Floral Resource Integration: Enhancing Biocontrol of Tuta absoluta Within Sustainable IPM Frameworks
Abstract
1. Introduction
2. Biological Control Agents and Habitat Enhancements for Effective T. absoluta Management
2.1. Key Biocontrol Agents for T. absoluta
2.2. Role of Supplemental Floral Resources in Supporting Biocontrol Agent Performance Needs
2.3. Shelter, Habitat Structure, and Environmental Stability
| Habitat Feature | Biocontrol Agent(s) | Observed Benefits | Description | Citation |
|---|---|---|---|---|
| Natural vegetation strips | N. tenuis, M. pygmaeus | Enhance persistence and dispersal of predators; provides continuous cover for natural enemies | Effective in reducing pest populations by maintaining predator stability in regions such as Northeast Spain and Southeast France | [41] |
| Artificial shelters | N. artynes, Bracon nigricans | Protect from adverse environmental factors | Offer refuge, especially during climatic extremes or pesticide application | [56] |
| Hedge rows | N. tenuis | Reduce pesticide drift and enhance biodiversity | Acts as a buffer zone, providing environmental stability and habitat diversity | [57] |
| Mulch layers | M. pygmaeus | Create a favorable microclimate and support predator establishment | Conserve soil moisture and improve local microhabitats | [58] |
| Perennial ground cover | N. tutae | Support continuous habitat for reproduction | Provide constant habitat for overwintering biocontrol agents | [33] |
| Shelter belts | N. tenuis, N. artynes | Reduce wind speed and maintain stability for biocontrol agents | It is essential for providing consistent shelter and stabilizing agent populations in open fields | [59] |
3. Nutritional Ecology of Biocontrol Agents in Enhancing Biological Control Efficacy
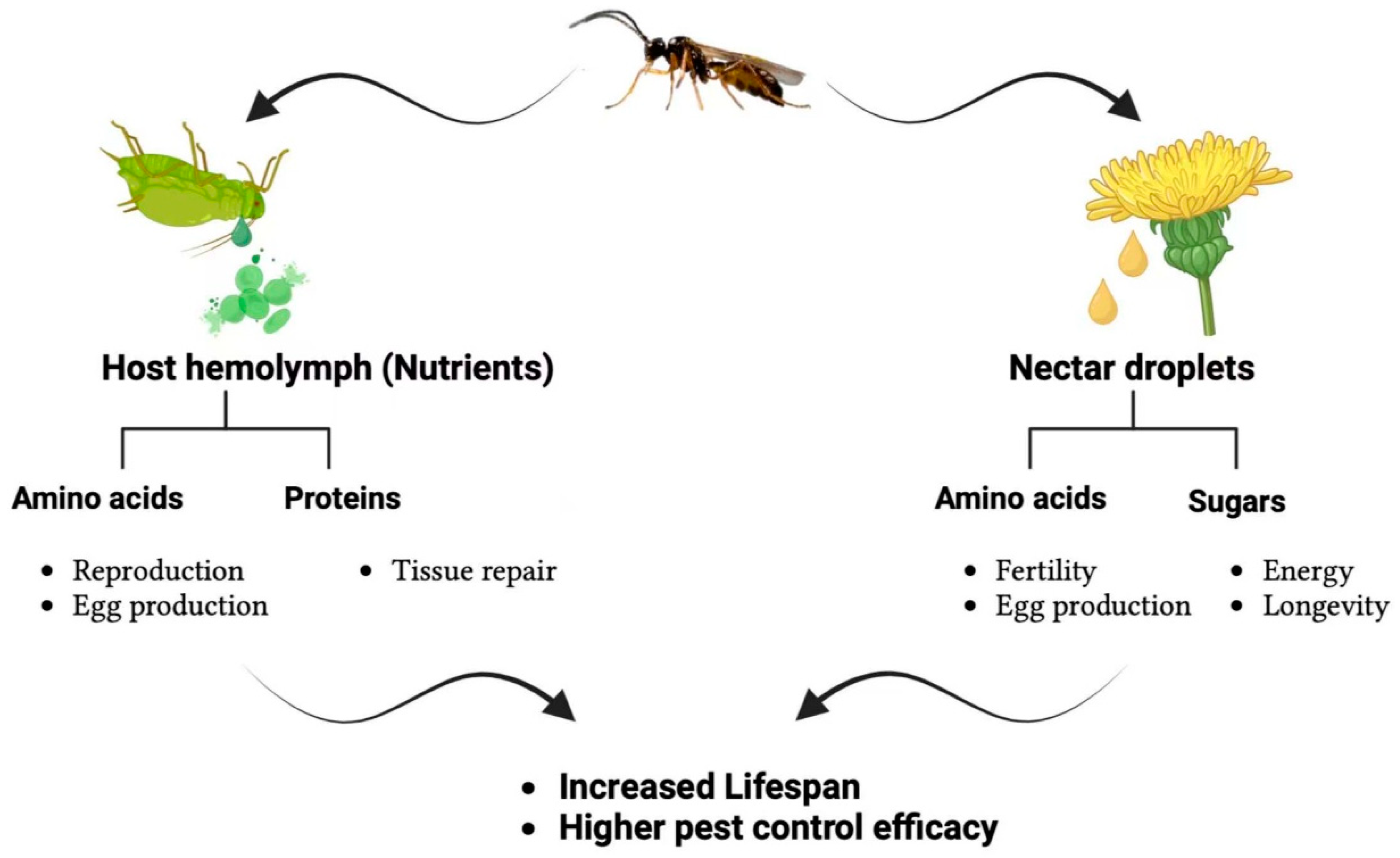
3.1. Sugar-Rich Resources and Their Impact on Biocontrol Agent Fitness
3.2. Protein and Amino Acid Contributions to Reproduction and Longevity
3.3. Nectar Quality and Floral Accessibility as Drivers of Biocontrol Efficacy
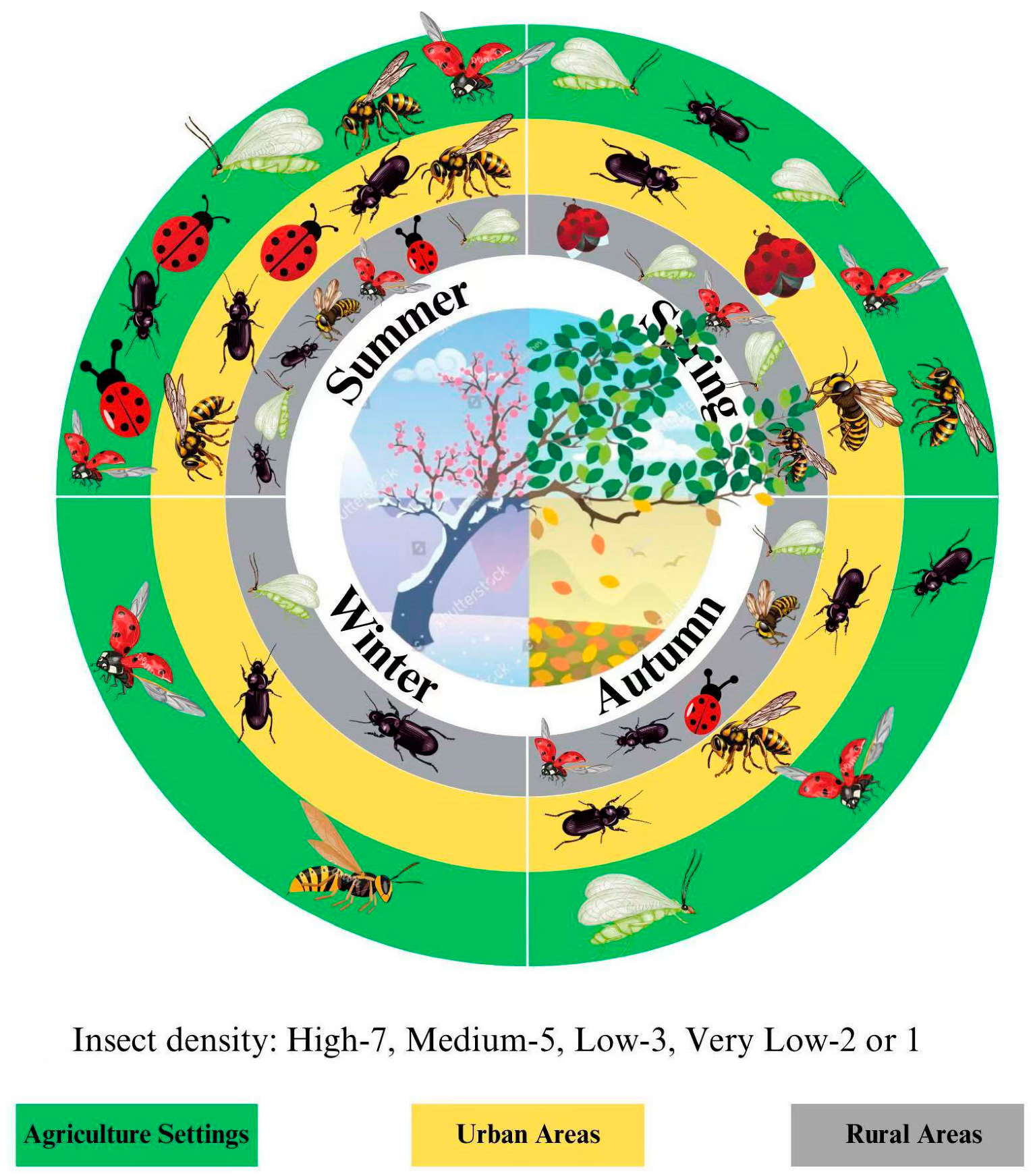
3.4. Temporal and Spatial Availability of Floral Resources in Supporting Biocontrol Populations
4. Synergistic Effects of Floral Resources on Biocontrol Agent Dynamics
4.1. Behavioral Patterns and Floral Resource Utilization
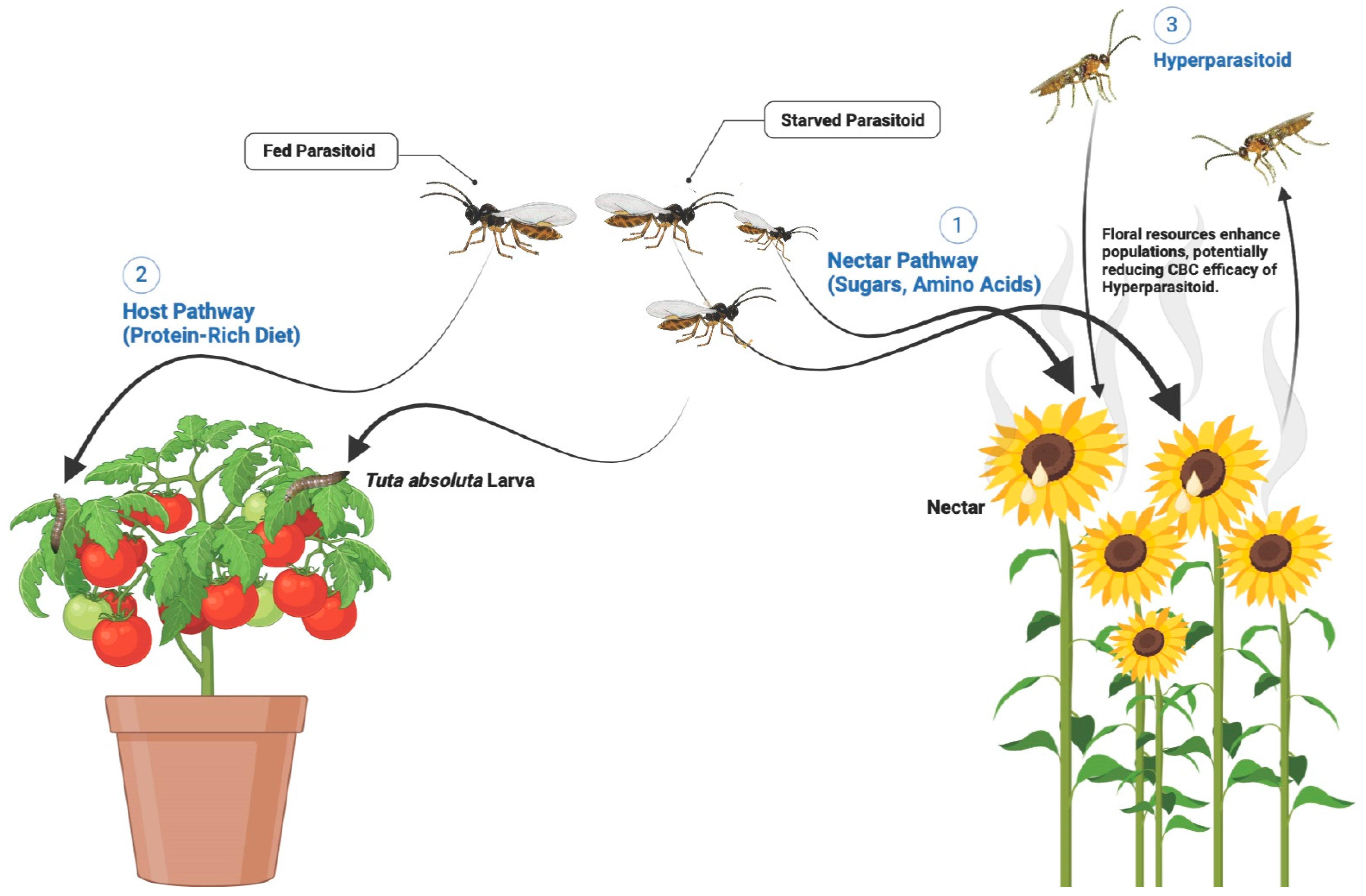
4.2. Influence of Floral Resources on Parasitoid-Host Interaction
5. Case Studies on the Use of Floral Resources to Enhance Biocontrol of T. absoluta
6. Challenges and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán Ruescas, D.; Tabone, E.; Frandon, J. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar]
- Chinchilla-Ramírez, M.; Pérez-Hedo, M.; Pannebakker, B.A.; Urbaneja, A. Genetic variation in the feeding behavior of isofemale lines of Nesidiocoris tenuis. Insects 2020, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Guedes, R.N.C.; Wan, F.H.; Desneux, N. Ecology, Worldwide Spread, and Management of the Invasive South American Tomato Pinworm, Tuta absoluta: Past, Present, and Future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Balzan, M.V.; Moonen, A.C. Management strategies for the control of Tuta absoluta (L epidoptera: G elechiidae) damage in open-field cultivations of processing tomato in Tuscany (Italy). EPPO Bull. 2012, 42, 217–225. [Google Scholar] [CrossRef]
- Guedes, R.; Picanço, M. The tomato borer Tuta absoluta in South America: Pest status, management and insecticide resistance. EPPO Bull. 2012, 42, 211–216. [Google Scholar] [CrossRef]
- Tarusikirwa, V.L.; Machekano, H.; Mutamiswa, R.; Chidawanyika, F.; Nyamukondiwa, C. Tuta absoluta (Meyrick)(lepidoptera: Gelechiidae) on the “offensive” in Africa: Prospects for integrated management initiatives. Insects 2020, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Konan, K.A.J.; Monticelli, L.S.; Ouali-N’Goran, S.-W.M.; Ramirez-Romero, R.; Martin, T.; Desneux, N. Combination of generalist predators, Nesidiocoris tenuis and Macrolophus pygmaeus, with a companion plant, Sesamum indicum: What benefit for biological control of Tuta absoluta? PLoS ONE 2021, 16, e0257925. [Google Scholar] [CrossRef]
- Gabarra, R.; Riudavets, J.; Rodríguez, G.A.; Pujade-Villar, J.; Arnó, J. Prospects for the biological control of Drosophila suzukii. BioControl 2015, 60, 331–339. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Riahi, C.; Urbaneja, A. Use of zoophytophagous mirid bugs in horticultural crops: Current challenges and future perspectives. Pest Manag. Sci. 2021, 77, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Arnó, J.; Molina, P.; Aparicio, Y.; Denis, C.; Gabarra, R.; Riudavets, J. Natural enemies associated with Tuta absoluta and functional biodiversity in vegetable crops. BioControl 2021, 66, 613–623. [Google Scholar] [CrossRef]
- Aynalem, B. Tomato leafminer [(Tuta absoluta Meyrick) (Lepidoptera: Gelechiidae)] and its current ecofriendly management strategies: A review. J. Agric. Biotechnol. Sustain. Dev. 2018, 10, 11–24. [Google Scholar]
- Ferracini, C.; Bueno, V.H.; Dindo, M.L.; Ingegno, B.L.; Luna, M.G.; Salas Gervassio, N.G.; Sánchez, N.E.; Siscaro, G.; Van Lenteren, J.C.; Zappalà, L. Natural enemies of Tuta absoluta in the Mediterranean basin, Europe and South America. Biocontrol Sci. Technol. 2019, 29, 578–609. [Google Scholar] [CrossRef]
- Chailleux, A.; Desneux, N.; Arnó, J.; Gabarra, R. Biology of two key Palaearctic larval ectoparasitoids when parasitizing the invasive pest Tuta absoluta. J. Pest Sci. 2014, 87, 441–448. [Google Scholar] [CrossRef]
- Mwangi, N.V.R.G.M. Prevalence of Tuta absoluta (Meyrick) and Chemical Management in Loitoktok, Kajiado County, Kenya. Prevalence 2019, 9. [Google Scholar]
- Wäckers, F. The parasitoids’ need for sweets: Sugars in mass rearing and biological control. In Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; CABI Publishing: Wallingford, UK, 2003; pp. 59–72. [Google Scholar]
- Benelli, G.; Giunti, G.; Tena, A.; Desneux, N.; Caselli, A.; Canale, A. The impact of adult diet on parasitoid reproductive performance. J Pest Sci. 2017, 90, 807–823. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ellers, J.; Harvey, J.A. Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu. Rev. Entomol. 2008, 53, 361–385. [Google Scholar] [CrossRef]
- Balzan, M.V.; Wäckers, F.L. Flowers to selectively enhance the fitness of a host-feeding parasitoid: Adult feeding by Tuta absoluta and its parasitoid Necremnus artynes. Biocontrol 2013, 67, 21–31. [Google Scholar] [CrossRef]
- Arnó, J.; Oveja, M.F.; Gabarra, R. Selection of flowering plants to enhance the biological control of Tuta absoluta using parasitoids. Biocontrol 2018, 122, 41–50. [Google Scholar] [CrossRef]
- Ma, R. Bottom-Up Effects of Fertilization on Biocontrol Agents. Ph.D. Thesis, Université Côte d’Azur, Nice, France, 2024. [Google Scholar]
- Colmenarez, Y.C.; Vasquez, C. Benefits associated with the implementation of biological control programmes in Latin America. BioControl 2024, 69, 1–18. [Google Scholar] [CrossRef]
- Han, P.; Bayram, Y.; Shaltiel-Harpaz, L.; Sohrabi, F.; Saji, A.; Esenali, U.T.; Jalilov, A.; Ali, A.; Shashank, P.R.; Ismoilov, K. Tuta absoluta continues to disperse in Asia: Damage, ongoing management and future challenges. J. Pest Sci. 2019, 92, 1317–1327. [Google Scholar] [CrossRef]
- Desneux, N.; Han, P.; Mansour, R.; Arnó, J.; Brévault, T.; Campos, M.R.; Chailleux, A.; Guedes, R.N.; Karimi, J.; Konan, K.A.J. Integrated pest management of Tuta absoluta: Practical implementations across different world regions. J. Pest Sci. 2022, 95, 1–23. [Google Scholar]
- Urbaneja, A.; Montón, H.; Mollá, O. Suitability of the tomato borer Tuta absoluta as prey for Macrolophus pygmaeus and Nesidiocoris tenuis. J. Appl. Entomol. 2009, 133, 292–296. [Google Scholar] [CrossRef]
- Urbaneja, A.; González-Cabrera, J.; Arno, J.; Gabarra, R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag. Sci. 2012, 68, 1215–1222. [Google Scholar] [CrossRef]
- Cock, M.J.; van Lenteren, J.C.; Brodeur, J.; Barratt, B.I.; Bigler, F.; Bolckmans, K.; Cônsoli, F.L.; Haas, F.; Mason, P.G.; Parra, J.R.P. Do new access and benefit sharing procedures under the convention on biological diversity threaten the future of biological control? BioControl 2010, 55, 199–218. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Urbaneja, A. The Zoophytophagous Predator Nesidiocoris tenuis: A Successful But Controversial Biocontrol Agent in Tomato Crops. In Advances in Insect Control and Resistance Management; Horowitz, A., Ishaaya, I., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Arnó, J.; Castañé, C.; Riudavets, J.; Gabarra, R. Risk of damage to tomato crops by the generalist zoophytophagous predator Nesidiocoris tenuis (Reuter)(Hemiptera: Miridae). Bull. Entomol. Res. 2010, 100, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Vila, E.; Janssen, D.; Bretones, G.; Salvador, E.; Lara, L.; Tellez, M. Selection of refuges for Nesidiocoris tenuis (Het.: Miridae) and Orius laevigatus (Het.: Anthocoridae): Virus reservoir risk assessment. IOBC WPRS Bull 2009, 49, 281–286. [Google Scholar]
- Dumont, F.; Aubry, O.; Lucas, E. From evolutionary aspects of zoophytophagy to biological control. Front. Ecol. Evol. 2018, 6, 221. [Google Scholar] [CrossRef]
- Amodeo, V. Intraguild Interactions Between Egg Parasitoids: From Laboratory to Field Investigations. Ph.D. Thesis, University of Palermo, Palermo, Italy, 2023. [Google Scholar]
- Cherif, A.; Mansour, R.; Grissa-Lebdi, K. The egg parasitoids Trichogramma: From laboratory mass rearing to biological control of lepidopteran pests. Biocontrol Sci. Technol. 2021, 31, 661–693. [Google Scholar] [CrossRef]
- Gonthier, J. Improving the Efficiency of Biological Control with Parasitoids for Tuta absoluta Pest Management. Ph.D. Thesis, Universität Bern, Bern, Germany, 2023. [Google Scholar]
- White, B. Biological Control of Insects Pests; Scientific e-Resources: New Delhi, India, 2019. [Google Scholar]
- Luna, M.; Sánchez, N.E.; Pereyra, P.C.; Nieves, E.; Savino, V.; Luft, E.; Virla, E.; Speranza, S. Biological control of Tuta absoluta in A rgentina and I taly: Evaluation of indigenous insects as natural enemies. EPPO Bull. 2012, 42, 260–267. [Google Scholar] [CrossRef]
- Mansour, R.; Cherif, A.; Attia-Barhoumi, S.; Zappalà, L.; Grissa-Lebdi, K. Tuta absoluta in Tunisia: Ten years of invasion and pest management. Phytoparasitica 2019, 47, 461–474. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R. Trap crops and insectary plants in the order Brassicales. Ann. Entomol. Soc. Am. 2019, 112, 318–329. [Google Scholar] [CrossRef]
- Tompkins, J.-M.; Wratten, S.; Wäckers, F. Nectar to improve parasitoid fitness in biological control: Does the sucrose: Hexose ratio matter? BAAE 2010, 11, 264–271. [Google Scholar] [CrossRef]
- Urbaneja Bernat, P.; Riudavets, J.; Denis Lopez, C.; Ojeda, J.; Alomar, O.; Arnó, J. Lobularia maritima as a nutrient-rich floral food source for two parasitoid wasps of Tuta absoluta. Entomol. Gen. 2024, 44. [Google Scholar] [CrossRef]
- Fataar, S.E. Promoting Cotesia rubecula Marshall, 1885 (Hymenoptera: Braconidae) Against the Cabbage Pest Pieris rapae Linnaeus, 1758 (Lepidoptera: Pieridae) through Flowering Plants. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2021. [Google Scholar]
- Ardanuy Gabarra, A.; Figueras, M.; Matas, M.; Arnó i Pujol, J.; Agustí Abella, N.; Alomar, Ò.; Albajes Garcia, R.; Gabarra i Ambert, R. Banker plants and landscape composition influence colonisation precocity of tomato greenhouses by mirid predators. J. Pest Sci. 2021, 95, 447–459. [Google Scholar] [CrossRef]
- Lantero, E.; Matallanas, B.; Callejas, C. Current status of the main olive pests: Useful integrated pest management strategies and genetic tools. Appl. Sci. 2023, 13, 12078. [Google Scholar] [CrossRef]
- Aparicio, Y.; Riudavets, J.; Gabarra, R.; Agustí, N.; Rodríguez-Gasol, N.; Alins, G.; Blasco-Moreno, A.; Arnó, J. Can insectary plants enhance the presence of natural enemies of the green peach aphid (Hemiptera: Aphididae) in Mediterranean peach orchards? J. Econ. Entomol. 2021, 114, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hedo, M.; Bouagga, S.; Zhang, N.X.; Moerkens, R.; Messelink, G.; Jaques, J.A.; Flors, V.; Broufas, G.; Urbaneja, A.; Pappas, M.L. Induction of plant defenses: The added value of zoophytophagous predators. J. Pest Sci. 2022, 95, 1501–1517. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Jervis, M.A. Does floral nectar improve biological control by parasitoids. In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Cambridge University Press: Cambridge, UK, 2005; pp. 267–304. [Google Scholar]
- Kandori, I.; Miura, S.; Yano, E.; Yoneya, K.; Akino, T. V erbena× hybrida and Scaevola aemula flowers provide nutrients for the reproduction of Nesidiocoris tenuis used for biological pest control in greenhouses. J Pest Sci. 2022, 95, 1567–1575. [Google Scholar] [CrossRef]
- McGrath, H. Bespoke Field Margins Delivering Multiple Benefits to Fresh Produce. Ph.D. Thesis, University of Reading, Berkshire, UK, 2022. [Google Scholar]
- Amoabeng, B. Dual Ecosystem Services from Non-Food Crop Vegetation: Benefits for Cabbage Pest Management. Ph.D. Thesis, Charles Sturt University, New South Wales, Australia, 2019. [Google Scholar]
- Yadav, S.P.S.; Bhattarai, S.; Ghimire, N.P.; Yadav, B. A review on ecology, biology, and management of a detrimental pest, Tuta absoluta (Lepidoptera: Gelechiidae). J. Agric. Appl. Biol. 2022, 3, 77–96. [Google Scholar] [CrossRef]
- Chailleux, A.; Ndjiliw, S.; Diakhaté, M.; Akodjetin, G.F.; Correa, P.; Deletre, E.; Brévault, T. Approaches to conservation of Nesidiocoris tenuis for biological control of pests in field-grown tomato in Senegal. Biocontrol 2022, 172, 104984. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Swathy, K.; Sarayut, P.; Patcharin, K. Biology, classification, and entomopathogen-based management and their mode of action on Tuta absoluta (Meyrick) in Asia. Front. Microbiol. 2024, 15, 1429690. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Rodriguez-Saona, C.; Zalucki, M.P.; Liu, S.-s.; Desneux, N. A theoretical framework to improve the adoption of green Integrated Pest Management tactics. Commun. Biol. 2024, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Bottrell, D.; Schoenly, K. Integrated pest management for resource-limited farmers: Challenges for achieving ecological, social and economic sustainability. J. Agric. Sci. 2018, 156, 408–426. [Google Scholar] [CrossRef]
- Evans, E.W. Dispersal in host–parasitoid interactions: Crop colonization by pests and specialist enemies. Insects 2018, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Chailleux, A.; Bearez, P.; Pizzol, J.; Amiens-Desneux, E.; Ramirez-Romero, R.; Desneux, N. Potential for combined use of parasitoids and generalist predators for biological control of the key invasive tomato pest Tuta absoluta. J. Pest Sci. 2013, 86, 533–541. [Google Scholar] [CrossRef]
- Giorgini, M.; Guerrieri, E.; Cascone, P.; Gontijo, L. Current strategies and future outlook for managing the Neotropical tomato pest Tuta absoluta (Meyrick) in the Mediterranean Basin. Neotrop. Entomol. 2019, 48, 1–17. [Google Scholar]
- Bocca, F. Biology and Biocoenosis of Three Exotic Pests of the Vineyard Agroecosystem, with Particular Reference to New Associations Involving Palaearctic Parasitoids. Ph.D. Thesis, University of Torino, Torino, Italy, 2022. [Google Scholar]
- Kruidhof, H.M.; Elmer, W.H. Cultural methods for greenhouse pest and disease management. In Integrated Pest and Disease Management in Greenhouse Crops; Springer: Berlin/Heidelberg, Germany, 2020; pp. 285–330. [Google Scholar]
- Miall, J. The Parasitoid Community Associated with the Invasive Leek Moth, Acrolepiopsis assectella (Zeller) (Lepidoptera: Acrolepiidae): Can Conservation Biological Control Benefit an Introduced Classical Biological Control Agent in North America? Master’s Thesis, Carleton University, Ottawa, AB, Canada, 2018. [Google Scholar]
- Peri, E.; Moujahed, R.; Wajnberg, E.; Colazza, S. Applied chemical ecology to enhance insect parasitoid efficacy in the biological control of crop pests. In Chemical Ecology of Insects; CRC Press: Boca Raton, FL, USA, 2018; pp. 234–267. [Google Scholar]
- Thompson, M.N.; Medina, R.F.; Helms, A.M.; Bernal, J.S. Improving natural enemy selection in biological control through greater attention to chemical ecology and host-associated differentiation of target arthropod pests. Insects 2022, 13, 160. [Google Scholar] [CrossRef]
- Ali, J.; Abbas, A.; Abbas, S.; Ji, Y.; Khan, K.A.; Ghramh, H.A.; Mahamood, M.; Chen, R. Honeydew: A keystone in insect–plant interactions, current insights and future perspectives. J. Appl. Entomol. 2024, 148, 727–733. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Van Rijn, P.C. Pick and mix: Selecting flowering plants to meet the requirements of target biological control insects. In Biodiversity and Insect Pests: Key Issues for Sustainable Management; John Wiley and Sons Ltd: Hoboken, NJ, USA, 2012; pp. 139–165. [Google Scholar]
- Stone, C.M.; Foster, W.A. Plant-sugar feeding and vectorial capacity. In Ecology of Parasite-Vector Interactions; Wageningen Academic: Gelderland, The Netherlands, 2013; pp. 35–79. [Google Scholar]
- Dong, Y.C.; Han, P.; Niu, C.Y.; Zappalà, L.; Amiens-Desneux, E.; Bearez, P.; Lavoir, A.V.; Biondi, A.; Desneux, N. Nitrogen and water inputs to tomato plant do not trigger bottom-up effects on a leafminer parasitoid through host and non-host exposures. Pest Manag. Sci. 2018, 74, 516–522. [Google Scholar] [CrossRef]
- Gillespie, M.A.; Gurr, G.M.; Wratten, S.D. Beyond nectar provision: The other resource requirements of parasitoid biological control agents. Entomol. Exp. Appl. 2016, 159, 207–221. [Google Scholar] [CrossRef]
- Heimpel, G.E. Linking parasitoid nectar feeding and dispersal in conservation biological control. Biocontrol 2019, 132, 36–41. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, J.; Reynolds, O.L.; Chen, W.; He, W.; You, M.; Gurr, G.M. Alyssum (Lobularia maritima) selectively attracts and enhances the performance of Cotesia vestalis, a parasitoid of Plutella xylostella. Sci. Rep. 2020, 10, 6447. [Google Scholar] [CrossRef]
- Aparicio, Y.; Gabarra, R.; Arnó, J. Attraction of Aphidius ervi (Hymenoptera: Braconidae) and Aphidoletes aphidimyza (Diptera: Cecidomyiidae) to sweet alyssum and assessment of plant resources effects on their fitness. J. Econ. Entomol. 2018, 111, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zimmermann, O.; Hassan, S.A. Pollen as a source of food for egg parasitoids of the genus Trichogramma (Hymenoptera: Trichogrammatidae). Biocontrol Sci. Technol. 2004, 14, 201–209. [Google Scholar] [CrossRef]
- Hyder, M.; Li, Y.; Raza, M.F.; Zhang, M.; Chen, J.; Mao, J.; Bukero, A.; Zhang, L. Enhancing Coccinella Beetle Biological Pest Control via a Floral Approach in Cucumber Greenhouse. Life 2023, 13, 2080. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Sauerborn, J.; Martin, K.; Sauerborn, J. Crops and their Environment. Agroecology 2013, 1, 103–185. [Google Scholar]
- Álvarez-Pérez, S.; Lievens, B.; de Vega, C. Floral nectar and honeydew microbial diversity and their role in biocontrol of insect pests and pollination. Curr. Opin. Insect Sci. 2024, 61, 101138. [Google Scholar] [CrossRef]
- Cavallini, L. Can Conservation Biocontrol of Wheat Stem Sawfly Be Improved? Contributions of Supplemental Nutrition to Longevity, Egg Load, and Egg Volume of Bracon cephi and B. lissogaster. Master’s Thesis, Montana State University-Bozeman, Bozeman, MT, USA, 2022. [Google Scholar]
- Strand, M.R.; Casas, J. Parasitoid and host nutritional physiology in behavioral ecology. In Behavioral Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications; Blackwell Publishing Ltd: Oxford, UK, 2008; pp. 113–128. [Google Scholar]
- Jervis, M.A.; Heimpel, G.E.; Ferns, P.N.; Harvey, J.A.; Kidd, N.A. Life-history strategies in parasitoid wasps: A comparative analysis of ‘ovigeny’. J. Anim. Ecol. 2001, 70, 442–458. [Google Scholar] [CrossRef]
- Colazza, S.; Peri, E.; Cusumano, A. Chemical ecology of floral resources in conservation biological control. Ann. Rev. Entomol. 2023, 68, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.J.; Wajnberg, É. Optimal foraging behavior and efficient biological control methods. Behavioral Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications; Blackwell Publishing Ltd: Oxford, UK, 2008; pp. 1–30. [Google Scholar]
- Sivinski, J.; Wahl, D.; Holler, T.; Al Dobai, S.; Sivinski, R. Conserving natural enemies with flowering plants: Estimating floral attractiveness to parasitic Hymenoptera and attraction’s relationship to flower and plant morphology. BioControl 2011, 58, 208–214. [Google Scholar] [CrossRef]
- Abrahamczyk, S.; Kessler, M. Morphological and behavioural adaptations to feed on nectar: How feeding ecology determines the diversity and composition of hummingbird assemblages. J. Ornithol. 2015, 156, 333–347. [Google Scholar] [CrossRef]
- Rand, T.A.; Tylianakis, J.M.; Tscharntke, T. Spillover edge effects: The dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol. Lett. 2006, 9, 603–614. [Google Scholar] [CrossRef]
- Blitzer, E.J.; Dormann, C.F.; Holzschuh, A.; Klein, A.-M.; Rand, T.A.; Tscharntke, T. Spillover of functionally important organisms between managed and natural habitats. Agric. Ecosyst. Environ. 2012, 146, 34–43. [Google Scholar] [CrossRef]
- Ekbom, B.; Wiktelius, S.; Chiverton, P. Can polyphagous predators control the bird cherry-oat aphid (Rhopalosiphum padi) in spring cereals? A simulation study. Entomol. Exp. Appl. 1992, 65, 215–223. [Google Scholar] [CrossRef]
- van Rijn, P.C.; Kooijman, J.; Wäckers, F.L. The contribution of floral resources and honeydew to the performance of predatory hoverflies (Diptera: Syrphidae). Biol. Control 2013, 67, 32–38. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.; Lu, Y.; Morales, H.; Vazquez, L.L.; Legaspi, J.C.; Eliopoulos, P.A.; Hernandez, L.M. Current status and potential of conservation biological control for agriculture in the developing world. Biocontrol 2013, 65, 152–167. [Google Scholar] [CrossRef]
- Peñalver-Cruz, A.; Alvarez-Baca, J.K.; Alfaro-Tapia, A.; Gontijo, L.; Lavandero, B. Manipulation of agricultural habitats to improve conservation biological control in South America. Neotrop. Entomol. 2019, 48, 875–898. [Google Scholar]
- Hatt, S.; Xu, Q.; Francis, F.; Osawa, N. Aromatic plants of East Asia to enhance natural enemies towards biological control of insect pests. A review. Entomol. Gen. 2019, 38, 275–315. [Google Scholar] [CrossRef]
- Phanindra, P.; Vinay, M.; Paul, S.S.; Kotiyal, A. Regulations of Flowering in Fruit Crop for Higher Yield and Quality Production. In A Monthly Peer Reviewed Magazine for Agriculture and Allied Sciences; Scripown: New Delhi, India, 2024; 91p. [Google Scholar]
- Schellhorn, N.A.; Gagic, V.; Bommarco, R. Time will tell: Resource continuity bolsters ecosystem services. Trends Ecol. Evol. 2015, 30, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Balzan, M.; Moonen, A.C. Field margin vegetation enhances biological control and crop damage suppression from multiple pests in organic tomato fields. Entomol. Exp. Appl. 2014, 150, 45–65. [Google Scholar] [CrossRef]
- Iuliano, B.; Gratton, C. Temporal resource (dis) continuity for conservation biological control: From field to landscape scales. Food Sustain. Food Syst. 2020, 4, 127. [Google Scholar] [CrossRef]
- Spiesman, B.; Iuliano, B.; Gratton, C. Temporal resource continuity increases predator abundance in a metapopulation model: Insights for conservation and biocontrol. Land 2020, 9, 479. [Google Scholar] [CrossRef]
- Rayl, R.J.; Shields, M.W.; Tiwari, S.; Wratten, S.D. Conservation biological control of insect pests. Sustainable Agriculture Reviews 28: Ecology for Agriculture; Springer: Berlin/Heidelberg, Germany, 2018; pp. 103–124. [Google Scholar]
- Gardiner, M. Good Garden Bugs: Everything You Need to Know About Beneficial Predatory Insects; Quarry Books: Beverly, MA, USA, 2015. [Google Scholar]
- Khan, Z.R.; James, D.G.; Midega, C.A.; Pickett, J.A. Chemical ecology and conservation biological control. Biocontrol 2008, 45, 210–224. [Google Scholar] [CrossRef]
- Junker, R.R.; Romeike, T.; Keller, A.; Langen, D. Density-dependent negative responses by bumblebees to bacteria isolated from flowers. Apidologie 2014, 45, 467–477. [Google Scholar] [CrossRef]
- Bianchi, F.J.; Wäckers, F.L. Effects of flower attractiveness and nectar availability in field margins on biological control by parasitoids. Biocontrol 2008, 46, 400–408. [Google Scholar] [CrossRef]
- Lewis, W.; Stapel, J.O.; Cortesero, A.M.; Takasu, K. Understanding how parasitoids balance food and host needs: Importance to biological control. Biocontrol 1998, 11, 175–183. [Google Scholar] [CrossRef]
- Snyder, W.E. Give predators a complement: Conserving natural enemy biodiversity to improve biocontrol. Biocontrol 2019, 135, 73–82. [Google Scholar] [CrossRef]
- You, S.; You, M.; Niu, D. Identification of floral volatiles from Fagopyrum esculentum that attract Cotesia vestalis with potentially better biocontrol efficacy against Plutella xylostella. Pest Manag. Sci. 2024, 80, 763–775. [Google Scholar] [CrossRef]
- dos Reis, D.A. The Potential of Sugar Resources in the Reproductive Biology of Wheat Stem Sawfly Parasitoids. Ph.D. Thesis, Montana State University-Bozeman, Bozeman, MT, USA, 2018. [Google Scholar]
- Van Lenteren, J.C.; Alomar, O.; Ravensberg, W.J.; Urbaneja, A. Biological control agents for control of pests in greenhouses. In Integrated Pest and Disease Management in Greenhouse Crops; Springer: Berlin/Heidelberg, Germany, 2020; pp. 409–439. [Google Scholar]
- Mancoa, E.; Lombardi, N.; Cascone, P.; de Kogel, W. Application of pheromone-based control of Tuta absoluta in greenhouse tomato IPM in Campania, southern Italy. In Proceedings of the IPM Innovation in Europe, Poznan, Poland, 14–16 January 2015. 42p. [Google Scholar]
- Qasim, M.; Islam, W.; Rizwan, M.; Hussain, D.; Noman, A.; Khan, K.A.; Ghramh, H.A.; Han, X. Impact of plant monoterpenes on insect pest management and insect-associated microbes. Heliyon 2024, 10, e39120. [Google Scholar] [PubMed]
- Liambila, R.N. Characterisation of Essential Oil Compounds and Optimisation of Water and Potassium for Production of Lantana camara (L.) for Tuta absoluta Management. Ph.D. Thesis, JKUAT-CoANRE, Juja, Kenia, 2023. [Google Scholar]
- Lahiri, S.; Orr, D. Biological control in tomato production systems: Theory and practice. In Sustainable Management of Arthropod Pests of Tomato; Elsevier: Amsterdam, The Netherlands, 2018; pp. 253–267. [Google Scholar]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biot. 2021, 37, 90. [Google Scholar] [CrossRef] [PubMed]
- Supriyadi, S.; Bashir, H. Effects of Sweet Alyssum (Lobularia maritima) on Parasitoid and Predator Diversity and Abundance in Agroecosystems Especially in Rice. Int. J. Agric. Biosci. 2024, 13, 347–355. [Google Scholar]
- Scheper, J.; Holzschuh, A.; Kuussaari, M.; Potts, S.G.; Rundlöf, M.; Smith, H.G.; Kleijn, D. Environmental factors driving the effectiveness of European agri-environmental measures in mitigating pollinator loss—A meta-analysis. Ecol. Lett. 2013, 16, 912–920. [Google Scholar] [CrossRef]
- Brito Vera, G.A.; Pérez, F. Floral nectar (FN): Drivers of variability, causes, and consequences. Bras. J. Bot. 2024, 47, 1–11. [Google Scholar] [CrossRef]
- Thomas, M.B. Ecological approaches and the development of “truly integrated” pest management. Proc. Natl. Acad. Sci. USA 1999, 96, 5944–5951. [Google Scholar] [CrossRef]
- Morris, M.M.; Frixione, N.J.; Burkert, A.C.; Dinsdale, E.A.; Vannette, R.L. Microbial abundance, composition, and function in nectar are shaped by flower visitor identity. FEMS Microbiol. Ecol. 2020, 96, fiaa003. [Google Scholar] [CrossRef] [PubMed]
- Vannette, R.L. The floral microbiome: Plant, pollinator, and microbial perspectives. Ann. Rev. Ecol. Evol. Syst. 2020, 51, 363–386. [Google Scholar] [CrossRef]
- Cusumano, A.; Lievens, B. Microbe-mediated alterations in floral nectar: Consequences for insect parasitoids. Curr. Opin. Insect Sci. 2023, 60, 101116. [Google Scholar] [CrossRef]
- Lenaerts, M.; Goelen, T.; Paulussen, C.; Herrera-Malaver, B.; Steensels, J.; Van den Ende, W.; Verstrepen, K.J.; Wäckers, F.; Jacquemyn, H.; Lievens, B. Nectar bacteria affect life history of a generalist aphid parasitoid by altering nectar chemistry. Funct. Ecol. 2017, 31, 2061–2069. [Google Scholar] [CrossRef]
- Gonzalez, F.; Tkaczuk, C.; Dinu, M.M.; Fiedler, Ż.; Vidal, S.; Zchori-Fein, E.; Messelink, G.J. New opportunities for the integration of microorganisms into biological pest control systems in greenhouse crops. J. Pest Sci. 2016, 89, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Keyhani, N.O.; Xia, Y.; Xie, J. The potential and limitations of entomopathogenic fungi as biocontrol agents for insect pest management. Entomol. Gen. 2024, 44, 797–811. [Google Scholar] [CrossRef]
- Haan, N.L.; Iuliano, B.G.; Gratton, C.; Landis, D.A. Designing agricultural landscapes for arthropod-based ecosystem services in North America. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 64, pp. 191–250. [Google Scholar]
- Schütz, L.; Wenzel, B.; Rottstock, T.; Dachbrodt-Saaydeh, S.; Golla, B.; Kehlenbeck, H. How to promote multifunctionality of vegetated strips in arable farming: A qualitative approach for Germany. Ecosphere 2022, 13, e4229. [Google Scholar] [CrossRef]
- Le Billon, P. The political ecology of war: Natural resources and armed conflicts. Pol. Geogr. 2001, 20, 561–584. [Google Scholar] [CrossRef]
- Eustacchio, E.; Bonelli, M.; Minici, A.; Melotto, A.; Dinatale, E.; Gobbi, M.; Gianfranceschi, L.; Casartelli, M.; Caccianiga, M. Plants and flower-visiting arthropods in mountain ecosystems: The case study of the alpine species Androsace brevis (Primulaceae). In Proceedings of the 11th European PhD Network “Insect Science” Annual Meeting, Online, 14–16 November 2020. [Google Scholar]
- Jervis, M.A.; Lee, J.C.; Heimpel, G.E. Use of behavioural and life-history studies to understand the effects of habitat manipulation. In Ecological Engineering for Pest Management: Advances in Habitat Manipulation for Arthropods; CSIR: New Delhi, India, 2004; pp. 65–100. [Google Scholar]
- Fountain, M.T. Impacts of wildflower interventions on beneficial insects in fruit crops: A review. Insects 2022, 13, 304. [Google Scholar] [CrossRef]
- Isaacs, R.; Williams, N.; Ellis, J.; Pitts-Singer, T.L.; Bommarco, R.; Vaughan, M. Integrated crop pollination: Combining strategies to ensure stable and sustainable yields of pollination-dependent crops. BAAE 2017, 22, 44–60. [Google Scholar] [CrossRef]
- Holland, J.M.; Bianchi, F.J.; Entling, M.H.; Moonen, A.C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest Manag. Sci. 2016, 72, 1638–1651. [Google Scholar] [CrossRef] [PubMed]
- Ekström, G.; Ekbom, B. Pest control in agro-ecosystems: An ecological approach. Crit. Rev. Plant Sci. 2011, 30, 74–94. [Google Scholar] [CrossRef]
- Huang, N.; Enkegaard, A.; Osborne, L.S.; Ramakers, P.M.; Messelink, G.J.; Pijnakker, J.; Murphy, G. The banker plant method in biological control. Crit. Rev. Plant Sci. 2011, 30, 259–278. [Google Scholar] [CrossRef]
| Floral Species | Associated Biocontrol Agent(s) | Specific Benefits | Citation |
|---|---|---|---|
| Lobularia maritima (Sweet Alyssum) | N. artynes, N. tenuis | Increase longevity and fecundity; provides nectar and shelter for agents | [18] |
| Fagopyrum esculentum (Buckwheat) | Necremnus tutae, Bracon nigricans | Enhance parasitoid survival and egg load | [19] |
| Verbena × hybrida | N. tenuis | Supply essential sugars; supports reproduction in greenhouse settings | [46] |
| Scaevola aemula | N. tenuis | Provide fructose and glucose, enhancing predator survival | [46] |
| Centaurea cyanus (Cornflower) | N. artynes | Support parasitoid survival and foraging behavior | [47] |
| Calendula officinalis (Marigold) | M. pygmaeus | Promote predator population density; effective in tomato crop margins | [41] |
| Achillea millefolium (Yarrow) | B. nigricans, N. tutae | Enhance parasitoid survival; does not benefit T. absoluta | [10] |
| Sinapis alba (White Mustard) | N. tenuis | Provide pollen as a protein source; supports reproduction and fitness | [18] |
| Borago officinalis (Borage) | M. pygmaeus, N. tenuis | Increase predation rates; provides nectar with high nutritional value | [48] |
| Cosmos bipinnatus (Cosmos) | N. artynes | Boost parasitoid fecundity and longevity | [49] |
| Phacelia tanacetifolia (Lacy Phacelia) | N. tenuis, M. pygmaeus | Enhance foraging efficiency and lifespan | [24] |
| Sesamum indicum (Sesame) | N. tenuis | Reduce plant damage risk while enhancing pest control | [50] |
| Vicia sativa (Vetch) | N. artynes, B. nigricans | Provide necessary proteins for egg production | [8] |
| Lantana camara | N. tenuis | Increase longevity; provides rich nectar for biocontrol agents | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyder, M.; Ul Haq, I.; Younas, M.; Ghafar, M.A.; Akhtar, M.R.; Ahmed, Z.; Bukero, A.; Hou, Y. Floral Resource Integration: Enhancing Biocontrol of Tuta absoluta Within Sustainable IPM Frameworks. Plants 2025, 14, 319. https://doi.org/10.3390/plants14030319
Hyder M, Ul Haq I, Younas M, Ghafar MA, Akhtar MR, Ahmed Z, Bukero A, Hou Y. Floral Resource Integration: Enhancing Biocontrol of Tuta absoluta Within Sustainable IPM Frameworks. Plants. 2025; 14(3):319. https://doi.org/10.3390/plants14030319
Chicago/Turabian StyleHyder, Moazam, Inzamam Ul Haq, Muhammad Younas, Muhammad Adeel Ghafar, Muhammad Rehan Akhtar, Zubair Ahmed, Aslam Bukero, and Youming Hou. 2025. "Floral Resource Integration: Enhancing Biocontrol of Tuta absoluta Within Sustainable IPM Frameworks" Plants 14, no. 3: 319. https://doi.org/10.3390/plants14030319
APA StyleHyder, M., Ul Haq, I., Younas, M., Ghafar, M. A., Akhtar, M. R., Ahmed, Z., Bukero, A., & Hou, Y. (2025). Floral Resource Integration: Enhancing Biocontrol of Tuta absoluta Within Sustainable IPM Frameworks. Plants, 14(3), 319. https://doi.org/10.3390/plants14030319







