Harnessing Setaria as a Model for C4 Plant Adaptation to Abiotic Stress
Abstract
1. Introduction
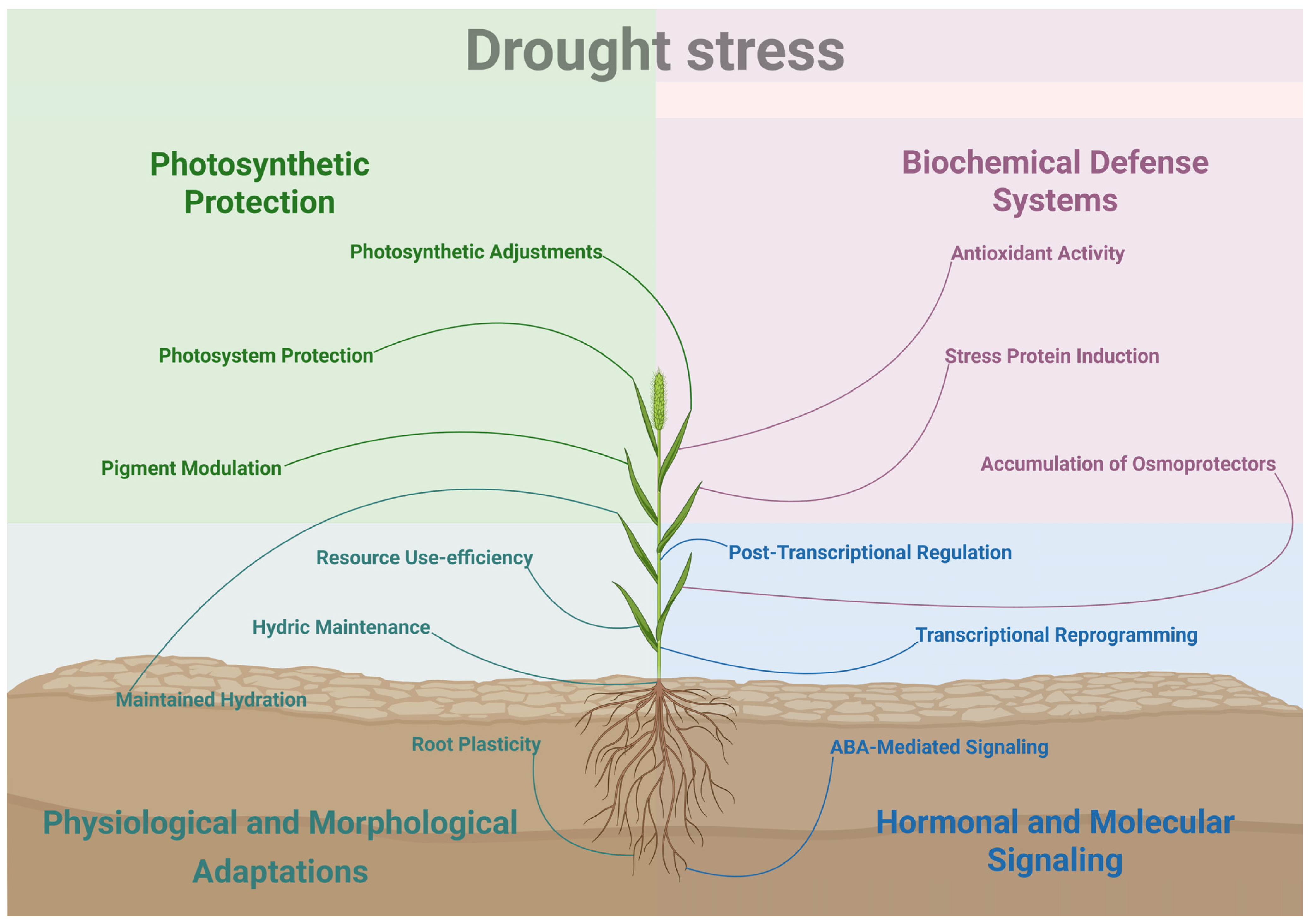
2. Drought Stress
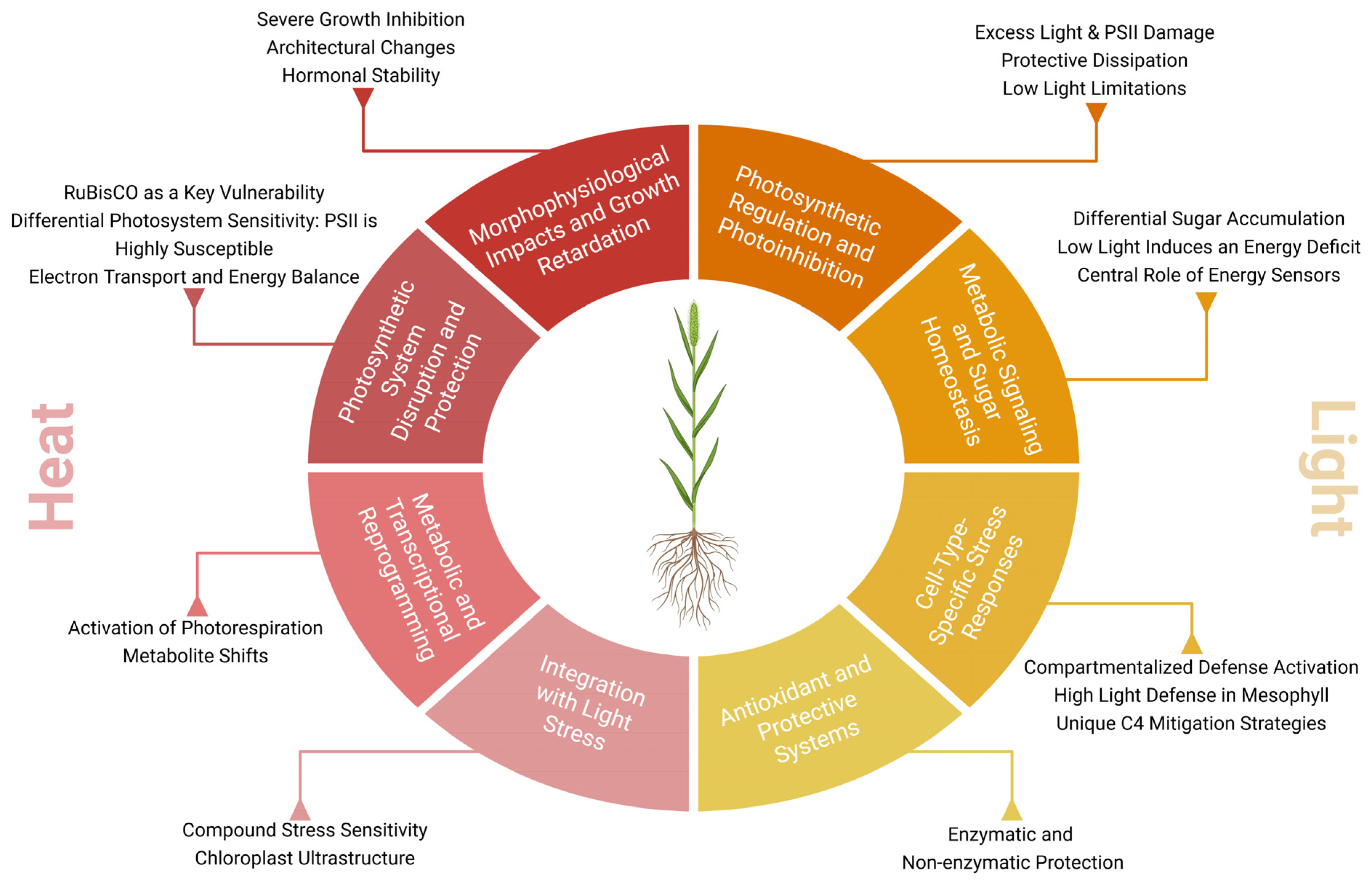
3. Extreme Temperatures
4. Light Stress
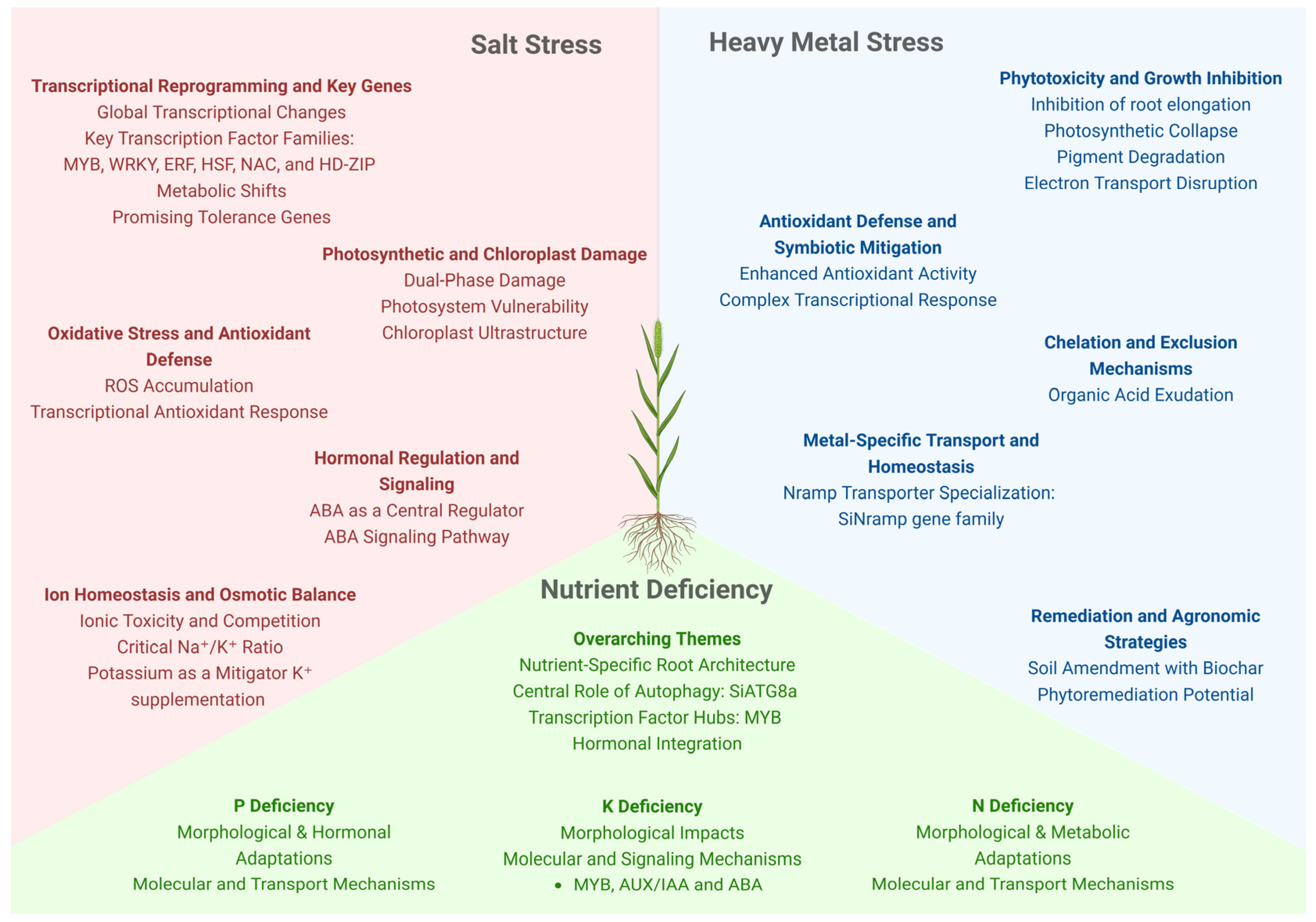
5. Salt Stress
6. Nutrient Deficiency
6.1. Nitrogen Deficiency
6.2. Phosphorus Deficiency
6.3. Potassium Deficiency
7. Heavy Metals
8. Combined Stimuli and Other Abiotic Stresses
9. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knutti, R.; Rogelj, J.; Sedlácek, J.; Fischer, E.M. A Scientific Critique of the Two-Degree Climate Change Target. Nat. Geosci. 2016, 9, 13–18. [Google Scholar]
- Coustenis, A.; Taylor, F.W.; Plainaki, C. Climate Issues from the Planetary Perspective and Insights for the Earth. In Global Change and Future Earth; Cambridge University Press: Cambridge, UK, 2018; pp. 40–54. [Google Scholar]
- Govindan, K. Sustainable Consumption and Production in the Food Supply Chain: A Conceptual Framework. Int. J. Prod. Econ. 2018, 195, 419–431. [Google Scholar] [CrossRef]
- Abberton, M.; Batley, J.; Bentley, A.; Bryant, J.; Cai, H.; Cockram, J.; Costa de Oliveira, A.; Cseke, L.J.; Dempewolf, H.; De Pace, C.; et al. Global Agricultural Intensification during Climate Change: A Role for Genomics. Plant Biotechnol. J. 2016, 14, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- IPCC. AR6 Synthesis Report: Climate Change 2023. Available online: https://www.ipcc.ch/report/ar6/syr/ (accessed on 1 November 2025).
- Oshunsanya, S.O.; Nwosu, N.J.; Li, Y. Abiotic Stress in Agricultural Crops Under Climatic Conditions. In Sustainable Agriculture, Forest and Environmental Management; Springer Singapore: Singapore, 2019; pp. 71–100. [Google Scholar]
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.K.; Ratnayake, S. Climate Change and Future of Agri-Food Production. In Future Foods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 49–79. [Google Scholar]
- Ghadirnezhad Shiade, S.R.; Fathi, A.; Taghavi Ghasemkheili, F.; Amiri, E.; Pessarakli, M. Plants’ Responses under Drought Stress Conditions: Effects of Strategic Management Approaches—A Review. J. Plant Nutr. 2023, 46, 2198–2230. [Google Scholar] [CrossRef]
- Seikh, T.A.; Liontou, P.; Korres, N.E. The Response of Weeds under Abiotic Stress as a Tool for Green Strategies in Agriculture. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2025; pp. 1–28. [Google Scholar]
- Zandalinas, S.I.; Sengupta, S.; Fritschi, F.B.; Azad, R.K.; Nechushtai, R.; Mittler, R. The Impact of Multifactorial Stress Combination on Plant Growth and Survival. New Phytol. 2021, 230, 1034–1048. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Pinnola, A.; Bassi, R. Molecular Mechanisms Involved in Plant Photoprotection. Biochem. Soc. Trans. 2018, 46, 467–482. [Google Scholar] [CrossRef]
- Chauhan, J.; Prathibha, M.; Singh, P.; Choyal, P.; Mishra, U.N.; Saha, D.; Kumar, R.; Anuragi, H.; Pandey, S.; Bose, B.; et al. Plant Photosynthesis under Abiotic Stresses: Damages, Adaptive, and Signaling Mechanisms. Plant Stress 2023, 10, 100296. [Google Scholar] [CrossRef]
- Opoku, E.; Sahu, P.P.; Findurová, H.; Holub, P.; Urban, O.; Klem, K. Differential Physiological and Production Responses of C3 and C4 Crops to Climate Factor Interactions. Front. Plant Sci. 2024, 15, 1345462. [Google Scholar] [CrossRef]
- Monson, R.K.; Li, S.; Ainsworth, E.A.; Fan, Y.; Hodge, J.G.; Knapp, A.K.; Leakey, A.D.B.; Lombardozzi, D.; Reed, S.C.; Sage, R.F.; et al. C4 Photosynthesis, Trait Spectra, and the Fast-efficient Phenotype. New Phytol. 2025, 246, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Stainbrook, S.C.; Aubuchon, L.N.; Chen, A.; Johnson, E.; Si, A.; Walton, L.; Ahrendt, A.J.; Strenkert, D.; Jez, J.M. C4 Grasses Employ Distinct Strategies to Acclimate Rubisco Activase to Heat Stress. Biosci. Rep. 2024, 44, BSR20240353. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Sui, N. Sensitivity and Responses of Chloroplasts to Salt Stress in Plants. Front. Plant Sci. 2024, 15, 1374086. [Google Scholar] [CrossRef] [PubMed]
- Brutnell, T.P.; Wang, L.; Swartwood, K.; Goldschmidt, A.; Jackson, D.; Zhu, X.-G.; Kellogg, E.; Van Eck, J. Setaria viridis: A Model for C4 Photosynthes. Plant Cell 2010, 22, 2537–2544. [Google Scholar] [CrossRef]
- Li, P.; Brutnell, T.P. Setaria viridis and Setaria italica, Model Genetic Systems for the Panicoid Grasses. J. Exp. Bot. 2011, 62, 3031–3037. [Google Scholar] [CrossRef]
- Bennetzen, J.L.; Schmutz, J.; Wang, H.; Percifield, R.; Hawkins, J.; Pontaroli, A.C.; Estep, M.; Feng, L.; Vaughn, J.N.; Grimwood, J.; et al. Reference Genome Sequence of the Model Plant Setaria. Nat. Biotechnol. 2012, 30, 555–561. [Google Scholar] [CrossRef]
- Mamidi, S.; Healey, A.; Huang, P.; Grimwood, J.; Jenkins, J.; Barry, K.; Sreedasyam, A.; Shu, S.; Lovell, J.T.; Feldman, M.; et al. A Genome Resource for Green Millet Setaria viridis Enables Discovery of Agronomically Valuable Loci. Nat. Biotechnol. 2020, 38, 1203–1210. [Google Scholar] [CrossRef]
- Doust, A.N.; Kellogg, E.A.; Devos, K.M.; Bennetzen, J.L. Foxtail Millet: A Sequence-Driven Grass Model System. Plant Physiol. 2009, 149, 137–141. [Google Scholar] [CrossRef]
- Van Eck, J. The Status of Setaria viridis Transformation: Agrobacterium-Mediated to Floral Dip. Front. Plant Sci. 2018, 9, 652. [Google Scholar] [CrossRef]
- Weiss, T.; Wang, C.; Kang, X.; Zhao, H.; Elena Gamo, M.; Starker, C.G.; Crisp, P.A.; Zhou, P.; Springer, N.M.; Voytas, D.F.; et al. Optimization of Multiplexed CRISPR/Cas9 System for Highly Efficient Genome Editing in Setaria viridis. Plant J. 2020, 104, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.; Romeiro, D.; Silva, J.P.; Basso, M.F.; Molinari, H.B.C.; Centeno, D.C. An Improved Protocol for Efficient Transformation and Regeneration of Setaria italica. Plant Cell Rep. 2020, 39, 501–510. [Google Scholar] [CrossRef]
- Hu, H.; Mauro-Herrera, M.; Doust, A.N. Domestication and Improvement in the Model C4 Grass, Setaria. Front. Plant Sci. 2018, 9, 719. [Google Scholar] [CrossRef]
- Liu, Y.; Xi, Y.; Zhang, F.; Wang, Z.; Wang, C.; Yu, S.; Chen, X. Charring-Induced Morphological Changes of Chinese “Five Grains”: An Experimental Study. Front. Plant Sci. 2023, 14, 1063617. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Muthamilarasan, M.; Prasad, M. Foxtail Millet: An Introduction. In The Foxtail Millet Genome; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–9. [Google Scholar]
- Diao, X.; Jia, G. Foxtail Millet Germplasm and Inheritance of Morphological Characteristics. In Genetics and Genomics of Setaria; Springer: Berlin/Heidelberg, Germany, 2017; pp. 73–92. [Google Scholar]
- Dekker, J. The Foxtail (Setaria) Species-Group. Weed Sci. 2003, 51, 641–656. [Google Scholar] [CrossRef]
- Sebastian, J.; Yee, M.-C.; Goudinho Viana, W.; Rellán-Álvarez, R.; Feldman, M.; Priest, H.D.; Trontin, C.; Lee, T.; Jiang, H.; Baxter, I.; et al. Grasses Suppress Shoot-Borne Roots to Conserve Water during Drought. Proc. Natl. Acad. Sci. USA 2016, 113, 8861–8866. [Google Scholar] [CrossRef]
- Saha, P.; Sade, N.; Arzani, A.; Rubio Wilhelmi, M.d.M.; Coe, K.M.; Li, B.; Blumwald, E. Effects of Abiotic Stress on Physiological Plasticity and Water Use of Setaria viridis (L.). Plant Sci. 2016, 251, 128–138. [Google Scholar] [CrossRef]
- Mauro-Herrera, M.; Doust, A.N. Development and Genetic Control of Plant Architecture and Biomass in the Panicoid Grass, Setaria. PLoS ONE 2016, 11, e0151346. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, X.; Li, H.; Cui, X.; Diao, X.; Qiao, Z. Genomic Analysis of Hexokinase Genes in Foxtail Millet (Setaria italica): Haplotypes and Expression Patterns Under Abiotic Stresses. Int. J. Mol. Sci. 2025, 26, 1962. [Google Scholar] [CrossRef]
- Supriya, L.; Shukla, P.; Dake, D.; Gudipalli, P.; Muthamilarasan, M. Physio-Biochemical and Molecular Analyses Decipher Distinct Dehydration Stress Responses in Contrasting Genotypes of Foxtail Millet (Setaria italica L.). J. Plant Physiol. 2025, 311, 154549. [Google Scholar] [CrossRef]
- Gao, H.; Ge, W.; Bai, L.; Zhang, T.; Zhao, L.; Li, J.; Shen, J.; Xu, N.; Zhang, H.; Wang, G.; et al. Proteomic Analysis of Leaves and Roots during Drought Stress and Recovery in Setaria italica L. Front. Plant Sci. 2023, 14, 1240164. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The Impact of Disasters and Crises on Agriculture and Food Security: 2021; FAO: Rome, Italy, 2021; ISBN 978-92-5-134071-4. [Google Scholar]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The Physiology of Plant Responses to Drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Zhang, R.; Zhi, H.; Li, Y.; Guo, E.; Feng, G.; Tang, S.; Guo, W.; Zhang, L.; Jia, G.; Diao, X. Response of Multiple Tissues to Drought Revealed by a Weighted Gene Co-Expression Network Analysis in Foxtail Millet [Setaria italica (L.) P. Beauv.]. Front. Plant Sci. 2022, 12, 746166. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, Y.; Xing, Q.; Duan, R.; Shen, J.; Zong, Y.; Zhang, D.; Shi, X.; Li, P.; Hao, X. Elevated CO2 Concentration Enhances Drought Tolerance by Mitigating Oxidative Stress and Enhancing Carbon Assimilation in Foxtail Millet (Setaria italica). J. Agron. Crop Sci. 2024, 210, e12778. [Google Scholar] [CrossRef]
- da Cunha Valença, D.; de Moura, S.M.; Travassos-Lins, J.; Alves-Ferreira, M.; Medici, L.O.; Ortiz-Silva, B.; Macrae, A.; Reinert, F. Physiological and Molecular Responses of Setaria viridis to Osmotic Stress. Plant Physiol. Biochem. 2020, 155, 114–125. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Yang, Q.; Luo, Y.; Gong, X.; Zhang, W.; Hu, Y.; Yang, T.; Dong, K.; Feng, B. Proteomic Changes in the Grains of Foxtail Millet (Setaria italica (L.) Beau) under Drought Stress. Span. J. Agric. Res. 2019, 17, e0802. [Google Scholar] [CrossRef]
- Shi, W.; Cheng, J.; Wen, X.; Wang, J.; Shi, G.; Yao, J.; Hou, L.; Sun, Q.; Xiang, P.; Yuan, X.; et al. Transcriptomic Studies Reveal a Key Metabolic Pathway Contributing to a Well-Maintained Photosynthetic System under Drought Stress in Foxtail Millet (Setaria italica L.). PeerJ 2018, 6, e4752. [Google Scholar] [CrossRef] [PubMed]
- Travassos-Lins, J.; de Oliveira Rocha, C.C.; de Souza Rodrigues, T.; Alves-Ferreira, M. Evaluation of the Molecular and Physiological Response to Dehydration of Two Accessions of the Model Plant Setaria viridis. Plant Physiol. Biochem. 2021, 169, 211–223. [Google Scholar] [CrossRef]
- Cal, A.J.; Sanciangco, M.; Rebolledo, M.C.; Luquet, D.; Torres, R.O.; McNally, K.L.; Henry, A. Leaf Morphology, Rather than Plant Water Status, Underlies Genetic Variation of Rice Leaf Rolling under Drought. Plant Cell Environ. 2019, 42, 1532–1544. [Google Scholar] [CrossRef]
- O’Toole, J.C.; Cruz, R.T.; Singh, T.N. Leaf Rolling and Transpiration. Plant Sci. Lett. 1979, 16, 111–114. [Google Scholar] [CrossRef]
- Guo, Y.; Hao, D.; Wang, X.; Wang, H.; Wu, Z.; Yang, P.; Zhang, B. Comparative Transcriptomics Reveals Key Genes Contributing to the Differences in Drought Tolerance among Three Cultivars of Foxtail Millet (Setaria italica). Plant Growth Regul. 2023, 99, 45–64. [Google Scholar] [CrossRef]
- Rodrigues, T.d.S.; Lins, J.T.; Cattem, M.V.; Jardim, V.C.; Buckeridge, M.S.; Grossi-de-Sá, M.F.; Reinert, F.; Alves-Ferreira, M. Evaluation of Setaria viridis Physiological and Gene Expression Responses to Distinct Water-Deficit Conditions. Biotechnol. Res. Innov. 2019, 3, 42–58. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and Drought: Can We Make Metabolic Connections from Available Data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Duarte, K.E.; Basso, M.F.; de Oliveira, N.G.; da Silva, J.C.F.; de Oliveira Garcia, B.; Cunha, B.A.D.B.; Cardoso, T.B.; Nepomuceno, A.L.; Kobayashi, A.K.; Santiago, T.R.; et al. MicroRNAs Expression Profiles in Early Responses to Different Levels of Water Deficit in Setaria viridis. Physiol. Mol. Biol. Plants 2022, 28, 1607–1624. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.J.; Ellsworth, P.Z.; Fahlgren, N.; Gehan, M.A.; Cousins, A.B.; Baxter, I. Components of Water Use Efficiency Have Unique Genetic Signatures in the Model C4 Grass Setaria. Plant Physiol. 2018, 178, 699–715. [Google Scholar] [CrossRef]
- Ajithkumar, I.P.; Panneerselvam, R. Osmolyte Accumulation, Photosynthetic Pigment and Growth of Setaria italica (L.) P. Beauv. under Drought Stress. Asian Pac. J. Reprod. 2013, 2, 220–224. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Wang, W.; Zhu, L. Photosynthetic Mechanisms of Carbon Fixation Reduction in Rice by Cadmium and Polycyclic Aromatic Hydrocarbons. Environ. Pollut. 2024, 344, 123436. [Google Scholar] [CrossRef]
- Nour, M.M.; Aljabi, H.R.; AL-Huqail, A.A.; Horneburg, B.; Mohammed, A.E.; Alotaibi, M.O. Drought Responses and Adaptation in Plants Differing in Life-Form. Front. Ecol. Evol. 2024, 12, 1452427. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does It Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Zhang, J.; Li, X.; Ma, F.; Duan, M.; Zhang, B.; Li, H. The Responses of the Lipoxygenase Gene Family to Salt and Drought Stress in Foxtail Millet (Setaria italica). Life 2021, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Deng, L.; Yao, H.; Zhang, Y.; Li, H.; Wang, H.; Zhang, B.; Han, Y.; Wang, J. Responses of the Lipoxygenase Gene Family to Drought Stress in Broomcorn Millet (Panicum milizaceum L.). Genes 2025, 16, 368. [Google Scholar] [CrossRef]
- Weng, Y.; Wang, Y.; Wang, K.; Wu, F.; Wei, Y.; Jiang, J.; Zhu, Y.; Wang, F.; Xie, H.; Xiao, Y.; et al. OsLOX1 Positively Regulates Seed Vigor and Drought Tolerance in Rice. Plant Mol. Biol. 2025, 115, 16. [Google Scholar] [CrossRef] [PubMed]
- Amoah, J.N.; Adu-Gyamfi, M.O.; Kwarteng, A.O. Effect of Drought Acclimation on Antioxidant System and Polyphenolic Content of Foxtail Millet (Setaria italica L.). Physiol. Mol. Biol. Plants 2023, 29, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, K.B.; Abdelly, C.; Savouré, A. How Reactive Oxygen Species and Proline Face Stress Together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Rai, G.K.; Khanday, D.M.; Choudhary, S.M.; Kumar, P.; Kumari, S.; Martínez-Andújar, C.; Martínez-Melgarejo, P.A.; Rai, P.K.; Pérez-Alfocea, F. Unlocking Nature’s Stress Buster: Abscisic Acid’s Crucial Role in Defending Plants against Abiotic Stress. Plant Stress 2024, 11, 100359. [Google Scholar] [CrossRef]
- de Oliveira, I.P.; Schaaf, C.; de Setta, N. Drought Responses in Poaceae: Exploring the Core Components of the ABA Signaling Pathway in Setaria italica and Setaria viridis. Plants 2024, 13, 1451. [Google Scholar] [CrossRef]
- Duarte, K.E.; de Souza, W.R.; Santiago, T.R.; Sampaio, B.L.; Ribeiro, A.P.; Cotta, M.G.; da Cunha, B.A.D.B.; Marraccini, P.R.R.; Kobayashi, A.K.; Molinari, H.B.C. Identification and Characterization of Core Abscisic Acid (ABA) Signaling Components and Their Gene Expression Profile in Response to Abiotic Stresses in Setaria viridis. Sci. Rep. 2019, 9, 4028. [Google Scholar] [CrossRef]
- Qi, X.; Xie, S.; Liu, Y.; Yi, F.; Yu, J. Genome-Wide Annotation of Genes and Noncoding RNAs of Foxtail Millet in Response to Simulated Drought Stress by Deep Sequencing. Plant Mol. Biol. 2013, 83, 459–473. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Qiao, Z.; Li, C.-L.; Zhang, W. WRKY1 Regulates Stomatal Movement in Drought-Stressed Arabidopsis thaliana. Plant Mol. Biol. 2016, 91, 53–65. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, L.-L.; Xu, Z.-S.; Fu, L.; Pang, H.-X.; Ma, Y.-Z.; Min, D.-H. Genome-Wide Analysis of MADS-Box Genes in Foxtail Millet (Setaria italica L.) and Functional Assessment of the Role of SiMADS51 in the Drought Stress Response. Front. Plant Sci. 2021, 12, 659474. [Google Scholar] [CrossRef]
- Kumar, K.; Muthamilarasan, M.; Prasad, M. Reference Genes for Quantitative Real-Time PCR Analysis in the Model Plant Foxtail Millet (Setaria italica L.) Subjected to Abiotic Stress Conditions. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 115, 13–22. [Google Scholar] [CrossRef]
- Martins, P.K.; Mafra, V.; de Souza, W.R.; Ribeiro, A.P.; Vinecky, F.; Basso, M.F.; Cunha, B.A.D.; Kobayashi, A.K.; Molinari, H.B.C. Selection of reliable reference genes for RT-qPCR analysis during developmental stages and abiotic stress in Setaria viridis. Sci. Rep. 2016, 6, 28348–28357. [Google Scholar] [CrossRef]
- Gao, J.; Lan, T. Functional Characterization of the Late Embryogenesis Abundant (LEA) Protein Gene Family from Pinus Tabuliformis (Pinaceae) in Escherichia coli. Sci. Rep. 2016, 6, 19467. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Q.; Li, D.; Yang, X.; Li, D. Multifunctional Roles of Plant Dehydrins in Response to Environmental Stresses. Front. Plant Sci. 2017, 8, 1018. [Google Scholar] [CrossRef] [PubMed]
- Qie, L.; Jia, G.; Zhang, W.; Schnable, J.; Shang, Z.; Li, W.; Liu, B.; Li, M.; Chai, Y.; Zhi, H.; et al. Mapping of Quantitative Trait Locus (QTLs) That Contribute to Germination and Early Seedling Drought Tolerance in the Interspecific Cross Setaria italica × Setaria viridis. PLoS ONE 2014, 9, e101868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, L.; Wang, Y.; Zhang, Y.; Jia, H.; Zhi, H.; Jia, G.; Han, Y.; Diao, X.; Tang, S. Multi-omics Analysis of Ubiquitin E2 Genes in Setaria: Evidence for the Roles of E2 Genes in Various Aspects of Plant Development, Stress Tolerance, and Domestication. Plant J. 2025, 123, e70473. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.M.; Knutti, R. Anthropogenic Contribution to Global Occurrence of Heavy-Precipitation and High-Temperature Extremes. Nat. Clim. Chang. 2015, 5, 560–564. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Sun, S.; Liu, Q.; Dai, X.; Wang, X. Transcriptomic Analysis Reveals the Correlation between End-of-Day Far Red Light and Chilling Stress in Setaria viridis. Genes 2022, 13, 1565. [Google Scholar] [CrossRef]
- Carvalho, C.G.I.; Santos, M.L.; Vieira, L.R.; Lopes, A.M.; Carmona, P.A.O.; Sousa, C.A.F.; Souza Junior, M.T. Morphophysiological Responses of Setaria viridis to Cold Stress. Pesqui. Agropecuária Bras. 2022, 57, e02424. [Google Scholar] [CrossRef]
- Swanton, C.J.; Huang, J.Z.; Deen, W.; Tollenaar, M.; Shrestha, A.; Rahimian, H. Effects of Temperature and Photoperiod on Setaria viridis. Weed Sci. 1999, 47, 446–453. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.-R.; Gao, J.; Lin, H.-X.; Lin, Y. The Molecular Basis of Heat Stress Responses in Plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, F.; Shao, Y.; He, J. Regulatory Mechanisms of Heat Stress Response and Thermomorphogenesis in Plants. Plants 2022, 11, 3410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sharwood, R.E.; Carroll, A.; Estavillo, G.M.; von Caemmerer, S.; Furbank, R.T. Systems Analysis of Long-Term Heat Stress Responses in the C4 Grass Setaria viridis. Plant Cell 2025, 37, koaf005. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, W.; Ren, B.; Zhao, B.; Zhang, J.; Liu, P.; Zhang, Z. High Temperature Reduces Photosynthesis in Maize Leaves by Damaging Chloroplast Ultrastructure and Photosystem II. J. Agron. Crop Sci. 2020, 206, 548–564. [Google Scholar] [CrossRef]
- Yang, D.; Qiu, H.; Pei, Y.; Hu, S.; Ma, S.; Liu, Z.; Zhang, Y.; Cao, M. Spatial and Temporal Evolution of the Infiltration Characteristics of a Loess Landslide. ISPRS Int. J. Geoinf. 2020, 9, 26. [Google Scholar] [CrossRef]
- Smith, A.; Gentile, B.R.; Xin, Z.; Zhao, D. The Effects of Heat Stress on Male Reproduction and Tillering in Sorghum Bicolor. Food Energy Secur. 2023, 12, e510. [Google Scholar] [CrossRef]
- Cocon, K.D.; Luis, P. The Potential of RuBisCO in CO2 Capture and Utilization. Prog. Energy Combust. Sci. 2024, 105, 101184. [Google Scholar] [CrossRef]
- Lobo, A.K.M.; Orr, D.J.; Carmo-Silva, E. Regulation of Rubisco Activity by Interaction with Chloroplast Metabolites. Biochem. J. 2024, 481, 1043–1056. [Google Scholar] [CrossRef]
- Murchie, E.H.; McAusland, L.; Burgess, A.J. Abiotic Stress, Acclimation, and Adaptation in Carbon Fixation Processes. In Photosynthesis in Action; Elsevier: Amsterdam, The Netherlands, 2022; pp. 103–132. [Google Scholar]
- Jespersen, D. Heat Shock Induced Stress Tolerance in Plants: Physiological, Biochemical, and Molecular Mechanisms of Acquired Tolerance. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–174. [Google Scholar]
- Nowicka, B.; Ciura, J.; Szymańska, R.; Kruk, J. Improving Photosynthesis, Plant Productivity and Abiotic Stress Tolerance—Current Trends and Future Perspectives. J. Plant Physiol. 2018, 231, 415–433. [Google Scholar] [CrossRef]
- Boyd, R.A.; Gandin, A.; Cousins, A.B. Temperature Response of C4 Photosynthesis: Biochemical Analysis of Rubisco, Phosphoenolpyruvate Carboxylase and Carbonic Anhydrase in Setaria viridis. Plant Physiol. 2015, 169, 1850–1861. [Google Scholar] [CrossRef]
- Lysenko, E.A.; Kozuleva, M.A.; Klaus, A.A.; Pshybytko, N.L.; Kusnetsov, V.V. Lower Air Humidity Reduced Both the Plant Growth and Activities of Photosystems I and II under Prolonged Heat Stress. Plant Physiol. Biochem. 2023, 194, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Kausar, A.; Zeidi, M.A.; Siddique, K.H.M.; Farooq, M. Plant Photosynthesis under Heat Stress: Effects and Management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Jha, Y. Regulation of Photosynthesis under Stress. In Improving Stress Resilience in Plants; Elsevier, Amsterdam, The Netherlands, 2024; pp. 35–48.
- Sack, L.; Holbrook, N.M. Leaf hydraulics. Annu. Rev. Plant Biol. 2006, 57, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sinclair, T.R.; Zhu, M.; Messina, C.D.; Cooper, M.; Hammer, G.L. Temperature Effect on Transpiration Response of Maize Plants to Vapour Pressure Deficit. Environ. Exp. Bot. 2012, 78, 157–162. [Google Scholar] [CrossRef]
- Yamori, W. Photosynthesis and Respiration. In Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–206. [Google Scholar]
- Nobel, P.S. Temperature and Energy Budgets. In Physicochemical and Environmental Plant Physiology; Elsevier; Amsterdam, The Netherlands, 2020; pp. 357–407.
- Heldt, H.-W.; Piechulla, B. Photosynthesis Is an Electron Transport Process. In Plant Biochemistry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 63–105. [Google Scholar]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to High Temperature Stress. J. Photochem. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Sirhindi, G.; Mushtaq, R.; Gill, S.S.; Sharma, P.; Abd_Allah, E.F.; Ahmad, P. Jasmonic Acid and Methyl Jasmonate Modulate Growth, Photosynthetic Activity and Expression of Photosystem II Subunit Genes in Brassica oleracea L. Sci. Rep. 2020, 10, 9322. [Google Scholar] [CrossRef]
- Zavafer, A.; Mancilla, C. Concepts of Photochemical Damage of Photosystem II and the Role of Excessive Excitation. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100421. [Google Scholar] [CrossRef]
- Yoshioka-Nishimura, M.; Yamamoto, Y. Quality Control of Photosystem II: The Molecular Basis for the Action of FtsH Protease and the Dynamics of the Thylakoid Membranes. J. Photochem. Photobiol. B 2014, 137, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W.; Niu, D.; Liu, X. Effects of Abiotic Stress on Chlorophyll Metabolism. Plant Sci. 2024, 342, 112030. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P.; Yamamoto, Y. Damage to Photosystem II by Lipid Peroxidation Products. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, T. Regulation of Photosynthesis by Cyclic Electron Transport around Photosystem I. In Advances in Botanical Research; Academic Press: Kyoto, Japan, 2020; pp. 177–204. [Google Scholar]
- Anderson, C.M.; Mattoon, E.M.; Zhang, N.; Becker, E.; McHargue, W.; Yang, J.; Patel, D.; Dautermann, O.; McAdam, S.A.M.; Tarin, T.; et al. High Light and Temperature Reduce Photosynthetic Efficiency through Different Mechanisms in the C4 Model Setaria viridis. Commun. Biol. 2021, 4, 1092. [Google Scholar] [CrossRef]
- Wang, L.; Peterson, R.B.; Brutnell, T.P. Regulatory Mechanisms Underlying C4 Photosynthesis. New Phytol. 2011, 190, 9–20. [Google Scholar] [CrossRef]
- Hu, M.; Yang, M.; Liu, J.; Huang, H.; Luan, R.; Yue, H.; Zhang, C. Physiological Investigation and Transcriptome Analysis Reveals the Mechanisms of Setaria italica’s Yield Formation under Heat Stress. Int. J. Mol. Sci. 2024, 25, 3171. [Google Scholar] [CrossRef]
- Modde, K.; Timm, S.; Florian, A.; Michl, K.; Fernie, A.R.; Bauwe, H. High Serine: Glyoxylate Aminotransferase Activity Lowers Leaf Daytime Serine Levels, Inducing the Phosphoserine Pathway in Arabidopsis. J. Exp. Bot. 2016, 68, erw467. [Google Scholar] [CrossRef]
- Hu, P.; Song, P.; Xu, J.; Wei, Q.; Tao, Y.; Ren, Y.; Yu, Y.; Li, D.; Hu, H.; Li, C. Genome-Wide Analysis of Serine Hydroxymethyltransferase Genes in Triticeae Species Reveals That TaSHMT3A-1 Regulates Fusarium Head Blight Resistance in Wheat. Front. Plant Sci. 2022, 13, 847087. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Q.; Zhang, J.; Hou, Y.; Dai, Q.; Lv, H.; Cao, P.; Zhao, L. Hydroxypyruvate Reductase Gene Family in Nicotiana Benthamiana: Genome-Wide Identification and Expression Pattern Profiling. Curr. Plant Biol. 2023, 35–36, 100305. [Google Scholar] [CrossRef]
- Liu, H.; Li, N.; Zhao, Y.; Kang, G.-Z.; Zhao, Y.-H.; Xu, H.-W. Serine Hydroxymethyltransferase (SHMT) Gene Family in Wheat (Triticum aestivum L.): Identification, Evolution, and Expression Analysis. Agronomy 2022, 12, 1346. [Google Scholar] [CrossRef]
- Liu, Y.; Mauve, C.; Lamothe-Sibold, M.; Guérard, F.; Glab, N.; Hodges, M.; Jossier, M. Photorespiratory Serine Hydroxymethyltransferase 1 Activity Impacts Abiotic Stress Tolerance and Stomatal Closure. Plant Cell Environ. 2019, 42, 2567–2583. [Google Scholar] [CrossRef]
- Timm, S.; Florian, A.; Jahnke, K.; Nunes-Nesi, A.; Fernie, A.R.; Bauwe, H. The Hydroxypyruvate-Reducing System in Arabidopsis: Multiple Enzymes for the Same End. Plant Physiol. 2011, 155, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Datta, D.; Pattnaik, D.; Dash, D.; Saha, D.; Panda, D.; Bhatta, B.B.; Parida, S.; Mishra, U.N.; Chauhan, J.; et al. Physiological, Biochemical, and Molecular Adaptation Mechanisms of Photosynthesis and Respiration under Challenging Environments. In Plant Perspectives to Global Climate Changes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 79–100. [Google Scholar]
- Dou, H.; Niu, G. Plant Responses to Light. In Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–166. [Google Scholar]
- Erickson, E.; Wakao, S.; Niyogi, K.K. Light Stress and Photoprotection in Chlamydomonas reinhardtii. Plant J. 2015, 82, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants Response to Light Stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Sembada, A.A.; Faizal, A.; Sulistyawati, E. Photosynthesis Efficiency as Key Factor in Decision-Making for Forest Design and Redesign: A Systematic Literature Review. Ecol. Front. 2024, 44, 1128–1139. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Kirilovsky, D. Transport of Electrons. In Photosynthesis in Action; Elsevier: Amsterdam, The Netherlands, 2022; pp. 17–29. [Google Scholar]
- Falk, J.; Munné-Bosch, S. Tocochromanol Functions in Plants: Antioxidation and Beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef]
- Pospíšil, P. The Role of Metals in Production and Scavenging of Reactive Oxygen Species in Photosystem II. Plant Cell Physiol. 2014, 55, 1224–1232. [Google Scholar] [CrossRef]
- Ksas, B.; Becuwe, N.; Chevalier, A.; Havaux, M. Plant Tolerance to Excess Light Energy and Photooxidative Damage Relies on Plastoquinone Biosynthesis. Sci. Rep. 2015, 5, 10919. [Google Scholar] [CrossRef]
- Gould, K.S. Nature′s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. Biomed. Res. Int. 2004, 2004, 314–320. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.; Yu, J.; Liu, H.; Cao, Z.; Manukovsky, N.S.; Liu, H. Interaction Effects of Light Intensity and Nitrogen Concentration on Growth, Photosynthetic Characteristics and Quality of Lettuce (Lactuca sativa L. Var. Youmaicai). Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Jonwal, S.; Verma, N.; Sinha, A.K. Regulation of Photosynthetic Light Reaction Proteins via Reversible Phosphorylation. Plant Sci. 2022, 321, 111312. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, H.; Wang, J.; Sui, X.; Xu, N. Adaptive Changes in Chlorophyll Content and Photosynthetic Features to Low Light in Physocarpus Amurensis Maxim and Physocarpus Opulifolius “Diabolo”. PeerJ 2016, 4, e2125. [Google Scholar] [CrossRef]
- Henry, C.; Watson-Lazowski, A.; Oszvald, M.; Griffiths, C.; Paul, M.J.; Furbank, R.T.; Ghannoum, O. Sugar Sensing Responses to Low and High Light in Leaves of the C4 Model Grass Setaria viridis. J. Exp. Bot. 2019, 71, 1039–1052. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Zhang, L.G.; Huang, L.; Qi, X.; Wen, Y.Y.; Dong, S.Q.; Song, X.E.; Wang, H.F.; Guo, P.Y. Photosynthetic and Physiological Responses of Foxtail Millet (Setaria italica L.) to Low-Light Stress during Grain-Filling Stage. Photosynthetica 2017, 55, 491–500. [Google Scholar] [CrossRef]
- Stavi, I.; Thevs, N.; Priori, S. Soil Salinity and Sodicity in Drylands: A Review of Causes, Effects, Monitoring, and Restoration Measures. Front. Environ. Sci. 2021, 9, 712831. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Sui, N.; Yang, Z.; Liu, M.; Wang, B. Identification and Transcriptomic Profiling of Genes Involved in Increasing Sugar Content during Salt Stress in Sweet Sorghum Leaves. BMC Genom. 2015, 16, 534. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.-L.; Liu, L.-N.; Xie, Q.; Sui, N. Photosynthetic Regulation Under Salt Stress and Salt-Tolerance Mechanism of Sweet Sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef]
- El-maarouf-bouteau, H.; Sajjad, Y.; Bazin, J.; Langlade, N.; Cristescu, S.M.; Balzergue, S.; Baudouin, E.; Bailly, C. Reactive Oxygen Species, Abscisic Acid and Ethylene Interact to Regulate Sunflower Seed Germination. Plant Cell Environ. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Wang, T.; Tohge, T.; Ivakov, A.; Mueller-Roeber, B.; Fernie, A.R.; Mutwil, M.; Schippers, J.H.M.; Persson, S. Salt-Related MYB1 Coordinates Abscisic Acid Biosynthesis and Signaling during Salt Stress in Arabidopsis. Plant Physiol. 2015, 169, 1027–1041. [Google Scholar] [CrossRef]
- Acharya, B.R.; Roy Choudhury, S.; Estelle, A.B.; Vijayakumar, A.; Zhu, C.; Hovis, L.; Pandey, S. Optimization of Phenotyping Assays for the Model Monocot Setaria viridis. Front. Plant Sci. 2017, 8, 2172. [Google Scholar] [CrossRef]
- Ferreira, T.M.M.; Santos, M.d.L.; Lopes, C.L.; Sousa, C.A.F.; Souza Junior, M.T. Effect of Salinity Stress in Setaria viridis (L.) P. Beauv. Accession A10.1 during Seed Germination and Plant Development. Ciência Agrotecnol. 2020, 44, e010020. [Google Scholar] [CrossRef]
- Han, F.; Sun, M.; He, W.; Guo, S.; Feng, J.; Wang, H.; Yang, Q.; Pan, H.; Lou, Y.; Zhuge, Y. Transcriptome Analysis Reveals Molecular Mechanisms under Salt Stress in Leaves of Foxtail Millet (Setaria italica L.). Plants 2022, 11, 1864. [Google Scholar] [CrossRef] [PubMed]
- Karunarathne, S.D.; Han, Y.; Zhang, X.-Q.; Dang, V.H.; Angessa, T.T.; Li, C. Using Chlorate as an Analogue to Nitrateto Identify Candidate Genes for Nitrogen Use Efficiency in Barley. Mol. Breed. 2021, 41, 47. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and Salinity Stress Responses and Microbe-Induced Tolerance in Plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Valeriano, F.R.; de Moura, S.M.; Travassos-Lins, J.; Alves-Ferreira, M.; Vieira, R.C.; Ortiz-Silva, B.; Reinert, F. Evaluation of Setaria viridis Responses to Salt Treatment and Potassium Supply: A Characterization of Three Contrasting Accessions. Braz. J. Bot. 2021, 44, 821–836. [Google Scholar] [CrossRef]
- Mendes Bezerra, A.C.; da Cunha Valença, D.; da Gama Junqueira, N.E.; Moll Hüther, C.; Borella, J.; Ferreira de Pinho, C.; Alves Ferreira, M.; Oliveira Medici, L.; Ortiz-Silva, B.; Reinert, F. Potassium Supply Promotes the Mitigation of NaCl-Induced Effects on Leaf Photochemistry, Metabolism and Morphology of Setaria viridis. Plant Physiol. Biochem. 2021, 160, 193–210. [Google Scholar] [CrossRef]
- Essemine, J.; Qu, M.; Lyu, M.-J.A.; Song, Q.; Khan, N.; Chen, G.; Wang, P.; Zhu, X.-G. Photosynthetic and Transcriptomic Responses of Two C4 Grass Species with Different NaCl Tolerance. J. Plant Physiol. 2020, 253, 153244. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Essemine, J.; Lyu, M.-J.A.; Qu, M.; Perveen, S.; Khan, N.; Song, Q.; Chen, G.; Zhu, X.-G. Contrasting Responses of Plastid Terminal Oxidase Activity Under Salt Stress in Two C4 Species With Different Salt Tolerance. Front. Plant Sci. 2020, 11, 1009. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and Plant Responses to Salinity Stress: A Review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.-H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and Salinity: Crosstalk in Crop-Mediated Stress Tolerance Mechanisms. Front. Plant Sci. 2019, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Lai, J.; Wu, Q.; Zhang, S.; Chen, L.; Dai, Y.-S.; Wang, C.; Du, J.; Xiao, S.; Yang, C. Jasmonate Complements the Function of Arabidopsis Lipoxygenase3 in Salinity Stress Response. Plant Sci. 2016, 244, 1–7. [Google Scholar] [CrossRef]
- Kuo, H.-Y.; Kang, F.-C.; Wang, Y.-Y. Glucosinolate Transporter1 Involves in Salt-Induced Jasmonate Signaling and Alleviates the Repression of Lateral Root Growth by Salt in Arabidopsis. Plant Sci. 2020, 297, 110487. [Google Scholar] [CrossRef]
- Zhou, M.; Li, D.; Li, Z.; Hu, Q.; Yang, C.; Zhu, L.; Luo, H. Constitutive Expression of a MiR319 Gene Alters Plant Development and Enhances Salt and Drought Tolerance in Transgenic Creeping Bentgrass. Plant Physiol. 2013, 161, 1375–1391. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Y.; Gao, H.; Li, Y.; Tang, W.; Ma, L.; Yang, G.; Sun, J. Genomic Characterization and Expression Analysis of TCP Transcription Factors in Setaria italica and Setaria viridis. Plant Signal Behav. 2022, 17, 2075158. [Google Scholar] [CrossRef]
- Pan, Y.; Li, J.; Jiao, L.; Li, C.; Zhu, D.; Yu, J. A Non-Specific Setaria italica Lipid Transfer Protein Gene Plays a Critical Role under Abiotic Stress. Front. Plant Sci. 2016, 7, 1752. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Singh, R.K.; Suresh, B.V.; Rana, S.; Dulani, P.; Prasad, M. Genomic Dissection and Expression Analysis of Stress-Responsive Genes in C4 Panicoid Models, Setaria italica and Setaria viridis. J. Biotechnol. 2020, 318, 57–67. [Google Scholar] [CrossRef]
- Zhang, D.; He, S.; Fu, Y.; Yu, R.; Gao, X.; Wang, Z.; Liu, Z.; Guo, Y.; Chen, M. Transcriptome Analysis Reveals Key Genes in Response to Salinity Stress during Seed Germination in Setaria italica. Environ. Exp. Bot. 2021, 191, 104604. [Google Scholar] [CrossRef]
- Abbas, S.; Amna; Javed, M.T.; Ali, Q.; Azeem, M.; Ali, S. Nutrient Deficiency Stress and Relation with Plant Growth and Development. In Engineering Tolerance in Crop Plants Against Abiotic Stress; CRC Press: Boca Raton, FL, USA, 2021; pp. 239–262. [Google Scholar]
- Cheng, J.; Tan, H.; Shan, M.; Duan, M.; Ye, L.; Yang, Y.; He, L.; Shen, H.; Yang, Z.; Wang, X. Genome-Wide Identification and Characterization of the NPF Genes Provide New Insight into Low Nitrogen Tolerance in Setaria. Front. Plant Sci. 2022, 13, 1043832. [Google Scholar] [CrossRef]
- Nadeem, F.; Ahmad, Z.; Wang, R.; Han, J.; Shen, Q.; Chang, F.; Diao, X.; Zhang, F.; Li, X. Foxtail Millet [Setaria italica (L.) Beauv.] Grown under Low Nitrogen Shows a Smaller Root System, Enhanced Biomass Accumulation, and Nitrate Transporter Expression. Front. Plant Sci. 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fan, X.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, F.; Mahmood, R.; Sabir, M.; Khan, W.-D.; Haider, M.S.; Wang, R.; Zhong, Y.; Ishfaq, M.; Li, X. Foxtail Millet [Setaria italica (L.) Beauv.] over-Accumulates Ammonium under Low Nitrogen Supply. Plant Physiol. Biochem. 2022, 185, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Dong, E.; Liu, Q.; Wang, L.; Chen, E.; Jiao, X.; Diao, X. Foxtail Millet [Setaria italica (L.) P. Beauv.] Grown under Nitrogen Deficiency Exhibits a Lower Folate Contents. Front. Nutr. 2023, 10, 1035739. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Zhong, L.; Liu, J.; Xu, Z.; Li, L.; Zhou, Y.-B.; Guo, C.-H.; Ma, Y.-Z. Overexpression of the Autophagy-Related Gene SiATG8a from Foxtail Millet (Setaria italica L.) Confers Tolerance to Both Nitrogen Starvation and Drought Stress in Arabidopsis. Biochem. Biophys. Res. Commun. 2015, 468, 800–806. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Wang, E.; Hu, L.; Hawkesford, M.J.; Zhong, L.; Chen, Z.; Xu, Z.; Li, L.; Zhou, Y.; et al. Genome-Wide Analysis of Autophagy-Associated Genes in Foxtail Millet (Setaria italica L.) and Characterization of the Function of SiATG8a in Conferring Tolerance to Nitrogen Starvation in Rice. BMC Genom. 2016, 17, 797. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Qi, X.; Li, M.; Liu, J.; Le, S.; Chen, K.; Wang, C.; Zhou, Y.; Xu, Z.; et al. Overexpression of MYB-like Transcription Factor SiMYB30 from Foxtail Millet (Setaria italica L.) Confers Tolerance to Low Nitrogen Stress in Transgenic Rice. Plant Physiol. Biochem. 2023, 196, 731–738. [Google Scholar] [CrossRef]
- Ahmad, Z.; Nadeem, F.; Wang, R.; Diao, X.; Han, Y.; Wang, X.; Li, X. A Larger Root System Is Coupled With Contrasting Expression Patterns of Phosphate and Nitrate Transporters in Foxtail Millet [Setaria italica (L.) Beauv.] Under Phosphate Limitation. Front. Plant Sci. 2018, 9, 1367. [Google Scholar] [CrossRef]
- Roch, G.V.; Maharajan, T.; Krishna, T.P.A.; Ignacimuthu, S.; Ceasar, S.A. Expression of PHT1 Family Transporter Genes Contributes for Low Phosphate Stress Tolerance in Foxtail Millet (Setaria italica) Genotypes. Planta 2020, 252, 98. [Google Scholar] [CrossRef]
- He, Z.; Chen, M.; Ling, B.; Cao, T.; Wang, C.; Li, W.; Tang, W.; Chen, K.; Zhou, Y.; Chen, J.; et al. Overexpression of the Autophagy-Related Gene SiATG8a from Foxtail Millet (Setaria italica L.) in Transgenic Wheat Confers Tolerance to Phosphorus Starvation. Plant Physiol. Biochem. 2023, 196, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Khan, N.U.; Dai, S.; Qin, N.; Han, Z.; Guo, B.; Li, J. Transcriptome Analysis and Identification of the Low Potassium Stress-Responsive Gene SiSnRK2.6 in Foxtail Millet (Setaria italica L.). Theor. Appl. Genet. 2024, 137, 22. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Hu, L.; Chen, X.; Zhang, R.; Cheng, D.; Li, H.; Xu, Z.; Li, L.; Zhou, Y.; Liu, A.; et al. Genome-Wide Analysis and Identification of the Low Potassium Stress Responsive Gene SiMYB3 in Foxtail Millet (Setariaitalica L.). BMC Genom. 2019, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy Metals, Occurrence and Toxicity for Plants: A Review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Singh Sidhu, G.P.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global Evaluation of Heavy Metal Content in Surface Water Bodies: A Meta-Analysis Using Heavy Metal Pollution Indices and Multivariate Statistical Analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium Toxicity and Tolerance in Plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Narayanan, M.; Ma, Y. Mitigation of Heavy Metal Stress in the Soil through Optimized Interaction between Plants and Microbes. J. Environ. Manage 2023, 345, 118732. [Google Scholar] [CrossRef]
- Bhat, S.A.; Bashir, O.; Ul Haq, S.A.; Amin, T.; Rafiq, A.; Ali, M.; Américo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of Heavy Metals in Soil and Water: An Eco-Friendly, Sustainable and Multidisciplinary Approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef]
- Iqbal, B.; Ahmad, N.; Li, G.; Jalal, A.; Khan, A.R.; Zheng, X.; Naeem, M.; Du, D. Unlocking Plant Resilience: Advanced Epigenetic Strategies against Heavy Metal and Metalloid Stress. Plant Sci. 2024, 349, 112265. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation Strategies for Soils Contaminated with Heavy Metals: Modifications and Future Perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Rachappanavar, V.; Gupta, S.K.; Jayaprakash, G.K.; Abbas, M. Silicon Mediated Heavy Metal Stress Amelioration in Fruit Crops. Heliyon 2024, 10, e37425. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium Toxicity in Plants: Reasons, Mechanisms and Remediation Possibilities—A Review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef]
- Poli, A.; Schmitt, C.; Moulouel, B.; Mirmiran, A.; Puy, H.; Lefèbvre, T.; Gouya, L. Iron, Heme Synthesis and Erythropoietic Porphyrias: A Complex Interplay. Metabolites 2021, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wu, Z.; Zheng, Y.; Shen, J.; Zhu, Y.; Chen, Q.; Wang, B.; Yang, F.; Ding, Y.; Liu, H.; et al. Selenite Affected Photosynthesis of Oryza Sativa L. Exposed to Antimonite: Electron Transfer, Carbon Fixation, Pigment Synthesis via a Combined Analysis of Physiology and Transcriptome. Plant Physiol. Biochem. 2023, 201, 107904. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and Functional Alterations in Photosynthetic Apparatus of Plants under Cadmium Stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef] [PubMed]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of Lead and Copper on Photosynthetic Apparatus in Citrus (Citrus aurantium L.) Plants. The Role of Antioxidants in Oxidative Damage as a Response to Heavy Metal Stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Singh, S.; Yadav, V.; Arif, N.; Singh, V.P.; Dubey, N.K.; Ramawat, N.; Prasad, R.; Sahi, S.; Tripathi, D.K.; Chauhan, D.K. Heavy Metal Stress and Plant Life: Uptake Mechanisms, Toxicity, and Alleviation. In Plant Life Under Changing Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 271–287. [Google Scholar]
- Liu, J.; Ni, J.; Mo, A.; Fan, X.; Jiang, Y.; Xie, H.; Hu, J.; Zhu, Y.; Peng, C.; Yang, F. Cadmium Affects the Growth, Antioxidant Capacity, Chlorophyll Content, and Homeostasis of Essential Elements in Soybean Plants. South Afr. J. Bot. 2023, 162, 604–610. [Google Scholar] [CrossRef]
- Song, C.; Manzoor, M.A.; Mao, D.; Ren, X.; Zhang, W.; Zhang, Y. Photosynthetic Machinery and Antioxidant Enzymes System Regulation Confers Cadmium Stress Tolerance to Tomato Seedlings Pretreated with Melatonin. Sci. Hortic. 2024, 323, 112550. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Che, J.; Lyu, L.; Wu, W.; Cao, F.; Li, W. Effects of Cadmium Stress on the Growth, Physiology, Mineral Uptake, Cadmium Accumulation and Fruit Quality of “Sharpblue” Blueberry. Sci. Hortic. 2024, 337, 113593. [Google Scholar] [CrossRef]
- An, T.; Kuang, Q.; Wu, Y.; Gao, Y.; Zhang, Y.; Mickan, B.S.; Xu, B.; Zhang, S.; Deng, X.; Yu, M.; et al. Variability in Cadmium Stress Tolerance among Four Maize Genotypes: Impacts on Plant Physiology, Root Morphology, and Chloroplast Microstructure. Plant Physiol. Biochem. 2023, 205, 108135. [Google Scholar] [CrossRef]
- Liang, L.; Chenchang, W.; Tao, C. Advances in Understanding Cadmium Stress and Breeding of Cadmium-Tolerant Crops. Rice Sci. 2024, 31, 507–525. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; de Souza, W.R.; Martins, P.K.; Vinecky, F.; Duarte, K.E.; Basso, M.F.; da Cunha, B.A.D.B.; Campanha, R.B.; de Oliveira, P.A.; Centeno, D.C.; et al. Overexpression of BdMATE Gene Improves Aluminum Tolerance in Setaria viridis. Front. Plant Sci. 2017, 8, 865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, Z.; Wang, X.; Zhao, X. Antioxidant Defense Response of Arbuscular Mycorrhizal Fungi and Setaria viridis. Pak. J. Bot. 2023, 55, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, D.; Luo, X.; Zhang, Y. Effects of Setaria viridis on Heavy Metal Enrichment Tolerance and Bacterial Community Establishment in High-Sulfur Coal Gangue. Chemosphere 2024, 351, 141265. [Google Scholar] [CrossRef] [PubMed]
- Jadid, N.; Puspaningtyas, I.; Jannah, A.L.; Safitri, C.E.; Hutahuruk, V.H.D. Growth Responses of Indonesian Foxtail Millet (Setaria italica (L.) Beauv.) to Cadmium Stress. Air Soil Water Res. 2022, 15, 11786221221114310. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, J.; Liang, Y.; Wang, X.; Li, K.; Chen, L.; Aduragbemi, A.; Han, Y.; Sun, Z.; Li, H.; et al. Natural Resistance-Associated Macrophage Protein (Nramp) Family in Foxtail Millet (Setaria italica): Characterization, Expression Analysis and Relationship with Metal Content under Cd Stress. Agronomy 2023, 13, 2000. [Google Scholar] [CrossRef]
- Kang, X.; Geng, N.; Li, X.; Yu, J.; Wang, H.; Pan, H.; Yang, Q.; Zhuge, Y.; Lou, Y. Biochar Alleviates Phytotoxicity by Minimizing Bioavailability and Oxidative Stress in Foxtail Millet (Setaria italica L.) Cultivated in Cd- and Zn-Contaminated Soil. Front. Plant Sci. 2022, 13, 782963. [Google Scholar] [CrossRef]
- Kulasza, M.; Sielska, A.; Szenejko, M.; Soroka, M.; Skuza, L. Effects of Copper, and Aluminium in Ionic, and Nanoparticulate Form on Growth Rate and Gene Expression of Setaria italica Seedlings. Sci. Rep. 2024, 14, 15897. [Google Scholar] [CrossRef]
- Shabbir, R.; Singhal, R.K.; Mishra, U.N.; Chauhan, J.; Javed, T.; Hussain, S.; Kumar, S.; Anuragi, H.; Lal, D.; Chen, P. Combined Abiotic Stresses: Challenges and Potential for Crop Improvement. Agronomy 2022, 12, 2795. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Plant Responses to Climate Change: Metabolic Changes under Combined Abiotic Stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Li, Y.; Prakash, C.S.; Hu, Z. Panomics to Manage Combined Abiotic Stresses in Plants. Trends Plant Sci. 2025, 30, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Gowsiga, S.; Vijayalakshmi, D.; Deepika, J.; Arunkumar, P.; Sivakumar, R.; Sivakumar, U.; Yamuna, V. Exploring the Physiological Mechanisms and Ecofriendly Mitigation Strategies to Combat the Synergistic Effects of Drought and High Temperature Stress in Setaria italica. Cereal Res. Commun. 2025, 53, 2303–2319. [Google Scholar] [CrossRef]
- Nematpour, A.; Eshghizadeh, H.R.; Zahedi, M. Drought-Tolerance Mechanisms in Foxtail Millet (Setaria italica) and Proso Millet (Panicum Miliaceum) under Different Nitrogen Supply and Sowing Dates. Crop Pasture Sci. 2019, 70, 442–452. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Zhang, L.; Li, H. Stem Lodging Parameters of the Basal Three Internodes Associated with Plant Population Densities and Developmental Stages in Foxtail Millet (Setaria italica) Cultivars Differing in Resistance to Lodging. Crop Pasture Sci. 2017, 68, 349–357. [Google Scholar] [CrossRef]
- Tian, B.; Luan, S.; Zhang, L.; Liu, Y.; Zhang, L.; Li, H. Penalties in Yield and Yield Associated Traits Caused by Stem Lodging at Different Developmental Stages in Summer and Spring Foxtail Millet Cultivars. Field Crops Res. 2018, 217, 104–112. [Google Scholar] [CrossRef]
- Matsuura, A.; An, P.; Murata, K.; Inanaga, S. Effect of Pre- and Post-Heading Waterlogging on Growth and Grain Yield of Four Millets. Plant Prod. Sci. 2016, 19, 348–359. [Google Scholar] [CrossRef]
- Smýkal, P.; Nelson, M.; Berger, J.; Von Wettberg, E. The Impact of Genetic Changes during Crop Domestication on Healthy Food Development. Agronomy 2018, 8, 26. [Google Scholar] [CrossRef]
- Barton, L.; Newsome, S.D.; Chen, F.-H.; Wang, H.; Guilderson, T.P.; Bettinger, R.L. Agricultural Origins and the Isotopic Identity of Domestication in Northern China. Proc. Natl. Acad. Sci. USA 2009, 106, 5523–5528. [Google Scholar] [CrossRef]
- Khan, A.W.; Garg, V.; Roorkiwal, M.; Golicz, A.A.; Edwards, D.; Varshney, R.K. Super-Pangenome by Integrating the Wild Side of a Species for Accelerated Crop Improvement. Trends Plant Sci. 2020, 25, 148–158. [Google Scholar] [CrossRef]
- Mohd Saad, N.S.; Severn-Ellis, A.A.; Pradhan, A.; Edwards, D.; Batley, J. Genomics Armed With Diversity Leads the Way in Brassica Improvement in a Changing Global Environment. Front. Genet. 2021, 12, 600789. [Google Scholar] [CrossRef]
| Drought Stress | ||
|---|---|---|
| Effect | Reference | |
| Growth and developmental effects | Shoot dry mass decreases, leaf and tiller emergence slow down, increasing root length and surface area. Water status effects: leaf water potential and relative water content decline, wilting, leaf rolling and bleaching, reducing transpiration | [33,34,38,41,42,43,44,45,47,48,49,50] |
| Photosynthesis & Biochemical effects | Lower photosynthetic assimilation, degradation of pigments, loss of photosystem II integrity, reduced photochemical efficiency (qP, ΦPSII), overproduction of reactive oxygen species, damage cellular components, activation of antioxidant defenses and accumulation of osmolytes (proline) | [19,33,34,38,41,42,45,46,47,48,49,50,51,52,53,54,55,56,59,60,61,62,63,65,66,68,69,707271,73,74] |
| Molecular effects | Modulation of stress-responsive genes, members of PP2Cs, SnRK2s, bZIPs families and metabolic enzymes (e.g., PGM) | [90,91] |
| Stress temperature | ||
| Effect | Reference | |
| Growth and Developmental effects | Dwarfism and atrophy, leaf narrowing and total biomass reduction | [79,808382,84,85,86] |
| Photosynthesis & Biochemical effects | Reduced photosynthetic efficiency, reduced activation of RuBisCO, reduction in chlorophyll biosynthesis, downregulation of PSII and cytochrome b6f and accumulation of osmoprotective sugars | [65,79,87,88,90,94,95,96,97,98,107,108,109,110] |
| Molecular effects | Repression of starch synthesis genes, upregulation of SGAT, SHMT, HPR and downregulation of the GLYK protein | [82,83,108,111,112,113,114,115,116] |
| Saline Stress | ||
| Effect | Reference | |
| Growth and developmental effects | Delayed germination and reduced germination rates, Root emergence reduced, Leaf curling, burnt margins, increased leaf thickness, and reduced biomass accumulation. | [138,139,140,141,143,144] |
| Photosynthesis & Biochemical effects | Stomatal closure, reduced photosynthesis and ROS (reactive oxygen species) accumulation. | [65,132,139,142,143,11,145] |
| Molecular effects | LOX gene upregulation | [58] |
| Nutritional Stress | ||
| Effect | Reference | |
| Growth and developmental effects | Delayed germination and reduced germination rates, reduced shoot and root growth, decreased shoot dry weight, shorter and fewer lateral and crown roots, reduction in leaf area, leaf chlorosis, necrosis, pale and thin panicles, reduced growth, leaf curling, burnt margins, chlorosis, increased leaf thickness, chlorosis, curling, necrosis in older leaves, shorter internodes, reduced biomass accumulation/diminished fresh and dry biomass, reduced aerial growth and increased lateral-root length, density, and number | [138,158,160,162,163,167,170,171] |
| Photosynthesis & Biochemical effects | Decline in chlorophyll and carotenoid levels, reduction in nitrogen content in shoots and roots; mobilization to shoots, decline in amino acids and protein content in shoots; soluble protein increase in roots, reduced photosynthesis and stomatal dysfunction and turgor loss | [138,158,160,162] |
| Molecular effects | Upregulation of nutrient-transport related genes, regulation by transcription factors (e.g., SiMYB30), upregulation of potassium transporters; repression of certain ion-binding genes (ion homeostasis) and induction of hormonal and signaling pathways (AUX/IAA, ethylene, ABA, gibberellin pathway genes) | [159,160,166,169,170,171] |
| Light Stress | ||
| Effect | Reference | |
| Growth and developmental effects | Reduced growth, turgor and photosynthetic capacity in low light and change in daily photosynthesis pattern (curve from double peak to single peak) | [130] |
| Photosynthesis & Biochemical effects | Photoinhibition with functional failure of PSII, damage to PSII reaction centers, excessive generation of ROS damaging PSI and PSII, reduction in the maximum quantum efficiency of PSII, drop in CO2 assimilation rate, stomatal conductance, and water-use efficiency, alteration of fluorescence and dissipation of energy as heat (non-photochemical energy dissipation/photoprotection), accumulation of sugars under high light without suppression of photosynthesis and Activation of antioxidant mechanisms | [15,118,119,120,121,122,123,124,127,128,129,130,131] |
| Molecular effects | Upregulation of antioxidant genes and heat-shock proteins (HSPs) in mesophyll cells under high light, downregulation of antioxidant genes in bundle sheath under high light and reduced mitochondrial activity (sub-optimal light) | [15,120,121,123,124,130] |
| Heavy-Metal Stress | ||
| Effect | Reference | |
| Growth and developmental effects | Inhibition of germination, reduction in root elongation, leaf area, and shoot growth, reduced biomass accumulation (fresh/dry weight) and pale or thin panicles and leaf chlorosis | [15,176,177,178] |
| Photosynthesis & Biochemical effects | Leaf chlorosis (loss of green pigment), damage to chloroplasts and damage to the photosynthetic electron transport (PET) chain, structural changes in chloroplast membranes and in photosystems PSI/PSII, reduction in photosynthetic pigments (chlorophyll, carotenoids), inhibition of chlorophyll biosynthesis, change in chloroplast development, inhibition of key enzymes involved in carbon fixation (e.g., Rubisco) and reduction in net photosynthesis/photosynthetic rate | [55,181,182,183] |
| Molecular effects | Expression of stress-responsive genes (e.g., ACT-1, CDPK, P5CS) and upregulation of detoxification and defense pathways (e.g., chelation, antioxidative responses) | [55,184,186,187,188,189,190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira Gomes, J.D.; Fernandes-Esteves, J.M.; Travassos-Lins, J.; Acevedo, A.F.G.; de Souza Rodrigues, T.; Alves-Ferreira, M. Harnessing Setaria as a Model for C4 Plant Adaptation to Abiotic Stress. Plants 2025, 14, 3710. https://doi.org/10.3390/plants14243710
Ferreira Gomes JD, Fernandes-Esteves JM, Travassos-Lins J, Acevedo AFG, de Souza Rodrigues T, Alves-Ferreira M. Harnessing Setaria as a Model for C4 Plant Adaptation to Abiotic Stress. Plants. 2025; 14(24):3710. https://doi.org/10.3390/plants14243710
Chicago/Turabian StyleFerreira Gomes, Juan David, João Marcos Fernandes-Esteves, João Travassos-Lins, Andres Felipe Gaona Acevedo, Tamires de Souza Rodrigues, and Marcio Alves-Ferreira. 2025. "Harnessing Setaria as a Model for C4 Plant Adaptation to Abiotic Stress" Plants 14, no. 24: 3710. https://doi.org/10.3390/plants14243710
APA StyleFerreira Gomes, J. D., Fernandes-Esteves, J. M., Travassos-Lins, J., Acevedo, A. F. G., de Souza Rodrigues, T., & Alves-Ferreira, M. (2025). Harnessing Setaria as a Model for C4 Plant Adaptation to Abiotic Stress. Plants, 14(24), 3710. https://doi.org/10.3390/plants14243710







