Pseudomonas simiae WCS417 and Debaryomyces hansenii Induce Iron Deficiency Responses in Rice (Oryza sativa L.) Through Phytosiderophore Production and Gene Expression Modulation
Abstract
1. Introduction
2. Results
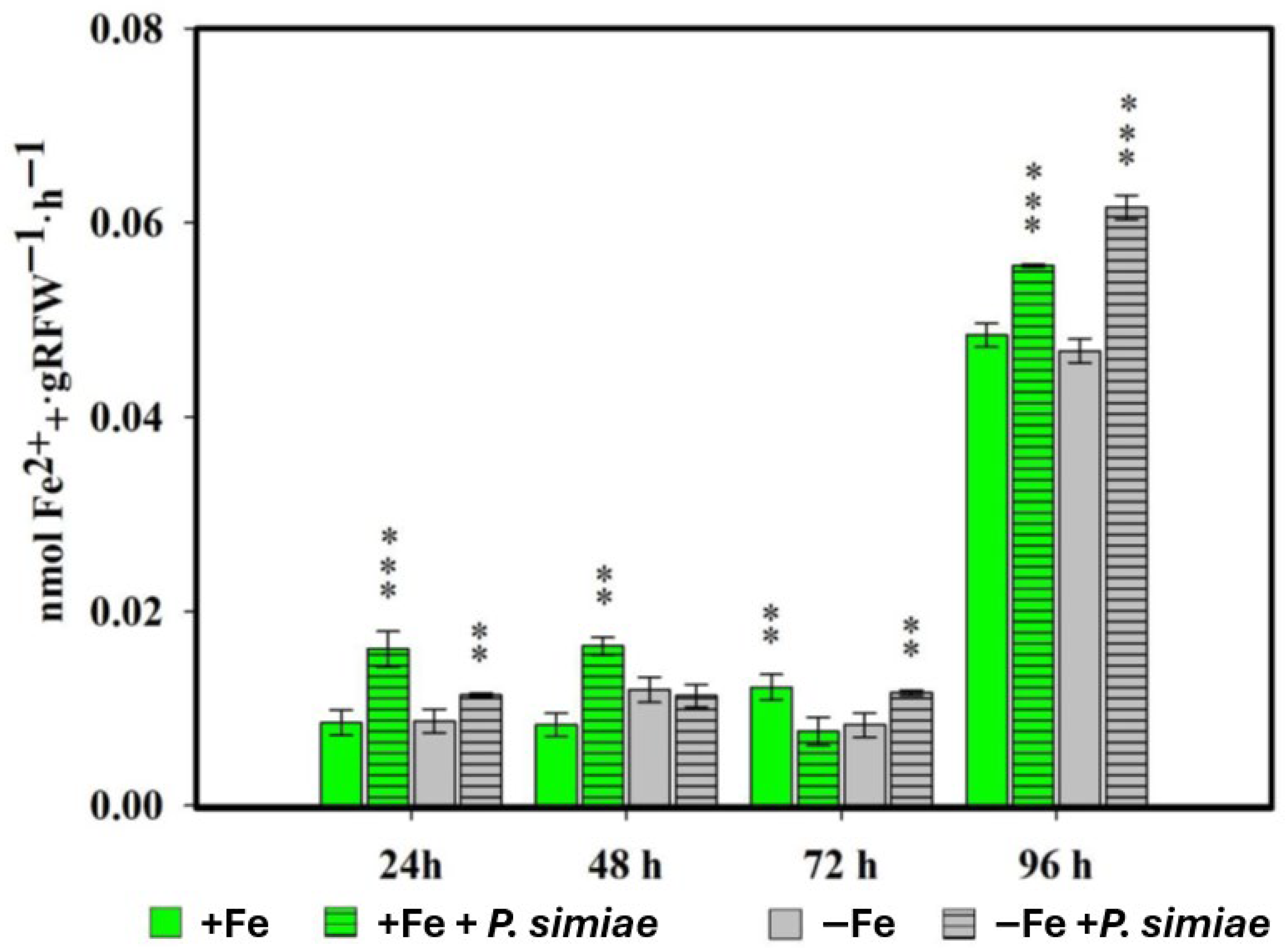
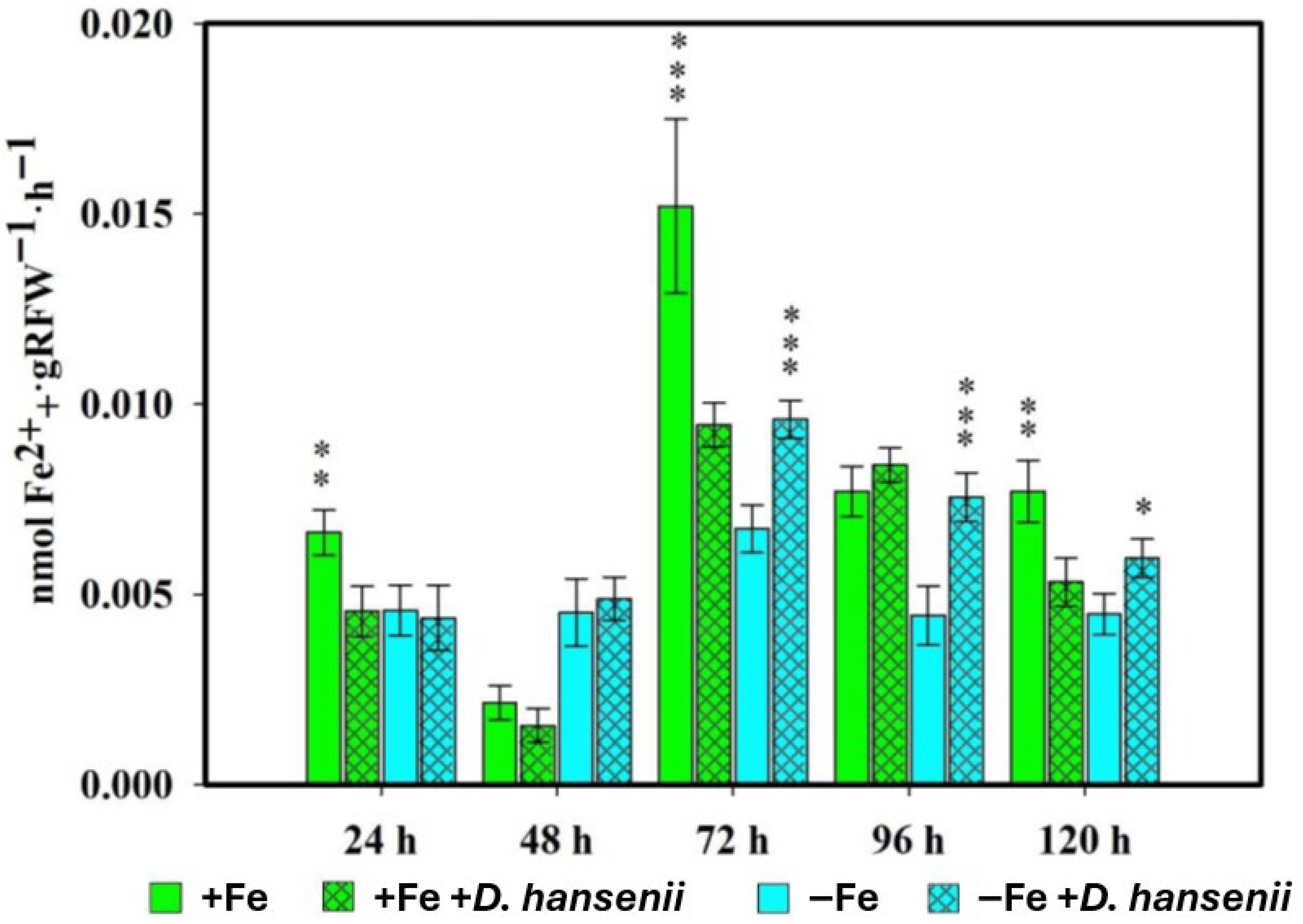
2.1. Phytosiderophore Production in Rice Plants Inoculated with Pseudomonas Simiae or Debaryomyces Hansenii
2.2. Effect of Inoculation with the Bacterium P. simiae (WCS417) or the Yeast D. hansenii (CBS767) on Gene Expression Levels Related to Responses to Iron Deficiency
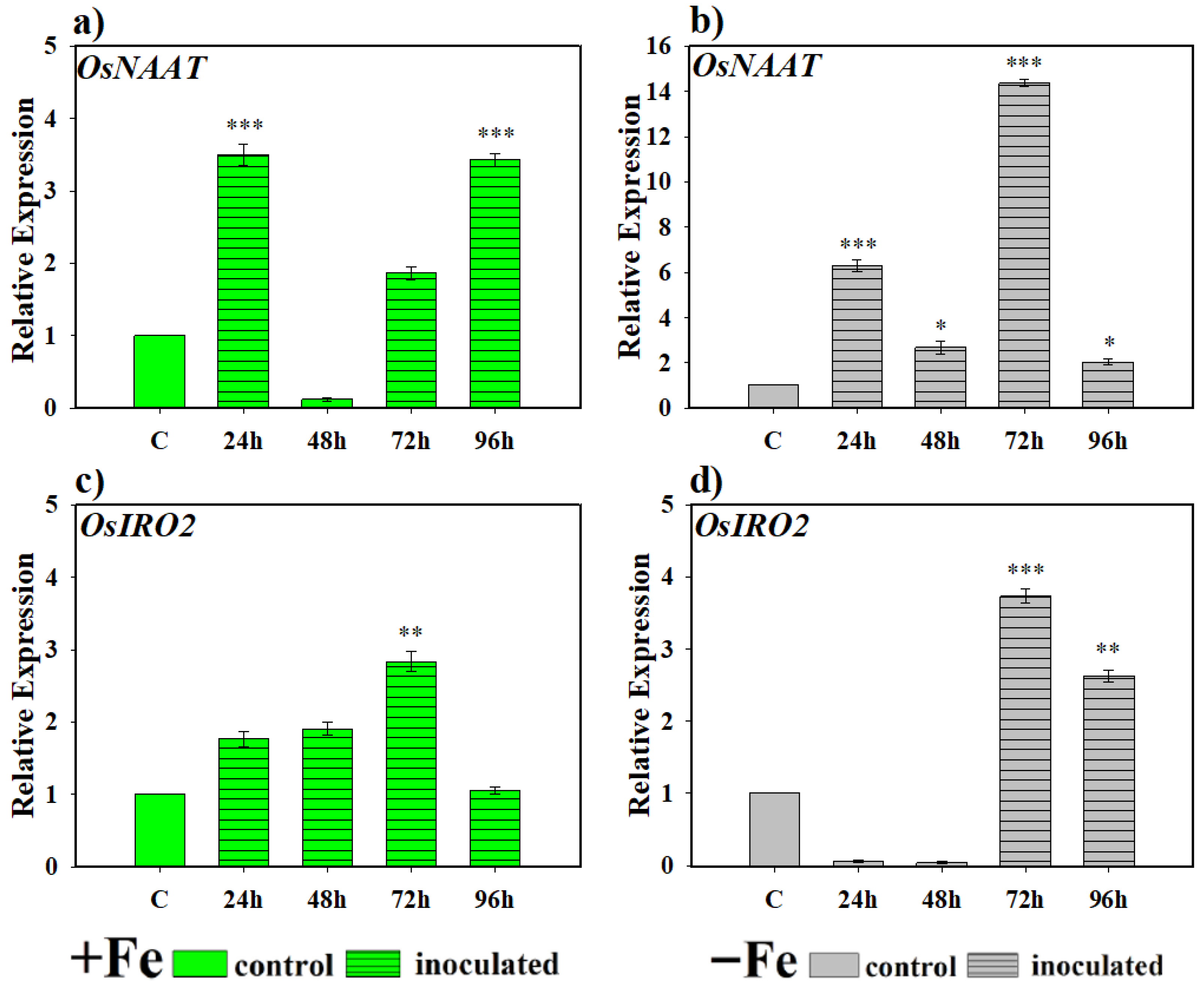
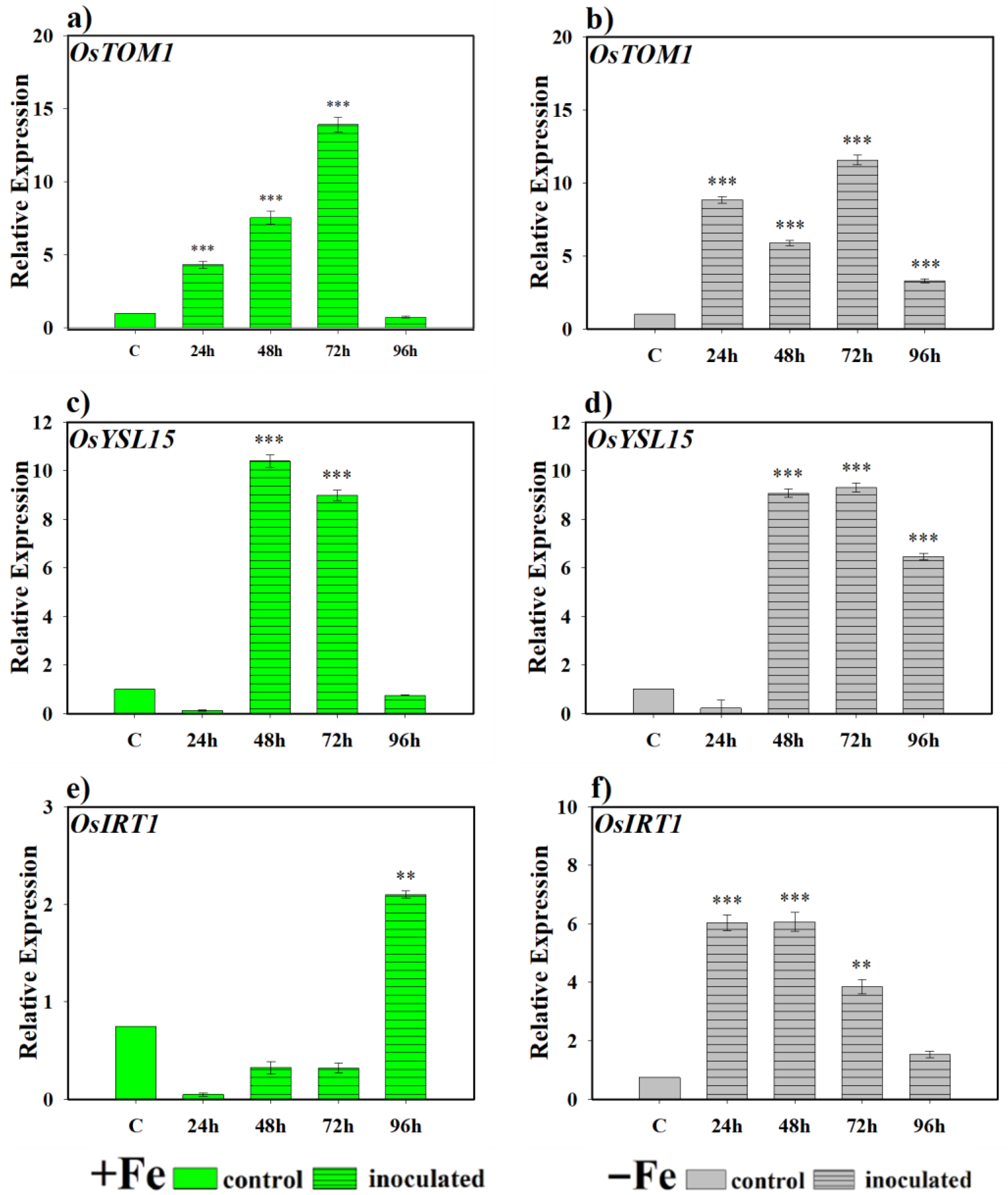
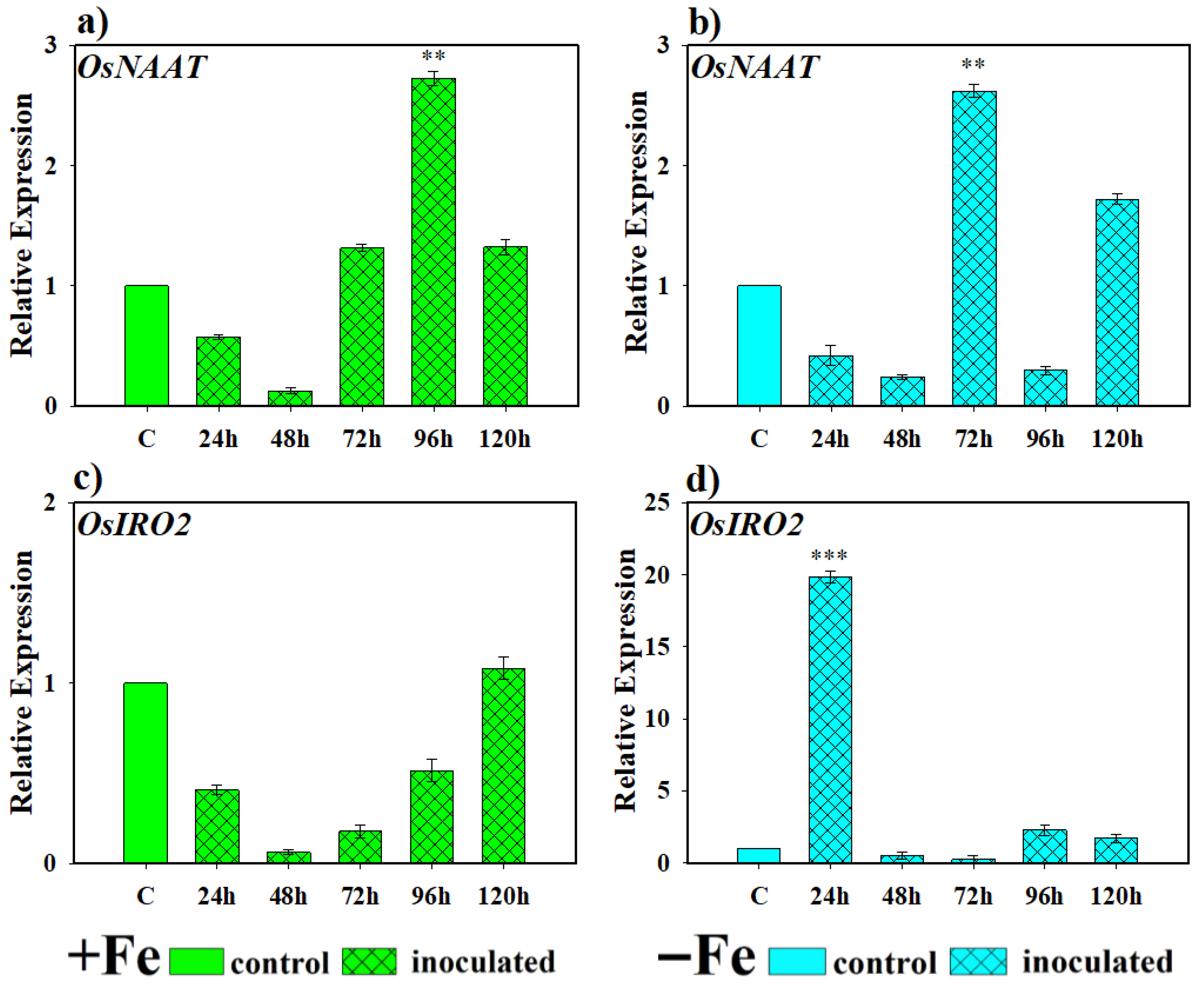
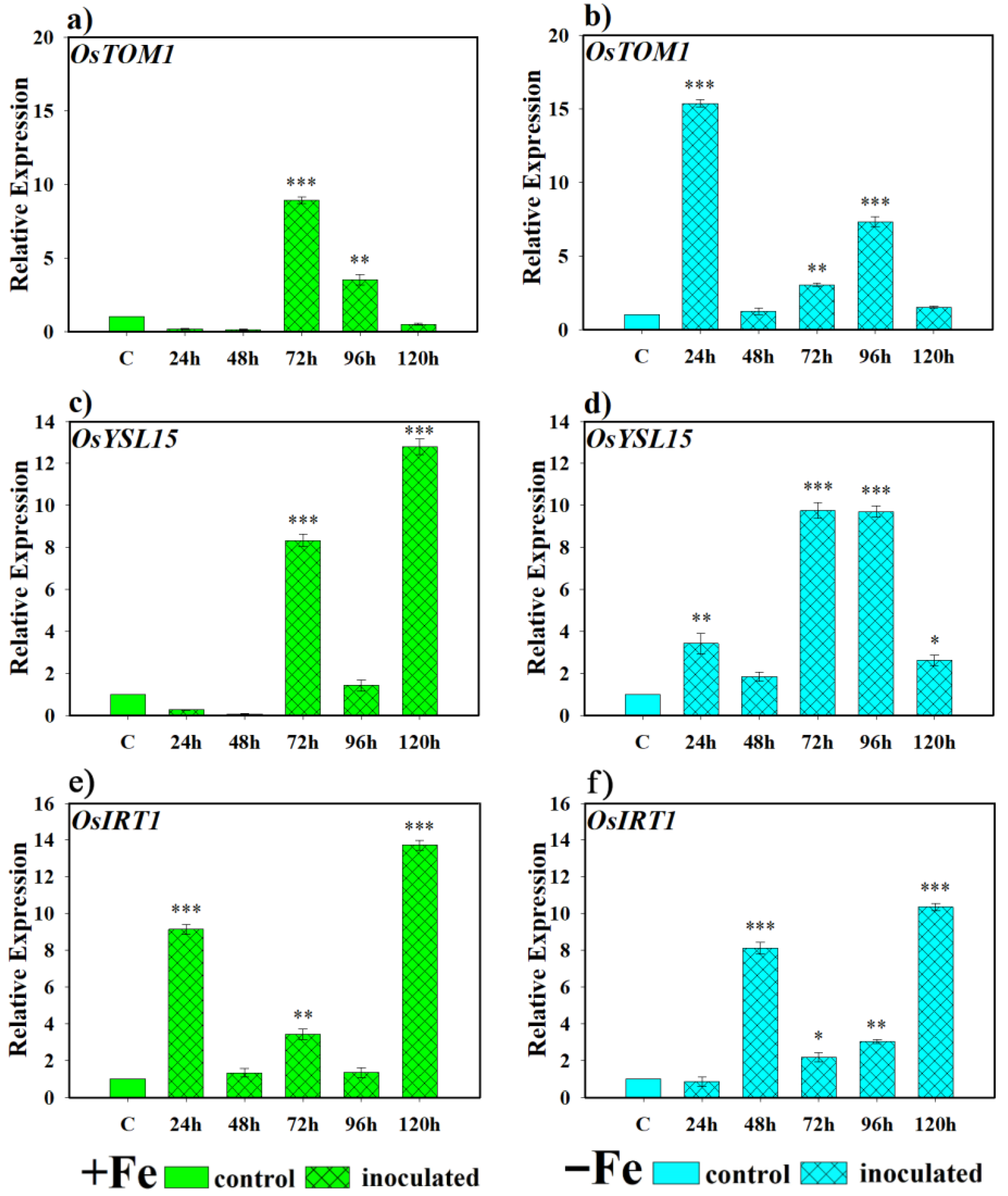
2.2.1. Effect of the Bacterium P. simiae (WCS417) on the Expression of the Genes OsNAAT, OsIRO2, OsTOM1, OsYSL15, and OsIRT1
2.2.2. Effect of the Yeast D. hansenii (CBS767) on the Expression of the Genes OsNAAT, OsIRO2, OsTOM1, OsYSL15, and OsIRT1
2.3. Effect of Inoculation with the Bacterium P. simiae (Strain WCS417) or the Yeast D. hansenii (CBS 767) on Gene Expression Levels Related to Responses to Ethylene
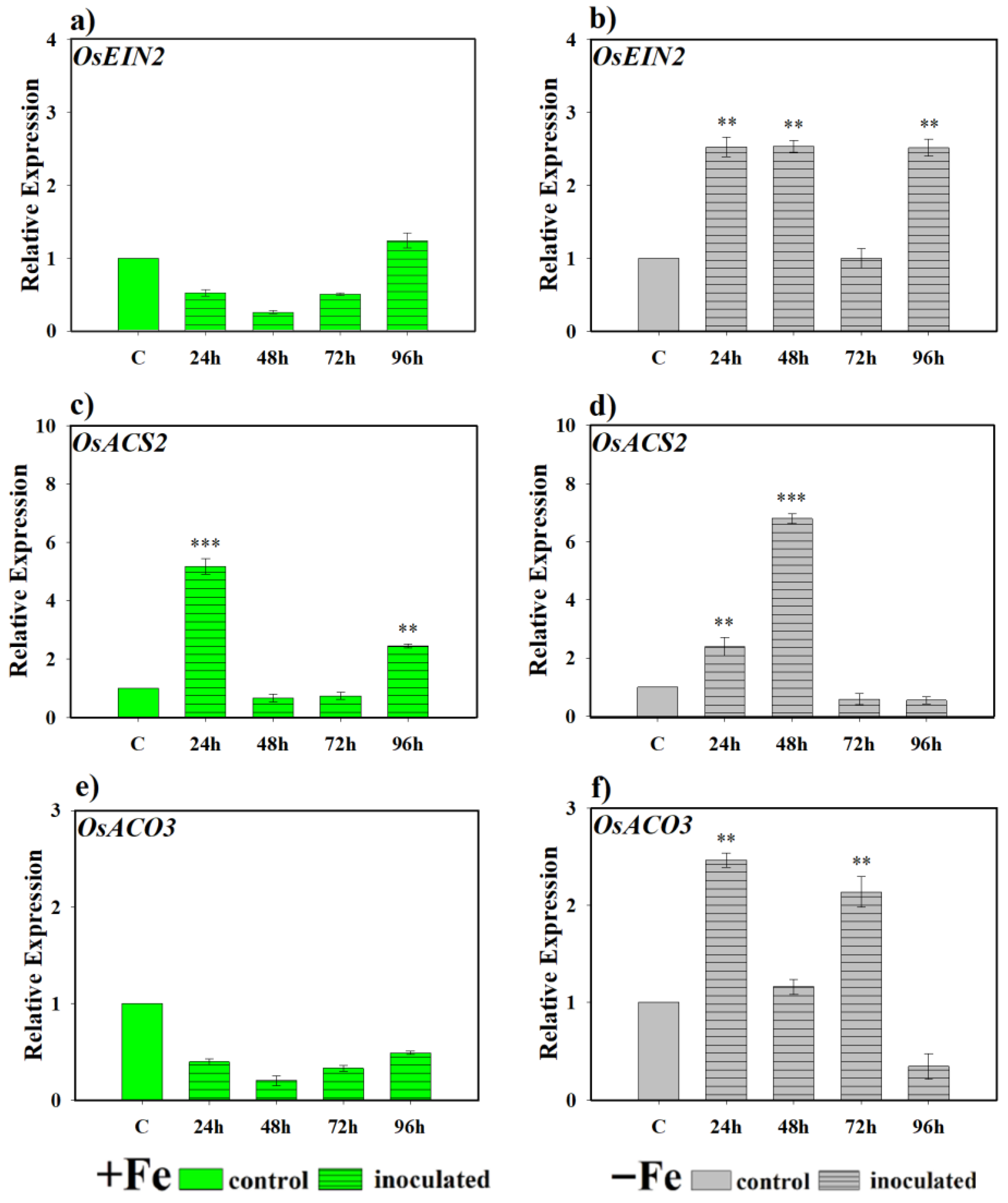
2.3.1. Effect of the Bacterium P. simiae (Strain WCS417) on the Expression of the Genes OsEIN2, OsACS2, OsACO3
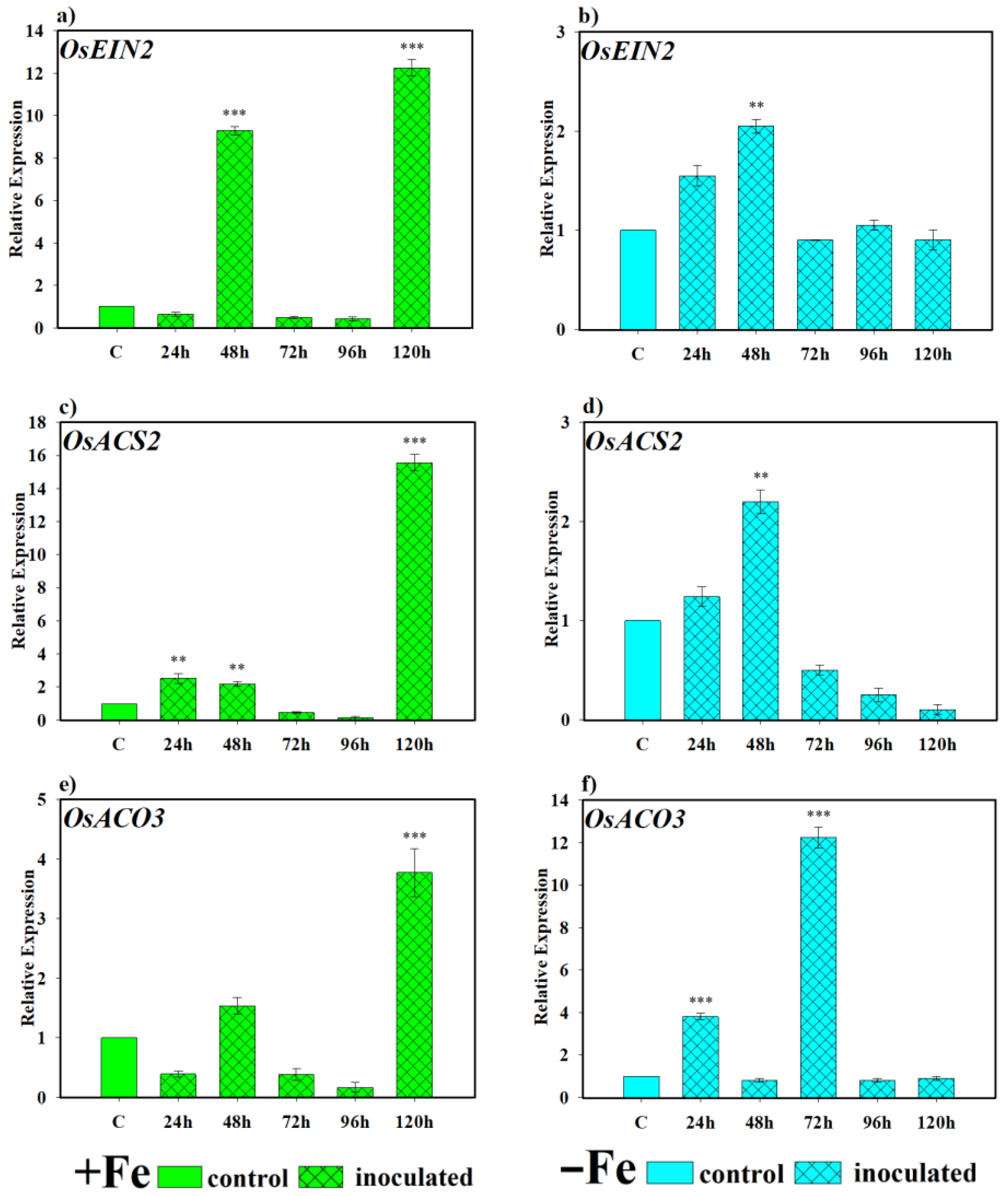
2.3.2. Effect of the Bacterium D. hansenii (Strain CBS767) on the Expression of the Genes OsEIN2, OsACS2, OsACO3
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Microbial Cultures and Plant Inoculation
4.3. Phytosiderophore Determinations
4.4. Gene Expression Analysis by qRT-PCR
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balk, J.; Pilon, M. Ancient and Essential: The Assembly of Iron–Sulfur Clusters in Plants. Trends Plant Sci. 2011, 16, 218–226. [Google Scholar] [CrossRef]
- Marschner, P. Rhizosphere Biology. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 369–388. ISBN 978-0-12-384905-2. [Google Scholar]
- Kobayashi, T.; Nishizawa, N.K. Iron Uptake, Translocation, and Regulation in Higher Plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef]
- Oertli, J.J.; Jacobson, L. Some Quantitative Considerations in Iron Nutrition of Higher Plants. Plant Physiol. 1960, 35, 683–688. [Google Scholar] [CrossRef]
- Brown, J.C.; Holmes, R.S.; Tiffin, L.O. Iron chlorosis in soybeans as related to the genotype of rootstalk: 3. Chlorosis susceptibility and reductive capacity at the root. Soil Sci. 1961, 91, 127–132. [Google Scholar] [CrossRef]
- Takagi, S. Naturally Occurring Iron-Chelating Compounds in Oat- and Rice-Root Washings: I. Activity Measurement and Preliminary Characterization. Soil Sci. Plant Nutr. 1976, 22, 423–433. [Google Scholar] [CrossRef]
- Römheld, V.; Marschner, H. Mobilization of Iron in the Rhizosphere of Different Plant Species. Adv. Plant Nutr. 1986, 2, 155–204. [Google Scholar]
- Bienfait, H.F. Mechanisms in Fe-efficiency Reactions of Higher Plants. J. Plant Nutr. 1988, 11, 605–629. [Google Scholar] [CrossRef]
- Hell, R.; Stephan, U.W. Iron Uptake, Trafficking and Homeostasis in Plants. Planta 2003, 216, 541–551. [Google Scholar] [CrossRef]
- Curie, C.; Briat, J.-F. Iron Transport and Signaling in Plants. Annu. Rev. Plant Biol. 2003, 54, 183–206. [Google Scholar] [CrossRef]
- Mori, S.; Nishizawa, N. Methionine as a Dominant Precursor of Phytosiderophores in Graminaceae Plants. Plant Cell Physiol. 1987, 28, 1081–1092. [Google Scholar] [CrossRef]
- Shojima, S.; Nishizawa, N.-K.; Fushiya, S.; Nozoe, S.; Irifune, T.; Mori, S. Biosynthesis of Phytosiderophores: In Vitro Biosynthesis of 2′-Deoxymugineic Acid from l-Methionine and Nicotianamine. Plant Physiol. 1990, 93, 1497–1503. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamaguchi, H.; Nakanishi, H.; Shioiri, T.; Nishizawa, N.-K.; Mori, S. Cloning Two Genes for Nicotianamine Aminotransferase, a Critical Enzyme in Iron Acquisition (Strategy II) in Graminaceous Plants. Plant Physiol. 1999, 121, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Inoue, H.; Nagasaka, S.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Cloning and Characterization of Deoxymugineic Acid Synthase Genes from Graminaceous Plants. J. Biol. Chem. 2006, 281, 32395–32402. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Takahashi, R.; Akhtar, S.; Ishimaru, Y.; Nakanishi, H.; Nishizawa, N.K. The Knockdown of OsVIT2 and MIT Affects Iron Localization in Rice Seed. Rice 2013, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Nozoye, T.; Nagasaka, S.; Kobayashi, T.; Takahashi, M.; Sato, Y.; Sato, Y.; Uozumi, N.; Nakanishi, H.; Nishizawa, N.K. Phytosiderophore Efflux Transporters Are Crucial for Iron Acquisition in Graminaceous Plants. J. Biol. Chem. 2011, 286, 5446–5454. [Google Scholar] [CrossRef]
- Inoue, H.; Takahashi, M.; Kobayashi, T.; Suzuki, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Identification and Localisation of the Rice Nicotianamine Aminotransferase Gene OsNAAT1 Expression Suggests the Site of Phytosiderophore Synthesis in Rice. Plant Mol. Biol. 2008, 66, 193–203. [Google Scholar] [CrossRef]
- Lee, S.; Chiecko, J.C.; Kim, S.A.; Walker, E.L.; Lee, Y.; Guerinot, M.L.; An, G. Disruption of OsYSL15 Leads to Iron Inefficiency in Rice Plants. Plant Physiol. 2009, 150, 786–800. [Google Scholar] [CrossRef]
- Kakei, Y.; Yamaguchi, I.; Kobayashi, T.; Takahashi, M.; Nakanishi, H.; Yamakawa, T.; Nishizawa, N.K. A Highly Sensitive, Quick and Simple Quantification Method for Nicotianamine and 2′-Deoxymugineic Acid from Minimum Samples Using LC/ESI-TOF-MS Achieves Functional Analysis of These Components in Plants. Plant Cell Physiol. 2009, 50, 1988–1993. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Suzuki, M.; Tsukamoto, T.; Suzuki, K.; Nakazono, M.; Kobayashi, T.; Wada, Y.; Watanabe, S.; Matsuhashi, S.; Takahashi, M.; et al. Rice Plants Take up Iron as an Fe3+ -phytosiderophore and as Fe2+. Plant J. 2006, 45, 335–346. [Google Scholar] [CrossRef]
- Ogo, Y.; Kakei, Y.; Itai, R.N.; Kobayashi, T.; Nakanishi, H.; Takahashi, H.; Nakazono, M.; Nishizawa, N.K. Spatial Transcriptomes of Iron-deficient and Cadmium-stressed Rice. New Phytol. 2014, 201, 781–794. [Google Scholar] [CrossRef]
- Ogo, Y.; Itai, R.N.; Kobayashi, T.; Aung, M.S.; Nakanishi, H.; Nishizawa, N.K. OsIRO2 Is Responsible for Iron Utilization in Rice and Improves Growth and Yield in Calcareous Soil. Plant Mol. Biol. 2011, 75, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Ogo, Y. Isolation and Characterization of IRO2, a Novel Iron-Regulated bHLH Transcription Factor in Graminaceous Plants. J. Exp. Bot. 2006, 57, 2867–2878. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ying, Y.; Narsai, R.; Ye, L.; Zheng, L.; Tian, J.; Whelan, J.; Shou, H. Identification of OsbHLH133 as a Regulator of Iron Distribution between Roots and Shoots in Oryza sativa. Plant Cell Environ. 2013, 36, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ness, E.; Hadar, Y.; Chen, Y.; Shanzer, A.; Libman, J. Iron Uptake by Plants from Microbial Siderophores: A Study with 7-Nitrobenz-2 Oxa-1,3-Diazole-Desferrioxamine as Fluorescent Ferrioxamine B Analog. Plant Physiol. 1992, 99, 1329–1335. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Van Wees, S.C.M.; Pieterse, C.M.J. Airborne Signals from Trichoderma Fungi Stimulate Iron Uptake Responses in Roots Resulting in Priming of Jasmonic Acid-dependent Defences in Shoots of Arabidopsis thalian and Solanum lycopersicum. Plant Cell Environ. 2017, 40, 2691–2705. [Google Scholar] [CrossRef]
- Yanni, Y.G.; Rizk, R.Y.; El-Fattah, F.K.A.; Squartini, A.; Corich, V.; Giacomini, A.; De Bruijn, F.; Rademaker, J.; Maya-Flores, J.; Ostrom, P.; et al. The Beneficial Plant Growth-Promoting Association of Rhizobium leguminosarum bv. trifolii with Rice Roots. Funct. Plant Biol. 2001, 28, 845. [Google Scholar] [CrossRef]
- Siddikee, M.A.; Chauhan, P.S.; Anandham, R.; Han, G.-H.; Sa, T. Isolation, Characterization, and Use for Plant Growth Promotion Under Salt Stress, of ACC Deaminase-Producing Halotolerant Bacteria Derived from Coastal Soil. J. Microbiol. Biotechnol. 2010, 20, 1577–1584. [Google Scholar] [CrossRef]
- Giri, K.; Mishra, G.; Chandra Suyal, D.; Kumar, N.; Doley, B.; Das, N.; Baruah, R.C.; Bhattacharyya, R.; Bora, N. Performance Evaluation of Native Plant Growth-Promoting Rhizobacteria for Paddy Yield Enhancement in the Jhum Fields of Mokokchung, Nagaland, North East India. Heliyon 2023, 9, e14588. [Google Scholar] [CrossRef]
- Lucena, C.; Alcalá-Jiménez, M.T.; Romera, F.J.; Ramos, J. Several Yeast Species Induce Iron Deficiency Responses in Cucumber Plants (Cucumis sativus L.). Microorganisms 2021, 9, 2603. [Google Scholar] [CrossRef]
- Kaur, J.; Anand, V.; Srivastava, S.; Bist, V.; Tripathi, P.; Naseem, M.; Nand, S.; Anshu; Khare, P.; Srivastava, P.K.; et al. Yeast Strain Debaryomyces hansenii for Amelioration of Arsenic Stress in Rice. Ecotoxicol. Environ. Saf. 2020, 195, 110480. [Google Scholar] [CrossRef]
- Romera, F.J.; Lucena, C.; García, M.J.; Alcántara, E.; Pérez-Vicente, R. The Role of Ethylene and Other Signals in the Regulation of Fe Deficiency Responses by Dicot Plants. In Stress Signaling in Plants: Genomics and Proteomics Perspective, Volume 2; Sarwat, M., Ahmad, A., Abdin, M.Z., Ibrahim, M.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 277–300. ISBN 978-3-319-42182-7. [Google Scholar]
- Aparicio, M.A.; Lucena, C.; García, M.J.; Ruiz-Castilla, F.J.; Jiménez-Adrián, P.; López-Berges, M.S.; Prieto, P.; Alcántara, E.; Pérez-Vicente, R.; Ramos, J.; et al. The Nonpathogenic Strain of Fusarium oxysporum FO12 Induces Fe Deficiency Responses in Cucumber (Cucumis sativus L.) Plants. Planta 2023, 257, 50. [Google Scholar] [CrossRef] [PubMed]
- Lucena, C.; Romera, F.J.; García, M.J.; Alcántara, E.; Pérez-Vicente, R. Ethylene Participates in the Regulation of Fe Deficiency Responses in Strategy I Plants and in Rice. Front. Plant Sci. 2015, 6, 1056. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Kakei, Y.; Shimo, H.; Bashir, K.; Sato, Y.; Sato, Y.; Uozumi, N.; Nakanishi, H.; Nishizawa, N.K. A Rice Phenolic Efflux Transporter Is Essential for Solubilizing Precipitated Apoplasmic Iron in the Plant Stele. J. Biol. Chem. 2011, 286, 24649–24655. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nakanishi Itai, R.; Nishizawa, N.K. Iron Deficiency Responses in Rice Roots. Rice 2014, 7, 27. [Google Scholar] [CrossRef]
- Alexander, D.B.; Zuberer, D.A. Use of Chrome Azurol S Reagents to Evaluate Siderophore Production by Rhizosphere Bacteria. Biol. Fert. Soils 1991, 12, 39–45. [Google Scholar] [CrossRef]
- Saharan, B.; Nehra, V. Plant Growth Promoting Rhizobacteria: A Critical Review. Life Sci. Med. Res. 2011, 21, 1–30. [Google Scholar]
- Jin, C.W.; Ye, Y.Q.; Zheng, S.J. An Underground Tale: Contribution of Microbial Activity to Plant Iron Acquisition via Ecological Processes. Ann. Bot. 2014, 113, 7–18. [Google Scholar] [CrossRef]
- Núñez-Cano, J.; Romera, F.J.; Prieto, P.; García, M.J.; Sevillano-Caño, J.; Agustí-Brisach, C.; Pérez-Vicente, R.; Ramos, J.; Lucena, C. Effect of the Nonpathogenic Strain Fusarium oxysporum FO12 on Fe Acquisition in Rice (Oryza sativa L.) Plants. Plants 2023, 12, 3145. [Google Scholar] [CrossRef]
- Inal, A.; Gunes, A.; Zhang, F.; Cakmak, I. Peanut/Maize Intercropping Induced Changes in Rhizosphere and Nutrient Concentrations in Shoots. Plant Physiol. Biochem. 2007, 45, 350–356. [Google Scholar] [CrossRef]
- Meziane, H.; Van Der Sluis, I.; Van Loon, L.C.; Höfte, M.; Bakker, P.A.H.M. Determinants of Pseudomonas putida WCS358 Involved in Inducing Systemic Resistance in Plants. Mol. Plant Pathol. 2005, 6, 177–185. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, J.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Ma, Z.; Wang, J. Paenibacillus polymyxa BFKC01 Enhances Plant Iron Absorption via Improved Root Systems and Activated Iron Acquisition Mechanisms. Plant Physiol. Biochem. 2016, 105, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Zamioudis, C.; Mastranesti, P.; Dhonukshe, P.; Blilou, I.; Pieterse, C.M.J. Unraveling Root Developmental Programs Initiated by Beneficial Pseudomonas spp. Bacteria. Plant Physiol. 2013, 162, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Zamioudis, C.; Korteland, J.; Van Pelt, J.A.; Van Hamersveld, M.; Dombrowski, N.; Bai, Y.; Hanson, J.; Van Verk, M.C.; Ling, H.; Schulze-Lefert, P.; et al. Rhizobacterial Volatiles and Photosynthesis-related Signals Coordinate MYB 72 Expression in Arabidopsis Roots during Onset of Induced Systemic Resistance and Iron-deficiency Responses. Plant J. 2015, 84, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Campbell, N.H.; Grotz, N.; Prichard, C.L.; Guerinot, M.L. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol. 2003, 133, 1102–1110. [Google Scholar] [CrossRef]
- Koryachko, A.; Matthiadis, A.; Haque, S.; Muhammad, D.; Ducoste, J.J.; Tuck, J.M.; Long, T.A.; Williams, C.M. Dynamic Modelling of the Iron Deficiency Modulated Transcriptome Response in Arabidopsis thaliana Roots. isP 2019, 1, diz005. [Google Scholar] [CrossRef]
- Yi, F.; Huo, M.; Li, J.; Yu, J. Time-series Transcriptomics Reveals a Drought-responsive Temporal Network and Crosstalk between Drought Stress and the Circadian Clock in Foxtail Millet. Plant J. 2022, 110, 1213–1228. [Google Scholar] [CrossRef]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef]
- Sauter, M.; Moffatt, B.; Saechao, M.C.; Hell, R.; Wirtz, M. Methionine Salvage and S-Adenosylmethionine: Essential Links between Sulfur, Ethylene and Polyamine Biosynthesis. Biochem. J. 2013, 451, 145–154. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Yu, K.; Feussner, K.; De Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.H.M.; Feussner, I.; Pieterse, C.M.J. MYB72-Dependent Coumarin Exudation Shapes Root Microbiome Assembly to Promote Plant Health. Proc. Natl. Acad. Sci. USA 2018, 115, E5213–E5222. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Berendsen, R.L.; De Jonge, R.; Stringlis, I.A.; Van Dijken, A.J.H.; Van Pelt, J.A.; Van Wees, S.C.M.; Yu, K.; Zamioudis, C.; Bakker, P.A.H.M. Pseudomonas simiae WCS417: Star Track of a Model Beneficial Rhizobacterium. Plant Soil 2021, 461, 245–263. [Google Scholar] [CrossRef]
- Nutaratat, P.; Srisuk, N.; Arunrattiyakorn, P.; Limtong, S. Plant Growth-Promoting Traits of Epiphytic and Endophytic Yeasts Isolated from Rice and Sugar Cane Leaves in Thailand. Fungal Biol. 2014, 118, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-Y.; Jiang, W.; Fu, S.-F.; Chou, J.-Y. Screening, Evaluation, and Selection of Yeasts with High Ammonia Production Ability under Nitrogen Free Condition from the Cherry Tomato (Lycopersicon esculentum var. cerasiforme) Rhizosphere as a Potential Bio-Fertilizer. Rhizosphere 2022, 23, 100580. [Google Scholar] [CrossRef]
- Núñez-Cano, J.; Ruiz-Castilla, F.J.; Ramos, J.; Romera, F.J.; Lucena, C. Debaryomyces hansenii Enhances Growth, Nutrient Uptake, and Yield in Rice Plants (Oryza sativa L.) Cultivated in Calcareous Soil. Agronomy 2025, 15, 1696. [Google Scholar] [CrossRef]
- Sevillano-Caño, J.; García, M.J.; Córdoba-Galván, C.; Luque-Cruz, C.; Agustí-Brisach, C.; Lucena, C.; Ramos, J.; Pérez-Vicente, R.; Romera, F.J. Exploring the Role of Debaryomyces hansenii as Biofertilizer in Iron-Deficient Environments to Enhance Plant Nutrition and Crop Production Sustainability. Int. J. Mol. Sci. 2024, 25, 5729. [Google Scholar] [CrossRef]
- Czarnecka, M.; Żarowska, B.; Połomska, X.; Restuccia, C.; Cirvilleri, G. Role of Biocontrol Yeasts Debaryomyces hansenii and Wickerhamomyces anomalus in Plants’ Defence Mechanisms against Monilinia fructicola in Apple Fruits. Food Microbiol. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- Ferreira, I.; De Sousa Melo, D.; Menezes, A.G.T.; Fonseca, H.C.; De Assis, B.B.T.; Ramos, C.L.; Magnani, M.; Dias, D.R.; Schwan, R.F. Evaluation of Potentially Probiotic Yeasts and Lactiplantibacillus plantarum in Co-Culture for the Elaboration of a Functional Plant-Based Fermented Beverage. Food Res. Int. 2022, 160, 111697. [Google Scholar] [CrossRef]
- Chacón-Navarrete, H.; Ruiz-Pérez, F.; Ruiz-Castilla, F.J.; Ramos, J. Exploring Biocontrol of Unwanted Fungi by Autochthonous Debaryomyces hansenii Strains Isolated from Dry Meat Products. J. Fungi 2022, 8, 873. [Google Scholar] [CrossRef]
- Römheld, V.; Marschner, H. Iron Deficiency Stress Induced Morphological and Physiological Changes in Root Tips of Sunflower. Physiol. Plant. 1981, 53, 354–360. [Google Scholar] [CrossRef]
- Reichman, S.M.; Parker, D.R. Critical Evaluation of Three Indirect Assays for Quantifying Phytosiderophores Released by the Roots of Poaceae. Eur. J. Soil Sci. 2007, 58, 844–853. [Google Scholar] [CrossRef]
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| OsNAAT1 | TAAGAGGATAATTGATTTGCTTAC | CTGATCATTCCAATCCTAGTACAAT |
| OsIRO2 | CTCCCATCGTTTCGGCTACCT | GCTGGGCACTCCTCGTTGATC |
| OsTOM1 | GCCCAAGAACGCCAAAATGA | GGCTTGAAGGTCAACGCAAG |
| OsYSL15 | AACATAAGGGGGACTG GTAC | TGATTACCGCAATGATGCTTAG |
| OsIRT1 | CGTC TTCTTCTTCTCCACCACGAC | GCAGCTGATGATCGAGTCTGACC |
| OsEIN2 | GCTGCGGTAGAGAAGCTATT | TGTACTGGATGTCTGCCTTATC |
| OsACS2 | TTTGGCGCCTTGACGGCCTC | AAAGGGAGCGCACCATGGCC |
| OsACO3 | TGCAACAGCACGCCACACCA | TGGATCGACGTCCAGCCCGT |
| OsActin | TGCTATGTACGTCGC CATCCAG | AATGAGTAACCACGCTCCGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Cano, J.; Ruiz-Castilla, F.J.; Romera, F.J.; Ramos, J.; Lucena, C. Pseudomonas simiae WCS417 and Debaryomyces hansenii Induce Iron Deficiency Responses in Rice (Oryza sativa L.) Through Phytosiderophore Production and Gene Expression Modulation. Plants 2025, 14, 3769. https://doi.org/10.3390/plants14243769
Núñez-Cano J, Ruiz-Castilla FJ, Romera FJ, Ramos J, Lucena C. Pseudomonas simiae WCS417 and Debaryomyces hansenii Induce Iron Deficiency Responses in Rice (Oryza sativa L.) Through Phytosiderophore Production and Gene Expression Modulation. Plants. 2025; 14(24):3769. https://doi.org/10.3390/plants14243769
Chicago/Turabian StyleNúñez-Cano, Jorge, Francisco J. Ruiz-Castilla, Francisco J. Romera, José Ramos, and Carlos Lucena. 2025. "Pseudomonas simiae WCS417 and Debaryomyces hansenii Induce Iron Deficiency Responses in Rice (Oryza sativa L.) Through Phytosiderophore Production and Gene Expression Modulation" Plants 14, no. 24: 3769. https://doi.org/10.3390/plants14243769
APA StyleNúñez-Cano, J., Ruiz-Castilla, F. J., Romera, F. J., Ramos, J., & Lucena, C. (2025). Pseudomonas simiae WCS417 and Debaryomyces hansenii Induce Iron Deficiency Responses in Rice (Oryza sativa L.) Through Phytosiderophore Production and Gene Expression Modulation. Plants, 14(24), 3769. https://doi.org/10.3390/plants14243769







