Antidepressant-like Effects of Garcinia nigrolineata Resin Extract in a Chronic Mild Stress Mouse Model: Modulation of Monoaminergic and HPA-Axis Pathways
Abstract
1. Introduction
2. Results
2.1. Monoamine Oxidase A and B (MAO-A and MAO-B) Inhibitory Activity of GNR-E
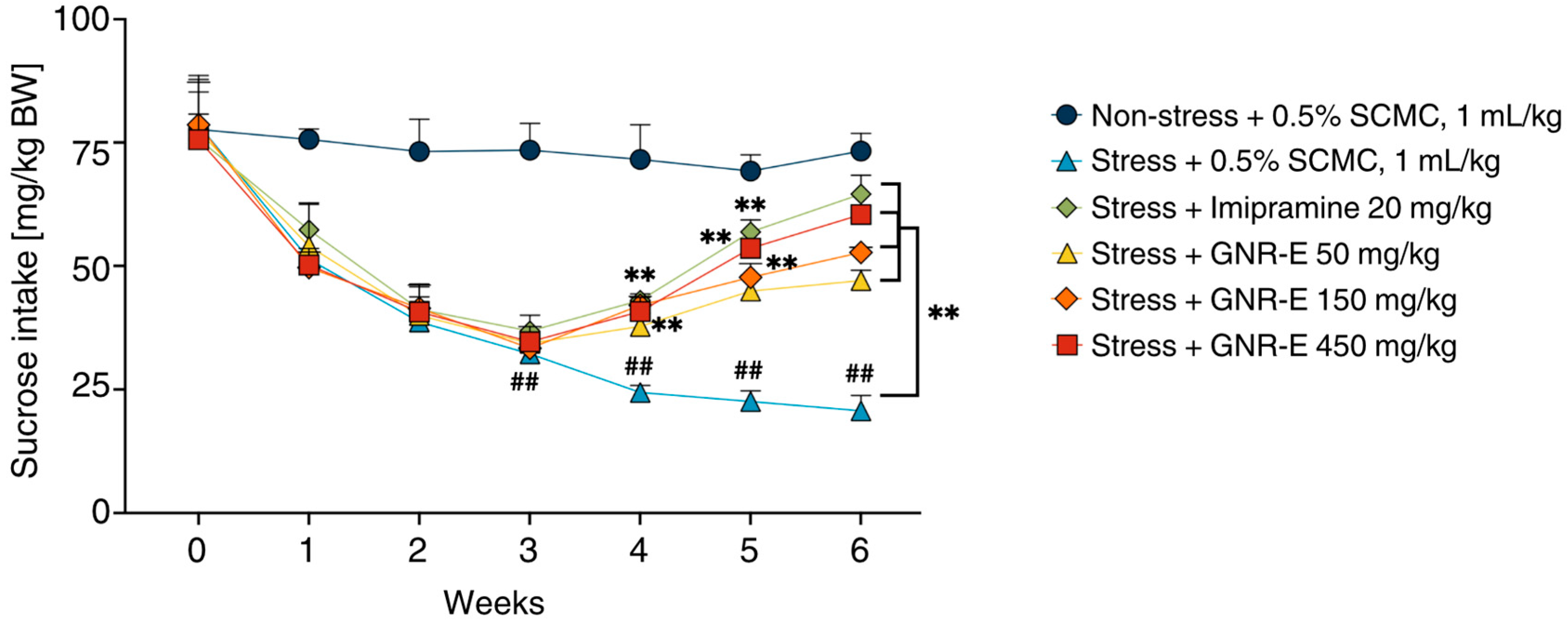
2.2. The Effect of GNR-E on Anhedonia Behavior in the Sucrose Preference Test
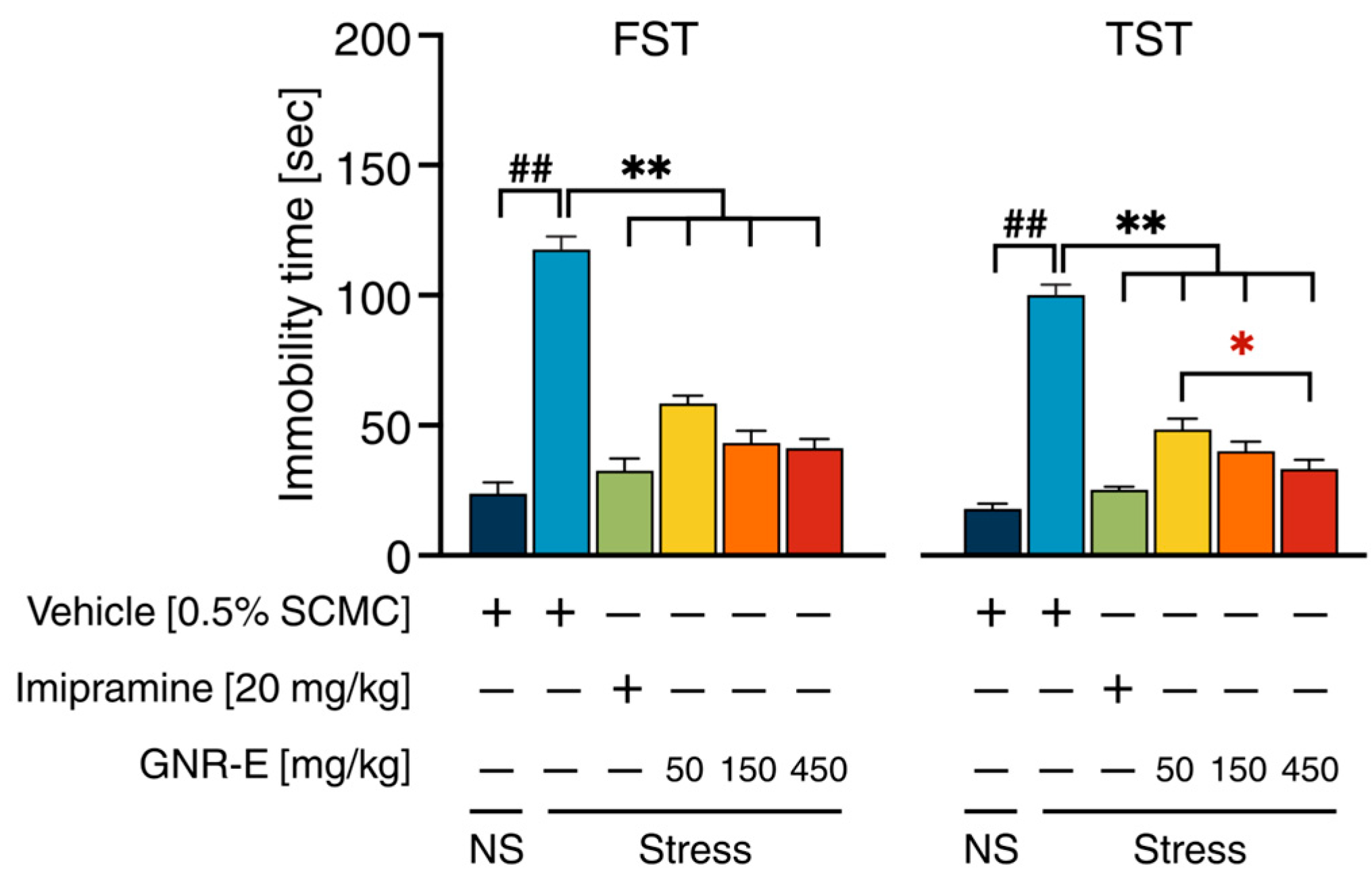
2.3. Effect of GNR-E on CMS-Induced Learned Helplessness Behavior in Forced Swimming Test and Tail Suspension Test
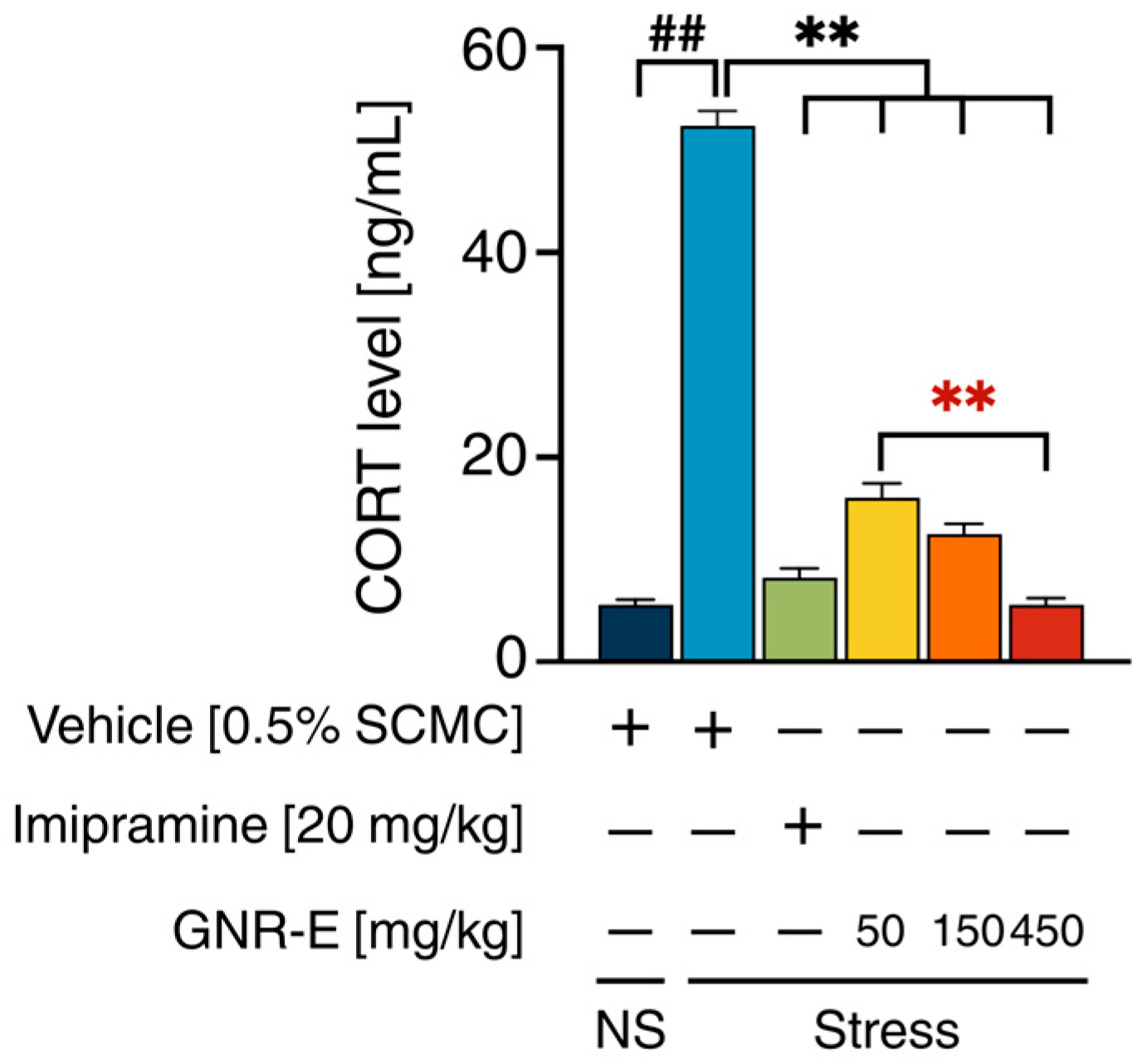
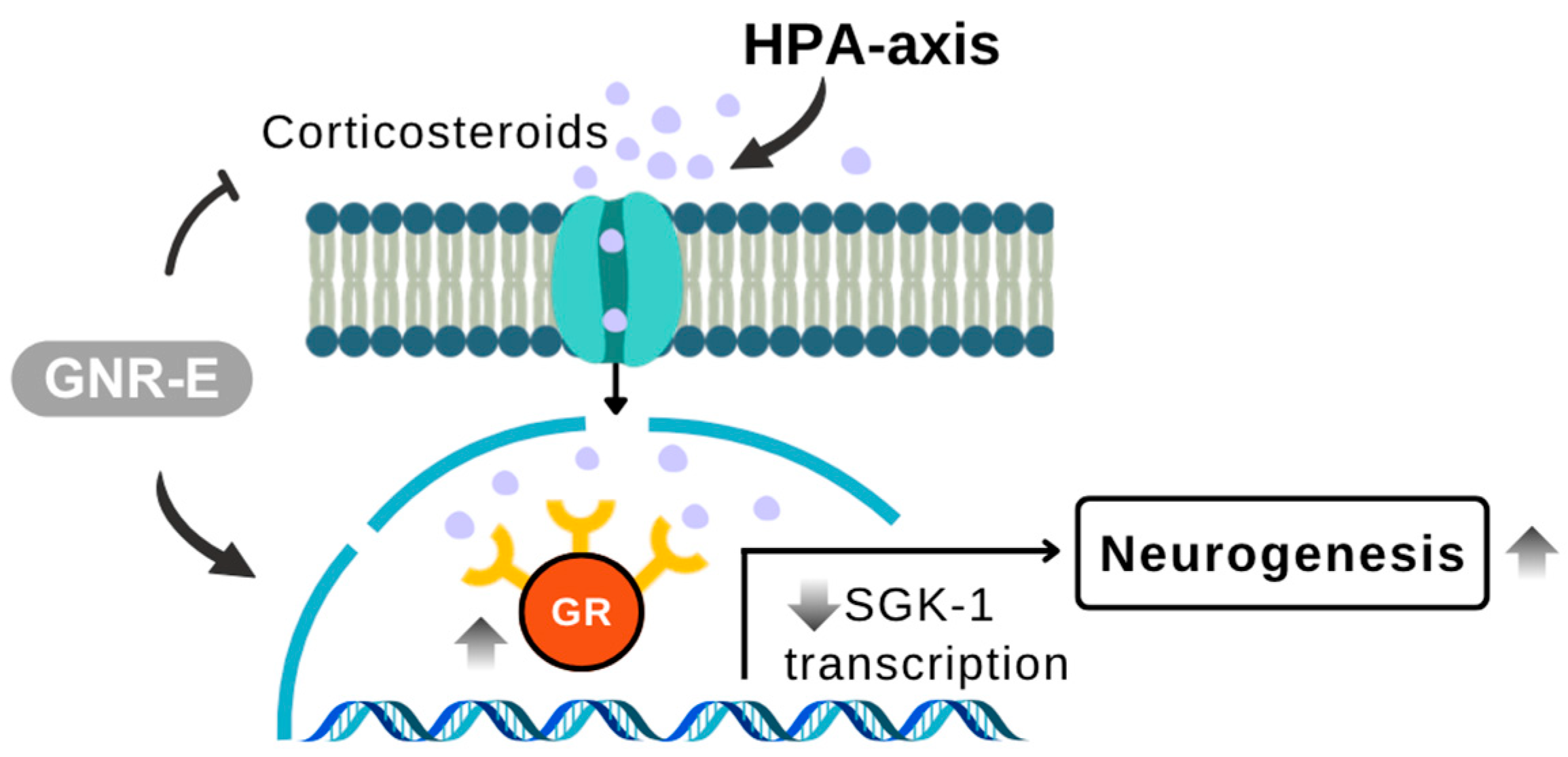
2.4. Effect of GNR-E on CMS-Induced Increase in Serum Corticosterone Levels
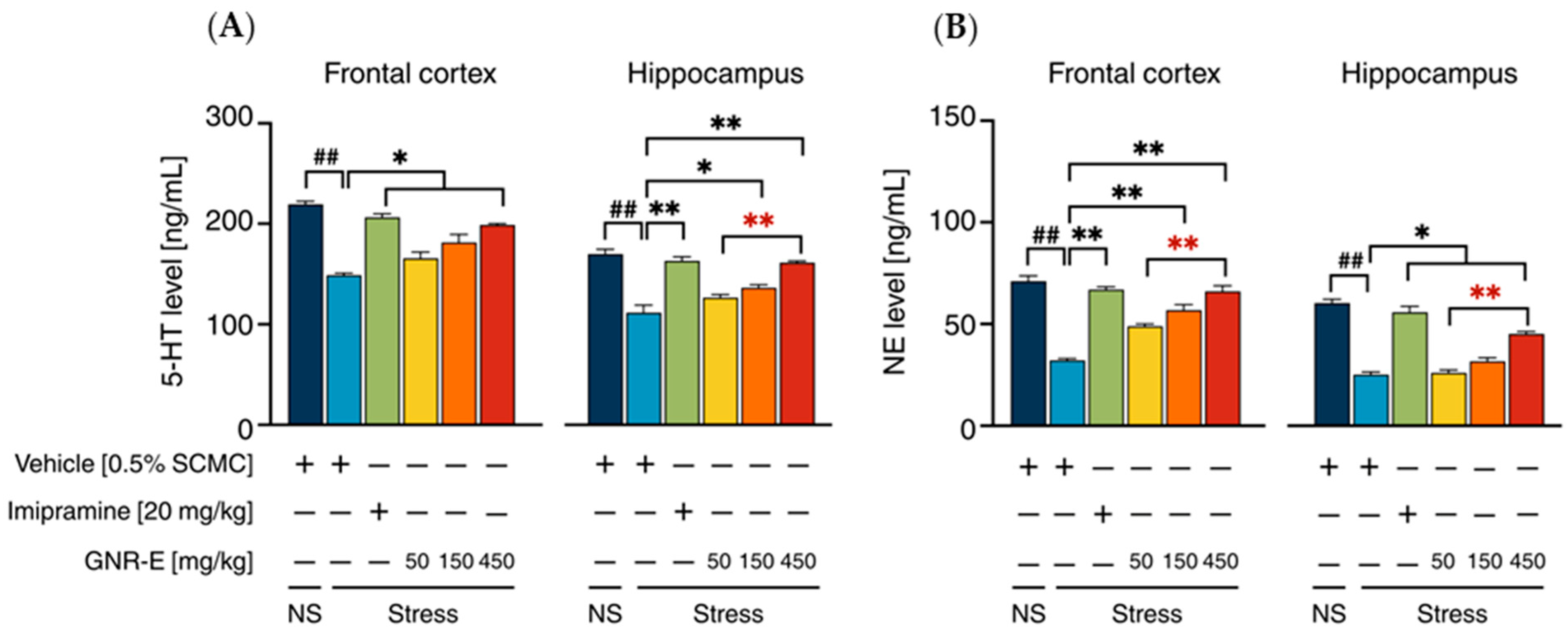
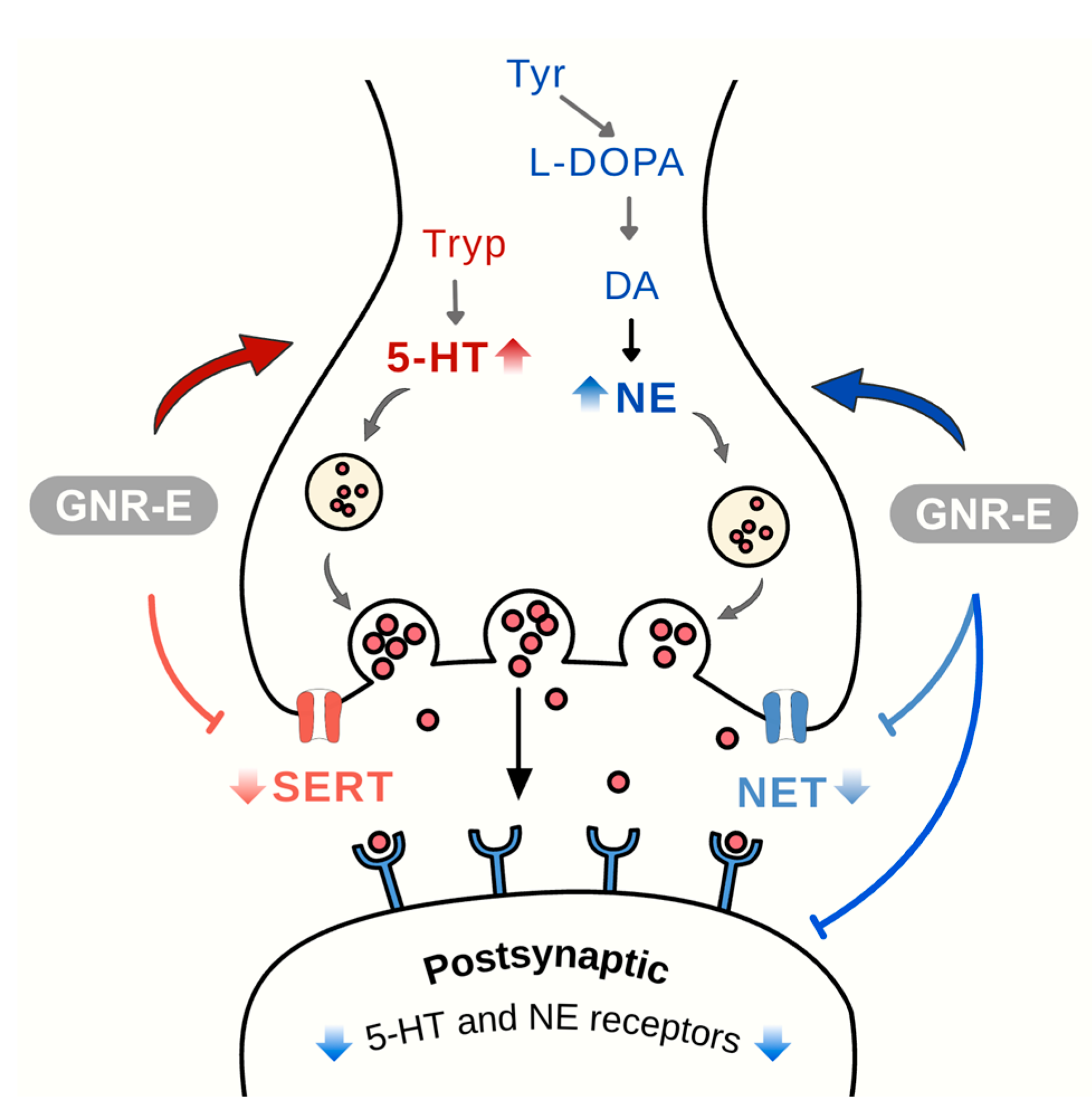
2.5. Effect of GNR-E on CMS-Induced Decrease in 5-HT and NE Levels
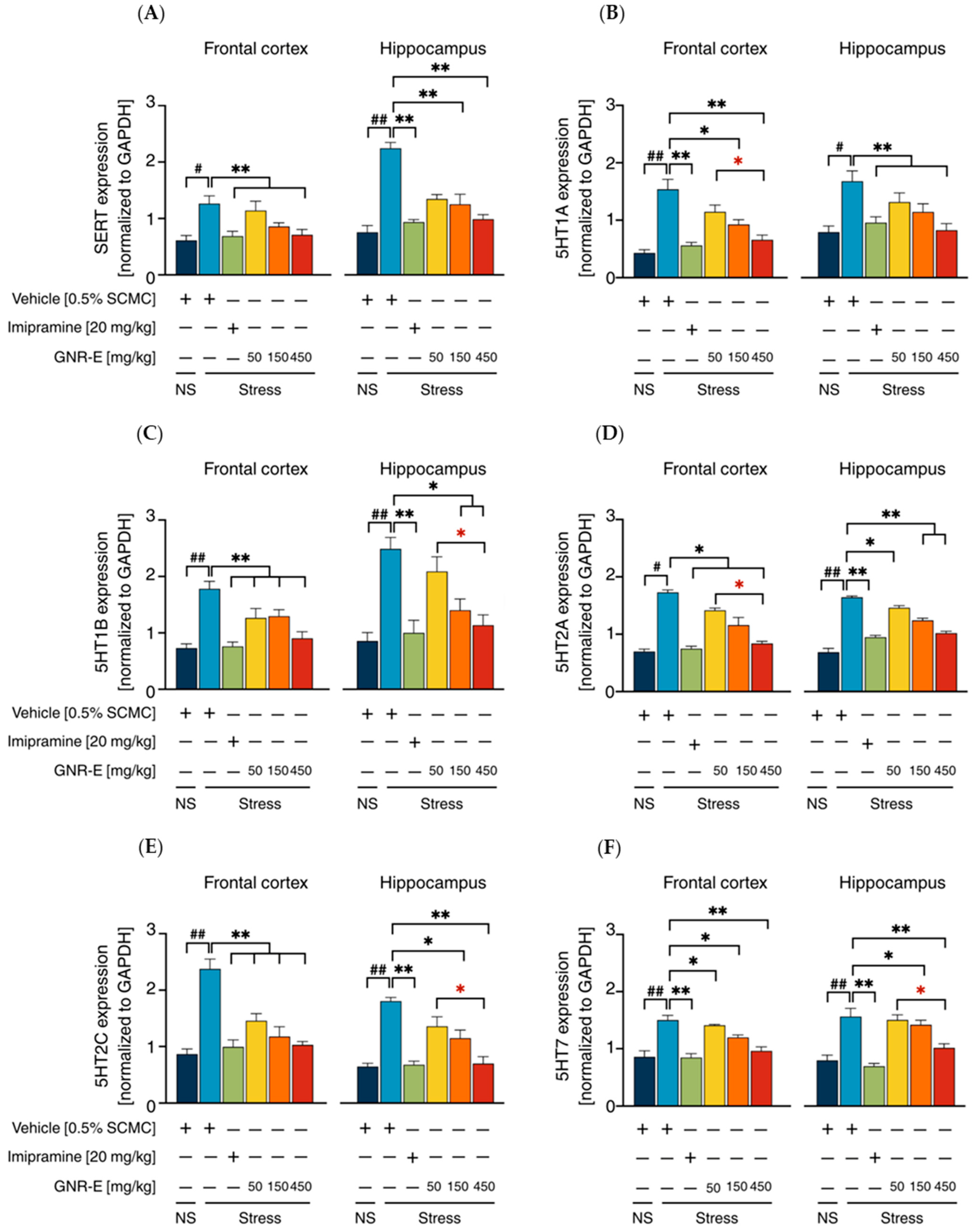
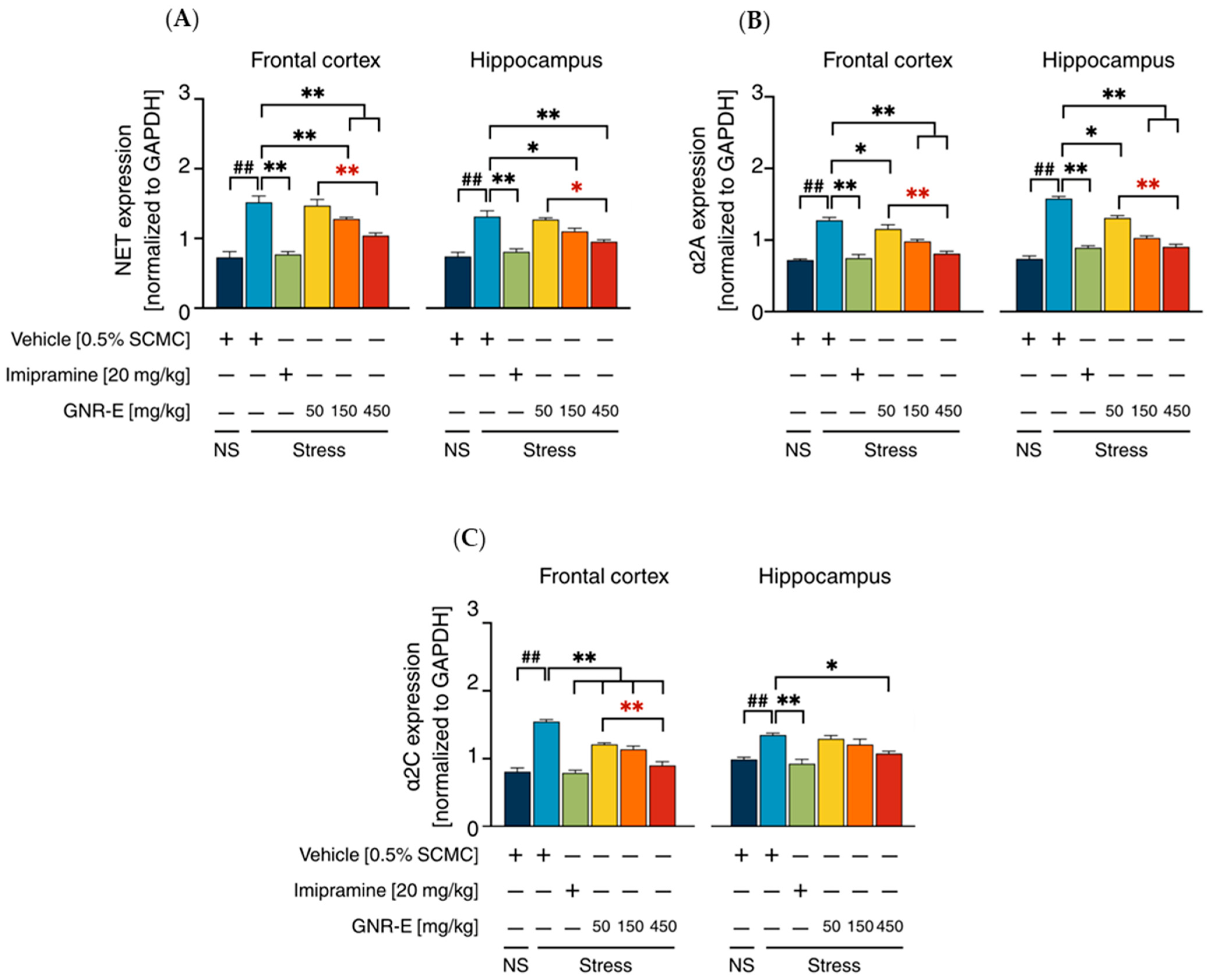
2.6. Effect of GNR-E on CMS-Induced Changes in Gene Expression
2.7. Phytochemical Profiling and HPLC-Based Identification of Major Constituents in GNR-E
3. Discussion
4. Materials and Methods
4.1. Plant Material, Extraction, Phytochemical Profiling, and HPLC Fingerprint
4.1.1. Plant Material and Extraction
4.1.2. Phytochemical Profiling
4.1.3. HPLC Fingerprint of the GNR-E
4.2. MAO-A and MAO-B Inhibitory Assay
4.3. In Vivo Studies
4.3.1. Animals, Husbandry, and Ethics Approval
4.3.2. Chronic Mild Stress (CMS)-Induced Depression Procedure
4.3.3. Drug Administration
4.3.4. Sucrose Consumption Test (SCT)
4.3.5. Tail Suspension Test (TST)
4.3.6. Forced Swimming Test (FST)
4.4. Neurochemical Studies
4.4.1. Blood Collection and Brain Preparation
4.4.2. Brain Extraction for Estimation of Neurotransmitter Level
4.4.3. Plasma Corticosterone (CORT) Assay
4.4.4. Determination of Serotonin (5-HT) Levels
4.4.5. Determination of Norepinephrine (NE) Levels
4.4.6. Quantitative Real-Time Polymerase Chain Reaction (QPCR)
5. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, H.; Xie, X.M.; Zhang, Q.; Cui, X.; Lin, J.X.; Sim, K.; Ungvari, G.S.; Zhang, L.; Xiang, Y.T. Prevalence of suicidality in major depressive disorder: A systematic review and meta-analysis of comparative studies. Front. Psychiatry 2021, 12, 690130. [Google Scholar] [CrossRef]
- Kasahara-Kiritani, M.; Kato, T.; Wakamatsu, A.; Webb, T.; Herr, K.; Vandervoort, L.; Li, N. Understanding anhedonia in major depressive disorder in Japan: Epidemiology and unmet needs from patients’ and physicians’ perspectives. BMC Psychiatry 2025, 25, 631. [Google Scholar] [CrossRef]
- Silva, R.C.; Maffioletti, E.; Gennarelli, M.; Baune, B.T.; Minelli, A. Biological correlates of early life stressful events in major depressive disorder. Psychoneuroendocrinology 2021, 125, 105103. [Google Scholar] [CrossRef]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef]
- Leiser, S.C.; Li, Y.; Pehrson, A.L.; Dale, E.; Smagin, G.; Sanchez, C. Serotonergic regulation of prefrontal cortical circuitries involved in cognitive processing: A review of individual 5-HT receptor mechanisms and concerted effects of 5-HT receptors exemplified by the multimodal antidepressant vortioxetine. ACS Chem. Neurosci. 2015, 6, 970–986. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Wu, Q.; Huang, H.; Li, S. Effects of α2A adrenoceptors on norepinephrine secretion from the locus coeruleus during chronic stress-induced depression. Front. Neurosci. 2017, 11, 243. [Google Scholar] [CrossRef]
- Bijata, M.; Wirth, A.; Wlodarczyk, J.; Ponimaskin, E. The interplay of serotonin 5-HT1A and 5-HT7 receptors in chronic stress. J. Cell Sci. 2024, 137, jcs262219. [Google Scholar] [CrossRef] [PubMed]
- Calzadilla, N.; Jayawardena, D.; Qazi, A.; Sharma, A.; Mongan, K.; Comiskey, S.; Eathara, A.; Saksena, S.; Dudeja, P.K.; Alrefai, W.A.; et al. Serotonin transporter deficiency induces metabolic alterations in the ileal mucosa. Int. J. Mol. Sci. 2024, 25, 4459. [Google Scholar] [CrossRef] [PubMed]
- Shanker, S.; Saroj, N.; Cordova, E.J.; Jarillo-Luna, R.A.; López-Sánchez, P.; Terrón, J.A. Chronic restraint stress induces serotonin transporter expression in the rat adrenal glands. Mol. Cell. Endocrinol. 2020, 518, 110935. [Google Scholar] [CrossRef] [PubMed]
- Gans, I.M.; Coffman, J.A. Glucocorticoid-mediated developmental programming of vertebrate stress responsivity. Front. Physiol. 2021, 12, 812195. [Google Scholar] [CrossRef] [PubMed]
- Ring, M. An integrative approach to HPA axis dysfunction: From recognition to recovery. Am. J. Med. 2025, 138, 1451–1463. [Google Scholar] [CrossRef]
- Panthong, A.; Norkaew, P.; Kanjanapothi, D.; Taesotikul, T.; Anantachoke, N.; Reutrakul, V. Anti-inflammatory, analgesic and antipyretic activities of the extract of gamboge from Garcinia hanburyi Hook f. J. Ethnopharmacol. 2007, 111, 335–340. [Google Scholar] [CrossRef]
- Lin, F.; Luo, B.; Cheng, Z.; Li, P.; Long, C. Ethnobotanical study on Garcinia (Clusiaceae) in China. Acta Soc. Bot. Pol. 2021, 90, e9012. [Google Scholar] [CrossRef]
- Khamphukdee, C.; Turkmani, I.; Chotritthirong, Y.; Chulikhit, Y.; Boonyarat, C.; Sekeroglu, N.; Silva, A.M.S.; Monthakantirat, O.; Kijjoa, A. Effects of the bark resin extract of Garcinia nigrolineata on chronic stress-induced memory deficit in mice and the in vitro monoamine oxidase and β-amyloid aggregation inhibitory activities of its prenylated xanthone constituents. Molecules 2022, 27, 3014. [Google Scholar] [CrossRef]
- Ruan, J.; Zheng, C.; Liu, Y.; Qu, L.; Yu, H.; Han, L.; Zhang, Y.; Wang, T. Chemical and biological research on herbal medicines rich in xanthones. Molecules 2017, 22, 1698. [Google Scholar] [CrossRef]
- Tiang, N.; Ahad, M.A.; Murugaiyah, V.; Hassan, Z. Xanthone-enriched fraction of Garcinia mangostana and α-mangostin improve the spatial learning and memory of chronic cerebral hypoperfusion rats. J. Pharm. Pharmacol. 2020, 72, 1629–1644. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Liu, X.; Liu, J.; Cai, E.; Zhao, Y.; Li, H.; Zhang, L.; Li, P.; Gao, Y. α-Mangostin exhibits antidepressant-like effects mediated by the modification of GABAergic, serotonergic and dopaminergic systems. Nat. Prod. Res. 2020, 34, 868–871. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). ICH Q2(R2) Validation of Analytical Procedures—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 10 October 2025).
- Das, T.; Saha, S.C.; Sunita, K.; Majumder, M.; Ghorai, M.; Mane, A.B.; Prasanth, D.A.; Kumar, P.; Pandey, D.K.; Al-Tawaha, A.R.; et al. Promising botanical-derived monoamine oxidase (MAO) inhibitors: Pharmacological aspects and structure-activity studies. S. Afr. J. Bot. 2022, 146, 127–145. [Google Scholar] [CrossRef]
- Aisha, A.F.A.; Abu-Salah, K.M.; Ismail, Z.; Majid, A.M.S.A. Determination of total xanthones in Garcinia mangostana fruit rind extracts by ultraviolet (UV) spectrophotometry. J. Med. Plants Res. 2013, 7, 29–35. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The chemistry behind the Folin–Ciocalteu method for the estimation of (poly)phenol content in food: Total phenolic intake in a Mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Chotritthirong, Y.; Chulikhit, Y.; Daodee, S.; Boonyarat, C.; Maneenet, J.; Khamphukdee, C.; Kwankhao, P.; Pitiporn, S.; Monthakantirat, O. Possible mechanisms for the prevention of anxiety and depressive-like behavior in a chronic mild stress mouse model by the Thai herbal medicine with Nelumbo nucifera, Centella asiatica, and Piper nigrum. Rev. Bras. Farmacogn. 2023, 33, 756–767. [Google Scholar] [CrossRef]
- Mizuki, D.; Matsumoto, K.; Tanaka, K.; Thi Le, X.; Fujiwara, H.; Ishikawa, T.; Higuchi, Y. Antidepressant-like effect of Butea superba in mice exposed to chronic mild stress and its possible mechanism of action. J. Ethnopharmacol. 2014, 156, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Markov, D.D. Sucrose preference test as a measure of anhedonic behavior in a chronic unpredictable mild stress model of depression: Outstanding issues. Brain Sci. 2022, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
- Armario, A. The forced swim test: Historical, conceptual and methodological considerations and its relationship with individual behavioral traits. Neurosci. Biobehav. Rev. 2021, 128, 74–86. [Google Scholar] [CrossRef]
- Saleem, U.; Hira, S.; Anwar, F.; Shah, M.A.; Bashir, S.; Baty, R.S.; Badr, R.H.; Blundell, R.; Batiha, G.E.-S.; Ahmad, B. Pharmacological screening of Viola odorata L. for memory-enhancing effect via modulation of oxidative stress and inflammatory biomarkers. Front. Pharmacol. 2021, 12, 664832. [Google Scholar] [CrossRef]
- Tadano, T.; Endo, Y.; Kisara, K. A simple determination of serotonin, 5-hydroxyindoleacetic acid and 5-hydroxytryptophan decarboxylase activity in rat brain areas and parallel correlation among the levels. Jpn. J. Pharmacol. 1980, 30, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Maickel, R.P.; Cox, R.H.; Saillant, J.; Miller, F.P. A method for the determination of serotonin and norepinephrine in discrete areas of rat brain. Int. J. Neuropharmacol. 1968, 7, 275–281. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Sample | IC50 (µg/mL) | Ki (µg/mL) | Si | |||
|---|---|---|---|---|---|---|
| MAO-A | MAO-B | MAO-A | MAO-B | MAO-A | MAO-B | |
| GNR-E | 8.81 ± 0.02 | 3.60 ± 0.04 | 2.32 | 1.55 | 1.50 | 0.67 |
| Clorgyline * | 0.01 ± 0.001 µM | 0.02 ± 0.002 µM | 0.001 | 0.07 | 0.14 | 7.0 |
| Deprenyl ** | 8.48 ± 0.14 µM | 0.10 ± 0.02 µM | 2.23 | 0.05 | 48.57 | 0.02 |
| Parameters | Standard Compounds | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| LOD | Concentration (µg/mL) | 0.5 | 0.5 | 0.1 | 0.5 | 0.2 |
| S/N | 3.08 ± 0.06 | 3.09 ± 0.07 | 3.05 ± 0.02 | 3.09 ± 0.06 | 3.08 ± 0.11 | |
| LOQ | Concentration (µg/mL) | 1.0 | 5.0 | 0.5 | 1.0 | 1.0 |
| S/N | 10.77 ± 0.66 | 9.81 ± 0.55 | 10.81 ± 0.69 | 10.86 ± 0.83 | 10.78 ± 0.92 | |
| Linearity | Range (µg/mL) | 1–20 | 5–30 | 1–20 | 0.5–15 | 5–13 |
| Equation | y = 7.2427x − 6.8218 | y = 0.6813x − 0.2263 | y = 19.705x − 5.7932 | y = 7.4318x − 1.3177 | y = 3.0641x − 10.690 | |
| (R2) | 0.9976 | 0.9945 | 0.9918 | 0.9970 | 0.9939 | |
| Precision (%RSD) | Repeatability | 0.18–1.64% | 0.07–1.13% | 0.02–1.19% | 0.03–1.75% | 0.03–1.93% |
| Intermediate precision | 0.07–1.62% | 0.53–3.02% | 0.35–1.37% | 0.52–1.36% | 0.18–1.59% | |
| Accuracy (%recovery) | Low concentration | 98.73 ± 0.05 | 108.34 ± 0.79 | 101.51 ± 0.25 | 100.47 ± 0.08 | 96.19 ± 0.43 |
| Medium concentration | 10.29 ± 0.45 | 95.25 ± 0.09 | 108.22 ± 0.58 | 104.33 ± 0.01 | 102.82 ± 0.83 | |
| High concentration | 98.35 ± 0.13 | 102.71 ± 0.75 | 98.99 ± 0.06 | 96.33 ± 0.39 | 98.78 ± 0.08 | |
| Robustness | 0.9 (mL/min) (% RSD) | 0.38 | 0.45 | 0.41 | 0.39 | 0.31 |
| 1.1 (mL/min) (% RSD) | 0.17 | 0.21 | 0.02 | 0.26 | 0.26 | |
| GNR-E (mg/g extract) | 0.070 ± 0.004 | 0.377 ± 0.069 | 0.101 ± 0.002 | 0.076 ± 0.006 | 1.916 ± 0.01 | |
| Genes | Forward (5′-3′) | Reward (5′-3′) | BP | Accession No. |
|---|---|---|---|---|
| SERT | CTCATCTTCACATTATCTACTTCAG | CTCACCAGCAGGACAGAAAG | 113 | NM_010484.2 |
| 5HT1A | CTGTGACCTGTTTATCGCCCTG | GTAGTCTATAGGGTCGGTGATTGC | 109 | NM_008308.5 |
| 5HT1B | ACATCCTCGGTCACCTCCATTA | CCCTAGCGGCCATGAGTTTC | 136 | NM_000863.3 |
| 5HT2A | CAGCGGTCCATCCACAGAG | CCACATTACAACAAACAGAAAGAACAC | 123 | NM_172812.3 |
| 5HT2B | AAGCCAATTCAGGCCAATC | GGGCACCACATAAGCAGAAA | 542 | NM_008311.3 |
| 5HT2C | GGTCCTTCGTGGCATTCTTCATC | CGCAGTTCCTCCTCGGTGTG | 121 | NM_001411391.1 |
| 5HT6 | CCTGGTGTCGCTCTTCACG | GGCATCACCACCAATCCC | 51 | NM_001377096.1 |
| 5HT7 | GTTAGTGTCACGGACCTCAT | ATCATTTTGGCCATACATTT | 255 | NM_001360300.1 |
| NET | TGCACGAGAGCAGTGGGAT | CGACCATCAGGCAGAGCAG | 69 | NM_009209.3 |
| α2A | GTGACACTGACGCTGGTTTG | CCAGTAACCCATAACCTCGTTG | 204 | NM_007417.5 |
| α2C | CTGTGGTGGGTTTCCTCATCG | ACTTGCCCGAAGTACCAGTAG | 199 | NM_007418.3 |
| GR | CACTAATCCTCTCCATCCTAC | AATGTCTGCTGCCTTCTG | 479 | NM_008173.4 |
| SGK-1 | GGGTGCCAAGGATGACTTTA | CTCGGTAAACTCGGGATAGA | 154 | NM_011361.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotritthirong, Y.; Sumanont, Y.; Daodee, S.; Mading, A.; Boonyarat, C.; Khamphukdee, C.; Kumla, D.; Maneenet, J.; Matsumoto, K.; Kijjoa, A.; et al. Antidepressant-like Effects of Garcinia nigrolineata Resin Extract in a Chronic Mild Stress Mouse Model: Modulation of Monoaminergic and HPA-Axis Pathways. Plants 2025, 14, 3651. https://doi.org/10.3390/plants14233651
Chotritthirong Y, Sumanont Y, Daodee S, Mading A, Boonyarat C, Khamphukdee C, Kumla D, Maneenet J, Matsumoto K, Kijjoa A, et al. Antidepressant-like Effects of Garcinia nigrolineata Resin Extract in a Chronic Mild Stress Mouse Model: Modulation of Monoaminergic and HPA-Axis Pathways. Plants. 2025; 14(23):3651. https://doi.org/10.3390/plants14233651
Chicago/Turabian StyleChotritthirong, Yutthana, Yaowared Sumanont, Supawadee Daodee, Abdulwaris Mading, Chantana Boonyarat, Charinya Khamphukdee, Decha Kumla, Juthamart Maneenet, Kinzo Matsumoto, Anake Kijjoa, and et al. 2025. "Antidepressant-like Effects of Garcinia nigrolineata Resin Extract in a Chronic Mild Stress Mouse Model: Modulation of Monoaminergic and HPA-Axis Pathways" Plants 14, no. 23: 3651. https://doi.org/10.3390/plants14233651
APA StyleChotritthirong, Y., Sumanont, Y., Daodee, S., Mading, A., Boonyarat, C., Khamphukdee, C., Kumla, D., Maneenet, J., Matsumoto, K., Kijjoa, A., Awale, S., & Monthakantirat, O. (2025). Antidepressant-like Effects of Garcinia nigrolineata Resin Extract in a Chronic Mild Stress Mouse Model: Modulation of Monoaminergic and HPA-Axis Pathways. Plants, 14(23), 3651. https://doi.org/10.3390/plants14233651











