Induction Culture
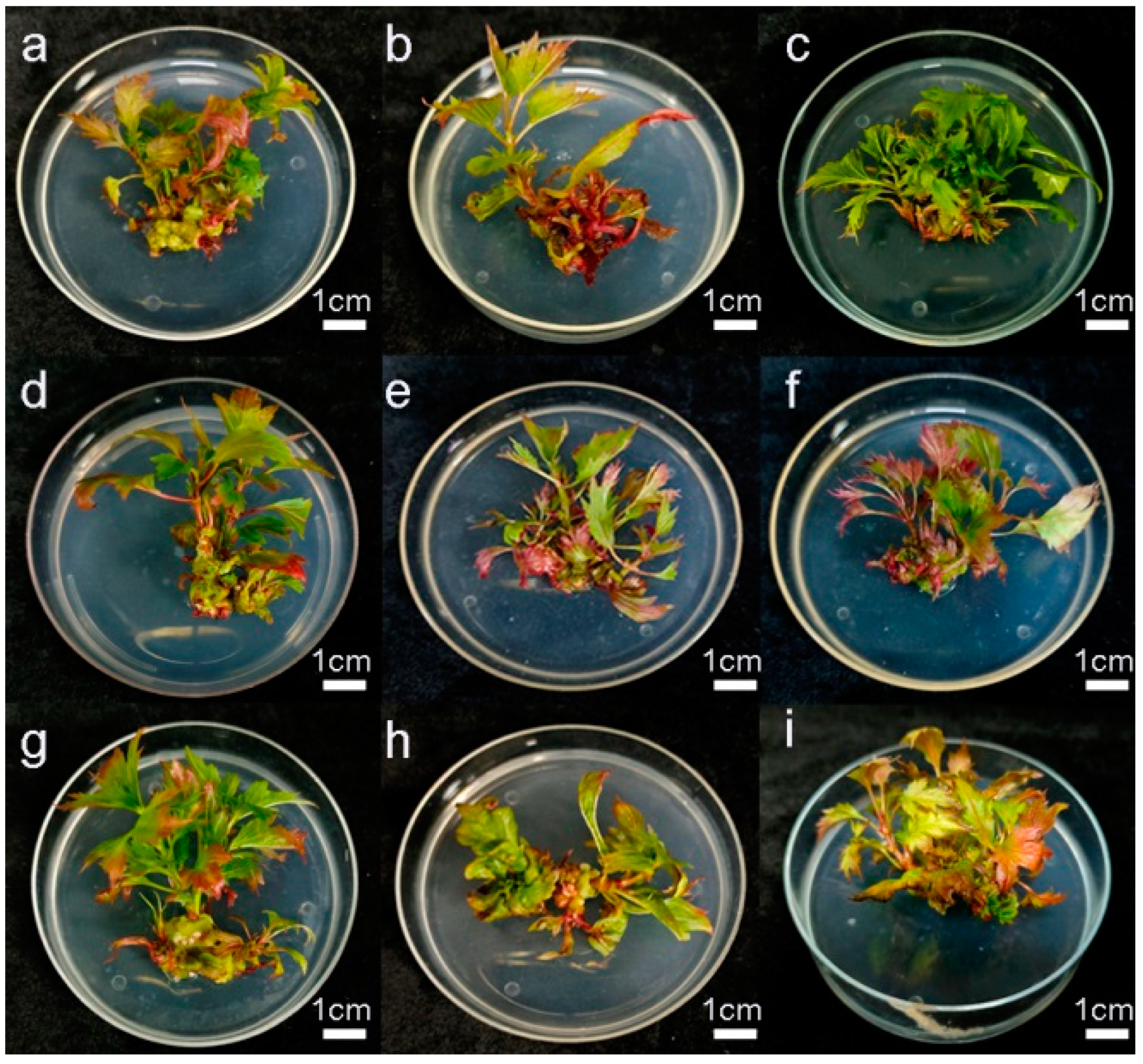
2.3. Shoot Proliferation
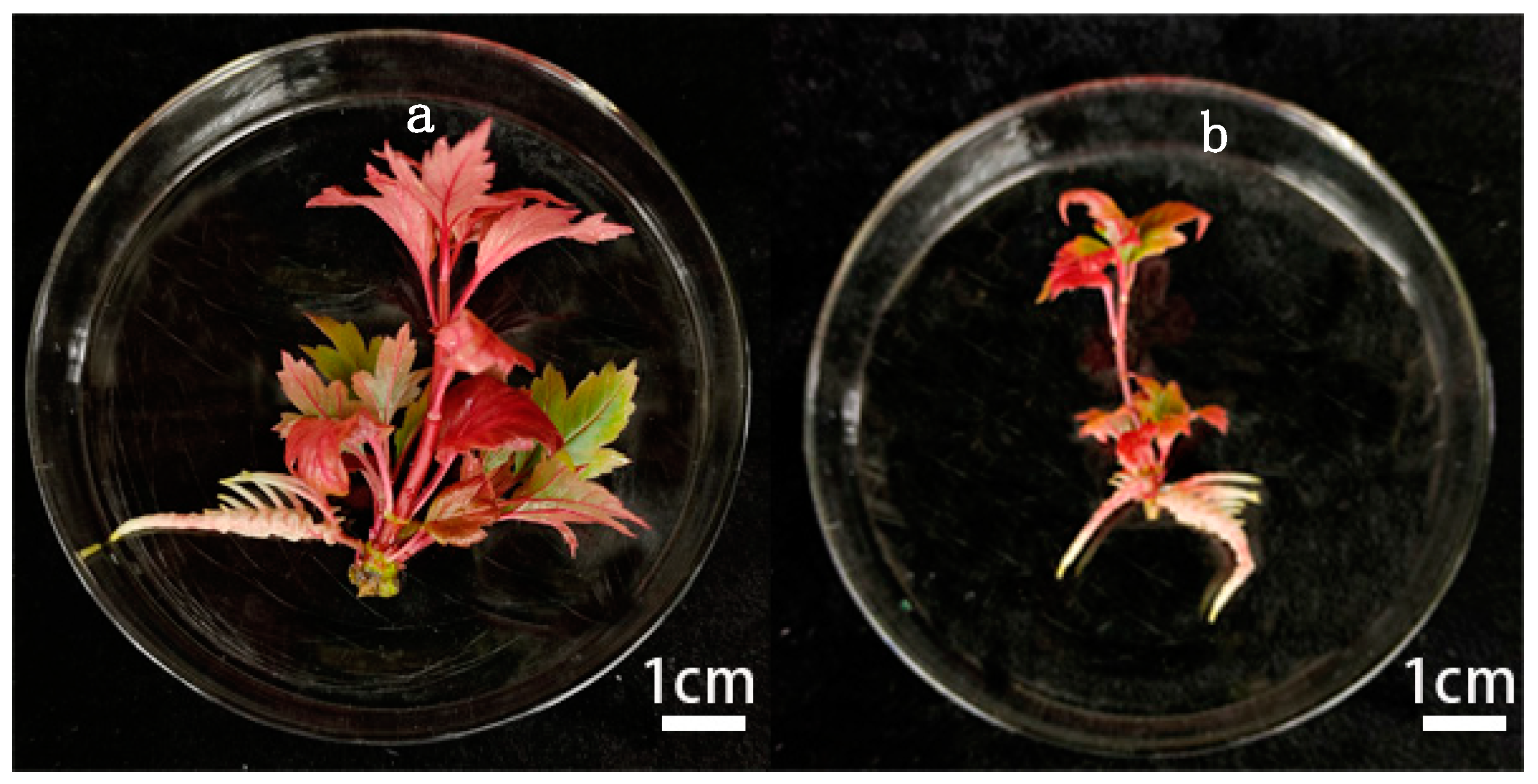
2.4. Induction of Adventitious Roots
2.5. Transplantation
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Components of the Medium
4.3. Cultivation Conditions
4.4. Sterilization of Explants
4.5. Primary Culture of Viburnum opulus L. ‘Roseum’
4.6. Induction of Bud Clusters from Young Stems
4.7. Rooting Culture
4.8. Ex Vitro Acclimatization
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.D.; Li, C.H.; Ai, X.R.; Xue, W.X.; Xu, L.X.; Xiang, Q.; Zhou, Y. Population Structure and Dynamic Characteristics of Five Wild Viburnum Plants in Southwest Hubei, China. Guihaia 2023, 43, 1747–1758. [Google Scholar]
- Ma, J.; Li, F. A Preliminary Study of Asexual Propagation Ways of Viburnum macrocephalum. J. Sichuan For. Sci. Technol. 2019, 40, 71–73+81. [Google Scholar]
- Du, X.; Shen, X.H. Effects of Cadmium Stress on Physiological and Biochemical Indices of Viburnum odoratissimum and V. tinus Seedlings. Chin. J. Ecol. 2010, 29, 899–904. [Google Scholar]
- Bi, B.; Liu, Y.C.; Chen, Q.; Zhou, Z.; Zhang, X.X.; Sun, H. Heavy Metal Accumulation Capability of Ten Broadleaved Tree Species to As, Hg, Pb, Cd and Cr. J. West China For. Sci. 2012, 41, 79–83. [Google Scholar]
- Chen, B.H.; Li, J.M.; Fan, H.H.; Zhang, J.; She, X.H.; Wang, S.F. Cutting Propagation Technique of Viburnum macrocephalum Fort. Bangladesh J. Bot. 2017, 46, 233–236. [Google Scholar]
- Bi, X.Y. Study Breeding Technologies of Viburnum sargenti Koehne. Master’s Thesis, Northeast Forestry University, Harbin, China, 2018. [Google Scholar]
- Wijerathna-Yapa, A.; Hiti-Bandaralage, J. Tissue Culture—A Sustainable Approach to Explore Plant Stresses. Life 2023, 13, 780. [Google Scholar] [CrossRef] [PubMed]
- VirVirginia, H.; Patricia, M.H. In Vitro Propagation of Viburnum opulus “Nanum”. J. Environ. Hort. 1985, 3, 41–45. [Google Scholar]
- Yaseen, S.A. Micorpropagation of Viburnum opulus (Roseum) by Using Single Nodes. Iraqi J. Agric. Sci. 2022, 53, 1388–1396. [Google Scholar] [CrossRef]
- Bhadrawale, D.; Mishra, J.P.; Mishra, Y. An Improvised in Vitro Vegetative Propagation Technique for Bambusa tulda: Influence of Season, Sterilization and Hormones. J. For. Res. 2018, 29, 1069–1074. [Google Scholar] [CrossRef]
- Wen, T.C.; Qian, Z.Y.; Li, B.Y. Research Progress on Explant Sterilization Technology of Plant Tissue Culture. Mod. Agric. Sci. Technol. 2024, 13, 72–76. [Google Scholar]
- Tian, H.; Hou, N. Study on Tissue Culture and Rapid Propagation Technology System of Polygonatum sibiricum Red. J. Nanjing Norm. Univ. Nat. Sci. Ed. 2020, 43, 129–135. [Google Scholar]
- Jian, D.; Gao, Y. Studies on the Technology of Primary Culture of Viburnum sargenti In Vitro. J. Jilin Agric. Sci. Technol. Univ. 2015, 24, 1–3. [Google Scholar]
- Wang, H.B.; Ding, D.; Zheng, L.J.; Wang, J.Y.; Zheng, L.J.; Guo, Y.C. Optimization of Tissue Culture and Rapid Propagation Technology System of Viburnum sargentii. Jiangsu Agric. Sci. 2021, 49, 59–63. [Google Scholar]
- Wu, J.; Fa, Y.L.; Zhu, C.Y.; Pang, X.W.; Wang, X.H. Microsomal Propagation of Viburnum sargentii. J. Shandong For. Sci. Technol. 2012, 42, 91–92. [Google Scholar]
- Zhen, X.H.; Hu, H.Y.; Xia, Y.S. Study on Tissue Culture of Viburnum opulus L. Anhui Agric. Sci. Bull. 2010, 16, 57–59. [Google Scholar]
- Guan, Q.Z.; Dong, X.C.; Dong, R.; Jiao, H.J.; Wang, H.W.; Wei, S.W. Study on Tissue Culture Rapid Propagation and Tissue Micro-Cuttage Technology of Pyrus Betulifolia. North. Hortic. 2024, 20, 23–29. [Google Scholar]
- Wang, Z.H.; Li, Q.; Zhang, S.F.; Deng, X.M.; Wu, A.M. Establishment and Optimization of Tissue Culture and Regeneration System of Ormosia henryi. Jiangsu Agric. Sci. 2024, 52, 50–55. [Google Scholar]
- Li, Z.J.; Cao, S.J.; Zhou, W. Tissue Culture and Rapid Propagation of Vaccinium ashei ‘Tifblue’. North. Hortic. 2022, 09, 33–39. [Google Scholar]
- Du, S.R.; Gu, T.T.; Pan, L.; Yang, W.X.; Jiang, C.Y. Studies on Rapid Propagation of Viburunm farreri in Tissue Culture. J. Hebei For. Sci. Technol. 2011, 05, 5–7. [Google Scholar]
- Cai, N.; Wang, X.M.; Li, Y.X.; Zeng, H.J.; Qiao, Z.Q. Establishment of a Tissue Culture and Rapid Propagation System of Lagerstroemia indica ‘Xiaoming 1’. Chin. Agric. Sci. Bull. 2016, 32, 22–27. [Google Scholar]
- Luo, M.W. Study on Rapid Propagation and Polyploid Induction of Platostoma palustr. Master’s Thesis, Guangxi Normal University, Guilin, China, 2023. [Google Scholar]
- Bi, X.Y.; Li, S.J. Establishment of Tissue Culture and Regeneration System of Viburnum sargentii. Hunan Agric. Sci. 2018, 4, 1–4+12. [Google Scholar]
- Liu, X.G.; Shan, M.H.; Su, S.; Wang, Y.Q.; Zhao, Y.; Dong, J.X. Optimization and Transplanting of Seeding Rooting Medium of Armeniaca sibirica. Mol. Plant Breed. 2020, 18, 1999–2005. [Google Scholar]
- Pan, L.M.; Huang, Q.F.; Chen, Q.P.; Wan, L.Y.; Fu, J.E.; Wei, S.G. Tissue Culture of Aristolochia fordiana Hemsl. Chin. J. Trop. Agric. 2021, 41, 73–77. [Google Scholar]



| Group | Sodium Hypochlorite Concentration | Sodium Hypochlorite Treatment Time | Contamination Rate % | Mortality Rate % | Recovery Rate% |
|---|---|---|---|---|---|
| 1 | 2% | 5 min | 80.00 ± 1.92 a | 0.00 | 20.00 ± 3.33 d |
| 2 | 2% | 10 min | 62.22 ± 1.11 b | 0.00 | 37.78 ± 2.94 b |
| 3 | 5% | 5 min | 24.44 ± 2.94 c | 2.22 ± 1.92 d | 73.33 ± 1.92 a |
| 4 | 5% | 10 min | 21.11 ± 2.22 c | 10.00 ± 1.92 c | 67.78 ± 2.22 a |
| 5 | 10% | 5 min | 13.33 ± 1.92 d | 53.33 ± 6.67 b | 28.89 ± 1.11 c |
| 6 | 10% | 10 min | 0.00 | 75.56 ± 3.85 a | 24.44 ± 1.11 cd |
| Group | Number of Inoculated Stem Segments/pc | Axillary Bud Sprouting Time/Day | Status of Axillary Buds |
|---|---|---|---|
| A | 30 | 8.67 | Leaf blade curled, apical dominance not obvious, more deformed buds |
| B | 30 | 10.33 | Axillary buds grow well, with spreading, bright green leaves |
| Groups | 6-BA Concentration (mg L−1) | IBA Concentration (mg L−1) | Sucrose Concentration (g·L−1) | Multiplication Coefficient | Growth State of Tissue-Culture Plantlets |
|---|---|---|---|---|---|
| 1 | 1.0 | 0.05 | 20 | 5.83 ± 0.31 abc | Leaves yellowing, differentiation buds turning red, growth is average. |
| 2 | 1.0 | 0.1 | 30 | 3.67 ± 0.42 e | Few differentiation buds, signs of vitrification, stems and leaves are thick and red. |
| 3 | 1.0 | 0.15 | 25 | 7.17 ± 0.48 a | More differentiation buds, leaf shape normal, growth is good. |
| 4 | 1.5 | 0.05 | 30 | 4.17 ± 0.31 de | Growth is average, stems have hyperplastic tissue proliferation. |
| 5 | 1.5 | 0.1 | 25 | 4.67 ± 0.49 cde | Growth is average, leaves are slender, leaf edges turning red. |
| 6 | 1.5 | 0.15 | 20 | 3.83 ± 0.31 de | Leaves are slender, most differentiation buds are malformed. |
| 7 | 2.0 | 0.05 | 25 | 5.17 ± 0.31 bcd | Growth is average, leaf edges slightly red, and some malformed buds present. |
| 8 | 2.0 | 0.1 | 20 | 3.50 ± 1.0.43 e | Growth is average, fewer differentiation buds, leaves curling. |
| 9 | 2.0 | 0.15 | 30 | 6.33 ± 0.766 ab | Growth is poor, leaves yellowing, more differentiation buds. |
| Groups | Sucrose Concentration (g·L−1) | NAA Concentration (mg L−1) | AC Concentration (mg L−1) | Rooting Rate % | Average Number of Primary Roots | Tissue-Culture Plantlets Grow and Root |
|---|---|---|---|---|---|---|
| 1 | 25 | 0.1 | 0.1 | 23.46 ± 1.23 d | 2.00 ± 50.58 d | Tissue-cultured plantlets grew poorly, browning at the base with only a few roots. |
| 2 | 25 | 0.2 | 0.5 | 56.79 ± 3.27 c | 2.80 ± 0.17 bc | Tissue-cultured plantlets have average growth with few roots. |
| 3 | 25 | 0.3 | 0.3 | 88.89 ± 3.70 a | 4.38 ± 0.08 a | Tissue-cultured plantlets have good growth with strong primary roots. |
| 4 | 30 | 0.1 | 0.5 | 77.78 ± 4.28 b | 4.29 ± 0.10 a | Tissue-cultured plantlets with good growth, weak primary root. |
| 5 | 30 | 0.2 | 0.3 | 77.78 ± 3.70 b | 2.29 ± 0.05 cd | Tissue culture plantlets were average in growth, with late root formation and short primary roots. |
| 6 | 30 | 0.3 | 0.1 | 55.56 ± 4.28 c | 3.40 ± 0.21 b | Tissue-culture plantlets have poor growth, with few main roots, thickened epidermis, and the growth of callus at the base. |
| 7 | 35 | 0.1 | 0.3 | 55.56 ± 2.14 c | 3.40 ± 0.10 b | Tissue-culture plantlets have average growth, with few roots and a small number of secondary roots. |
| 8 | 35 | 0.2 | 0.1 | 56.79 ± 3.27 c | 2.20 ± 0.07 cd | Tissue-culture plantlets have average growth, with a thickened epidermis on the main root. |
| 9 | 35 | 0.3 | 0.5 | 55.56 ± 3.70 c | 1.20 ± 0.05 e | Tissue-culture plantlets have poor growth, with weak and short main roots and browning at the base. |
| Group | 6-BA Concentration (mg·L−1) | IBA Concentration (mg·L−1) | Sucrose Concentration (g·L−1) |
|---|---|---|---|
| 1 | 1.0 | 0.05 | 20 |
| 2 | 1.0 | 0.1 | 30 |
| 3 | 1.0 | 0.15 | 25 |
| 4 | 1.5 | 0.05 | 30 |
| 5 | 1.5 | 0.1 | 25 |
| 6 | 1.5 | 0.15 | 20 |
| 7 | 2.0 | 0.05 | 25 |
| 8 | 2.0 | 0.1 | 20 |
| 9 | 2.0 | 0.15 | 30 |
| Group | Sucrose Concentration (g·L−1) | NAA Concentration (mg·L−1) | AC Concentration (mg·L−1) |
|---|---|---|---|
| 1 | 25 | 0.1 | 0.1 |
| 2 | 25 | 0.2 | 0.5 |
| 3 | 25 | 0.3 | 0.3 |
| 4 | 30 | 0.1 | 0.5 |
| 5 | 30 | 0.2 | 0.3 |
| 6 | 30 | 0.3 | 0.1 |
| 7 | 35 | 0.1 | 0.3 |
| 8 | 35 | 0.2 | 0.1 |
| 9 | 35 | 0.3 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, Y.; Dong, H.; Zhang, X.; Li, Y.; Cui, C.; Li, S. The Establishment of a Highly Efficient In Vitro Regeneration System for Viburnum opulus L. ‘Roseum’. Plants 2025, 14, 374. https://doi.org/10.3390/plants14030374
Ning Y, Dong H, Zhang X, Li Y, Cui C, Li S. The Establishment of a Highly Efficient In Vitro Regeneration System for Viburnum opulus L. ‘Roseum’. Plants. 2025; 14(3):374. https://doi.org/10.3390/plants14030374
Chicago/Turabian StyleNing, Yajing, Hao Dong, Xinxin Zhang, Yanhua Li, Chengpeng Cui, and Shujuan Li. 2025. "The Establishment of a Highly Efficient In Vitro Regeneration System for Viburnum opulus L. ‘Roseum’" Plants 14, no. 3: 374. https://doi.org/10.3390/plants14030374
APA StyleNing, Y., Dong, H., Zhang, X., Li, Y., Cui, C., & Li, S. (2025). The Establishment of a Highly Efficient In Vitro Regeneration System for Viburnum opulus L. ‘Roseum’. Plants, 14(3), 374. https://doi.org/10.3390/plants14030374






