Impact of Ficus deltoidea Aqueous Extract on Maternal Hepatic Drug Metabolism and Foetal Development in Rats
Abstract
1. Introduction
2. Results
2.1. Phytochemical Content of F. deltoidea Var Kunstleri Aqueous Extract
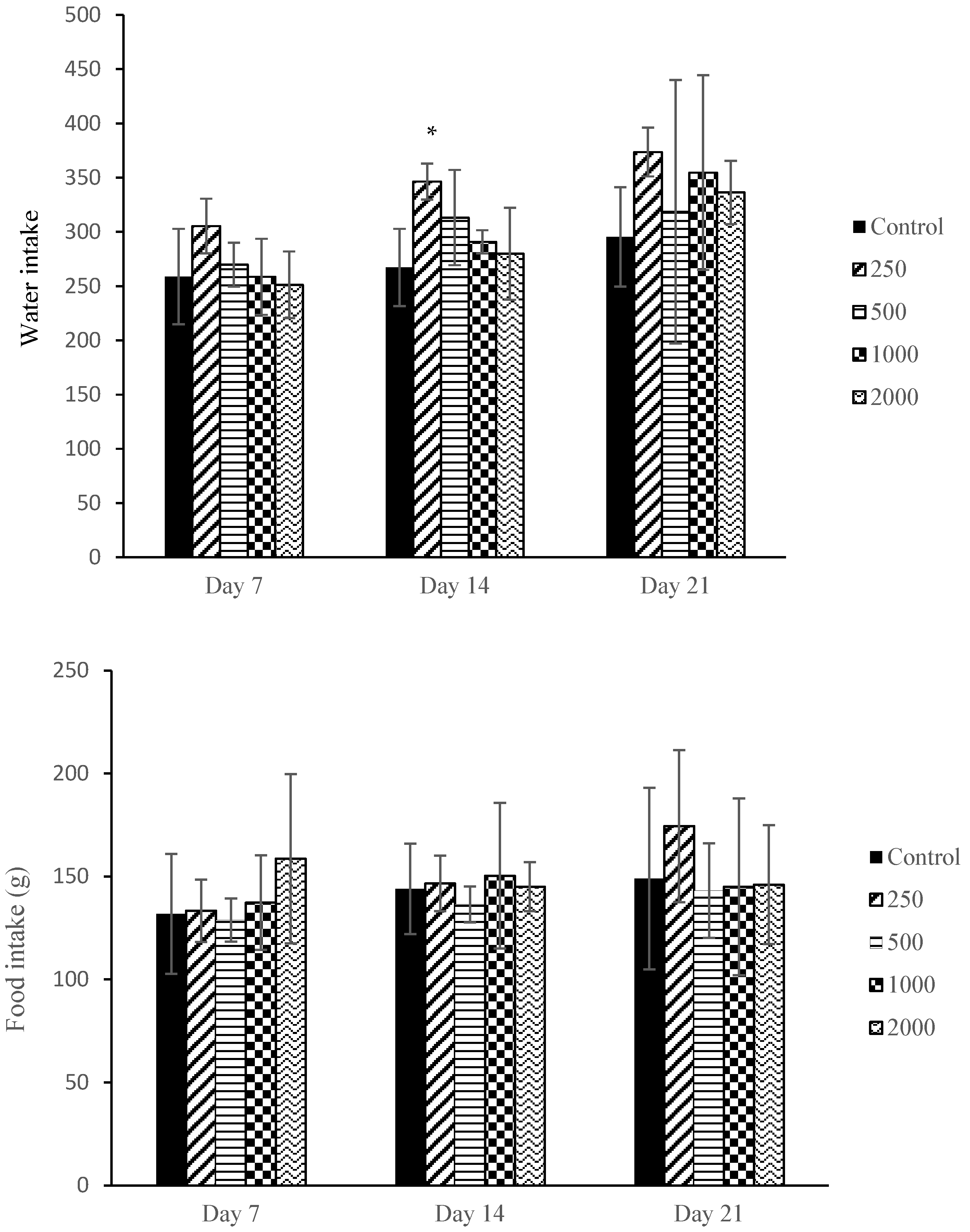
2.2. Maternal Mortality, Clinical Observations, and Food and Water Intake
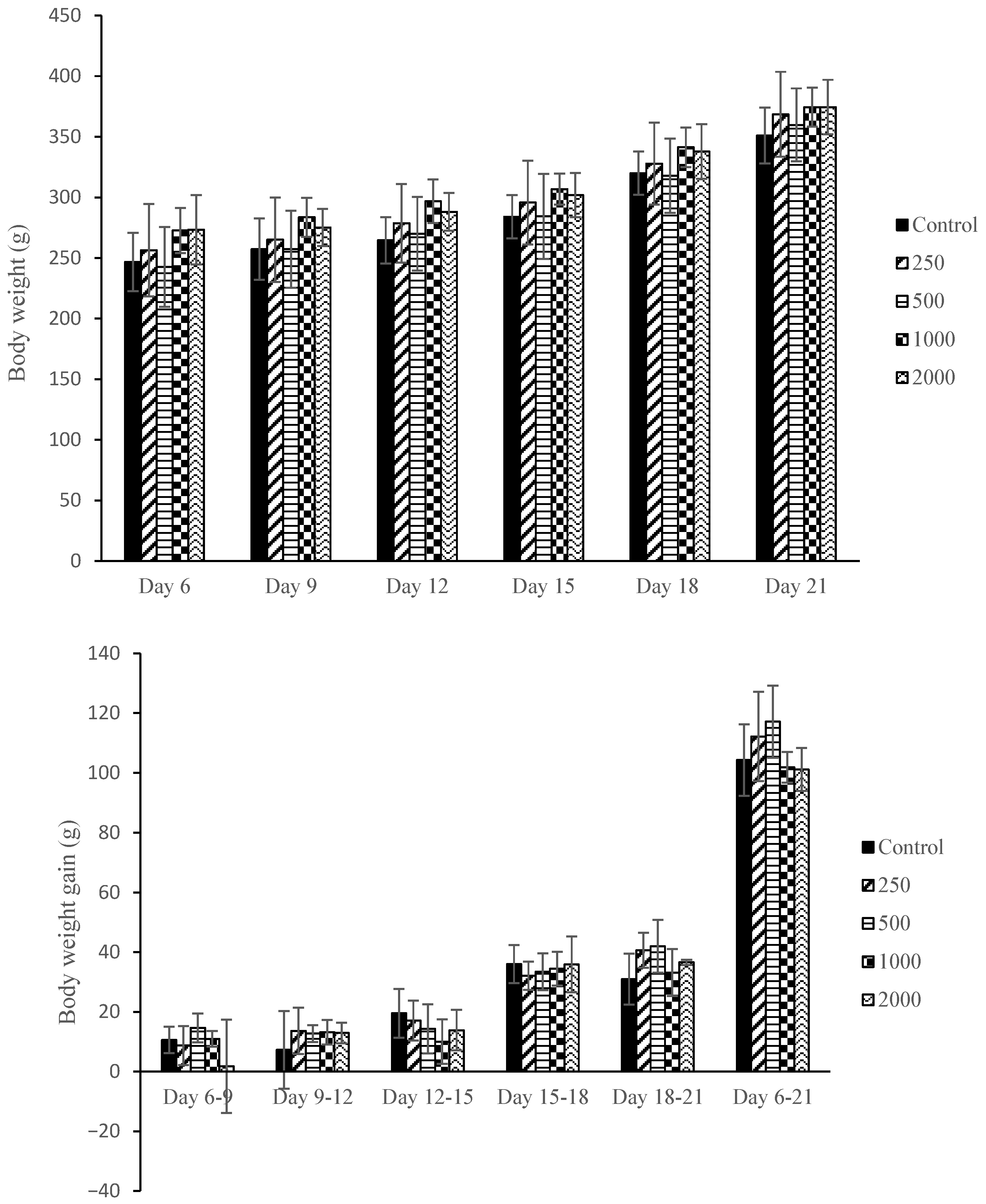
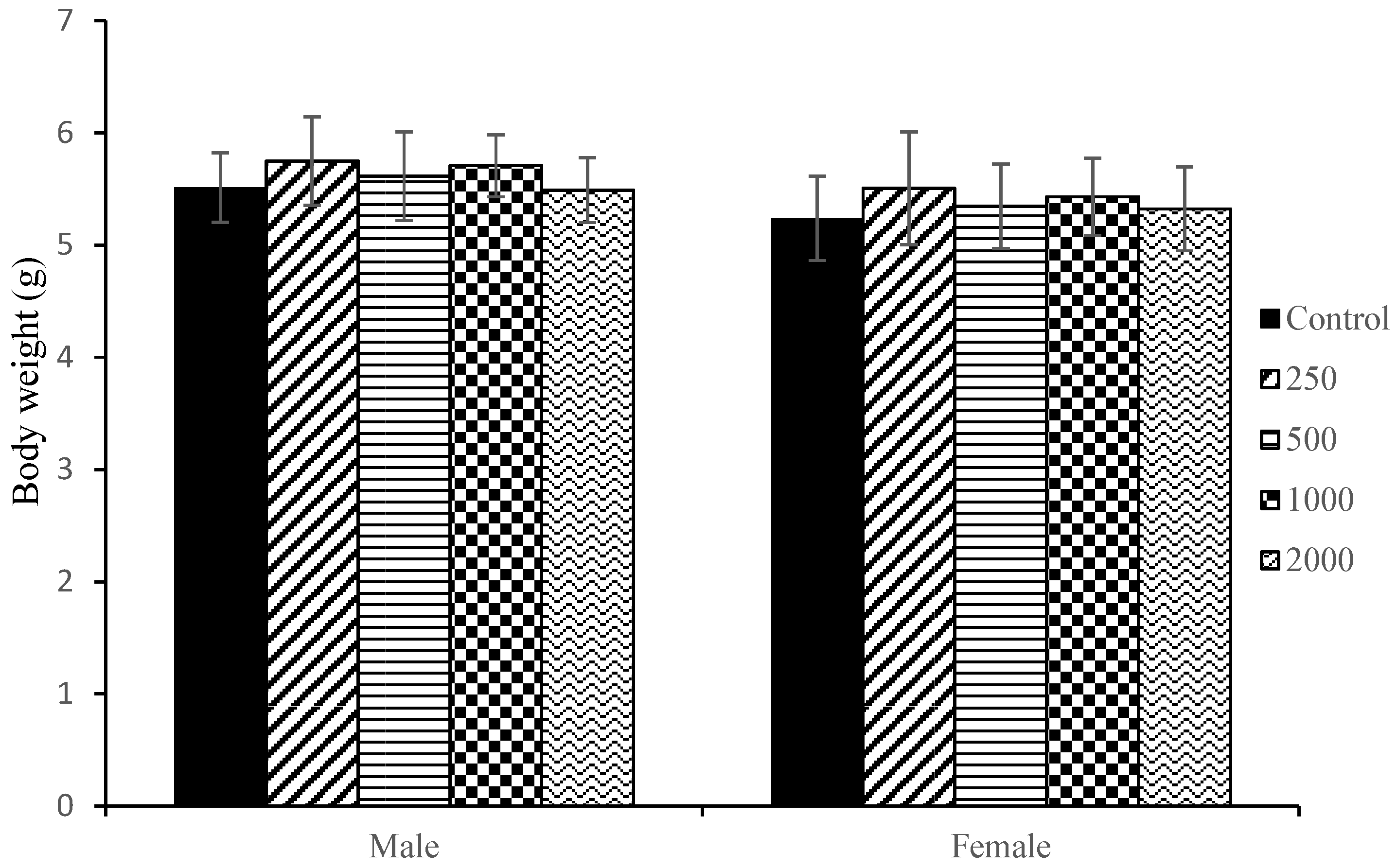
2.3. Maternal Body Weight and Body Weight Gain
2.4. Maternal Visceral Organs Examination
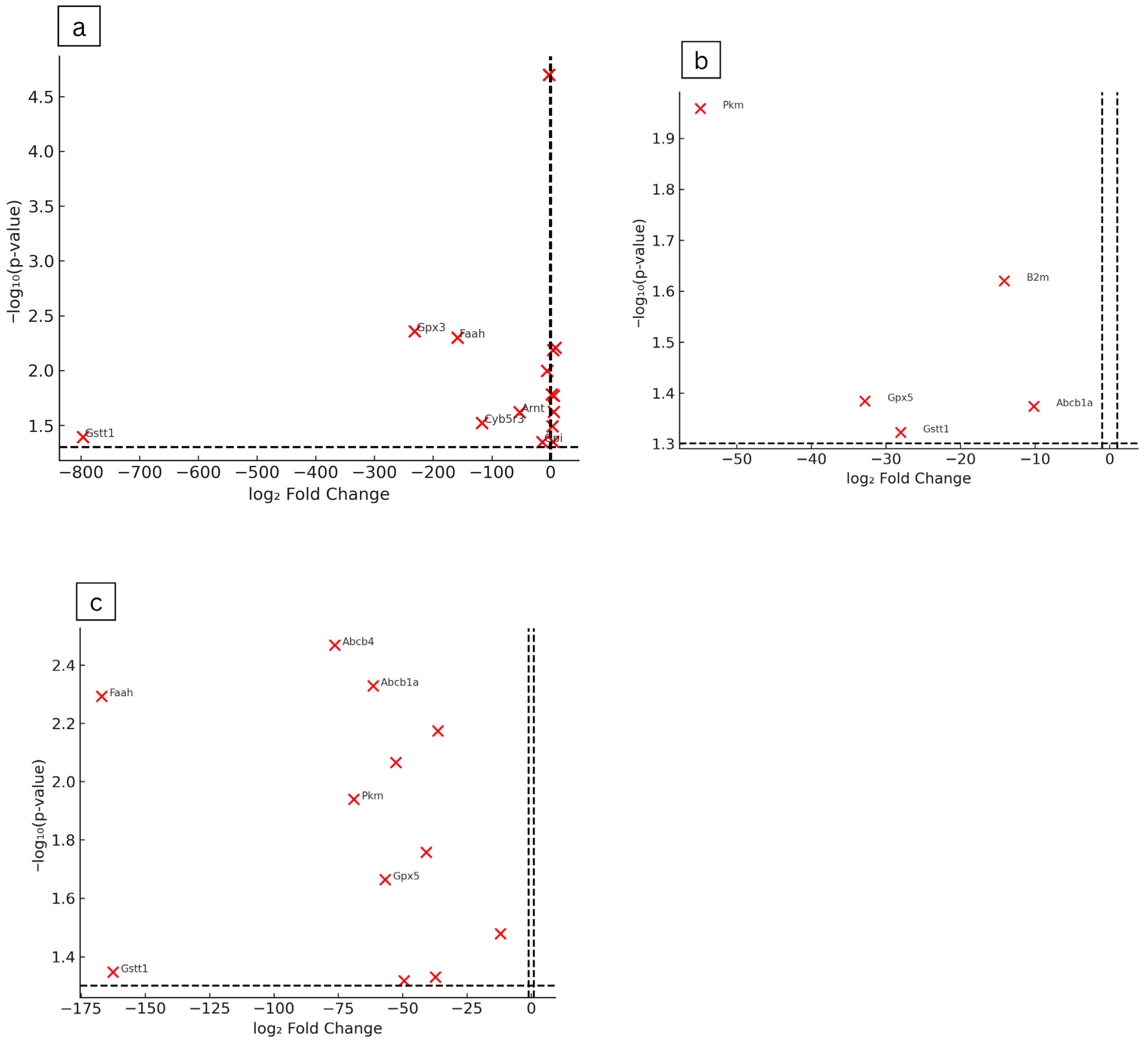
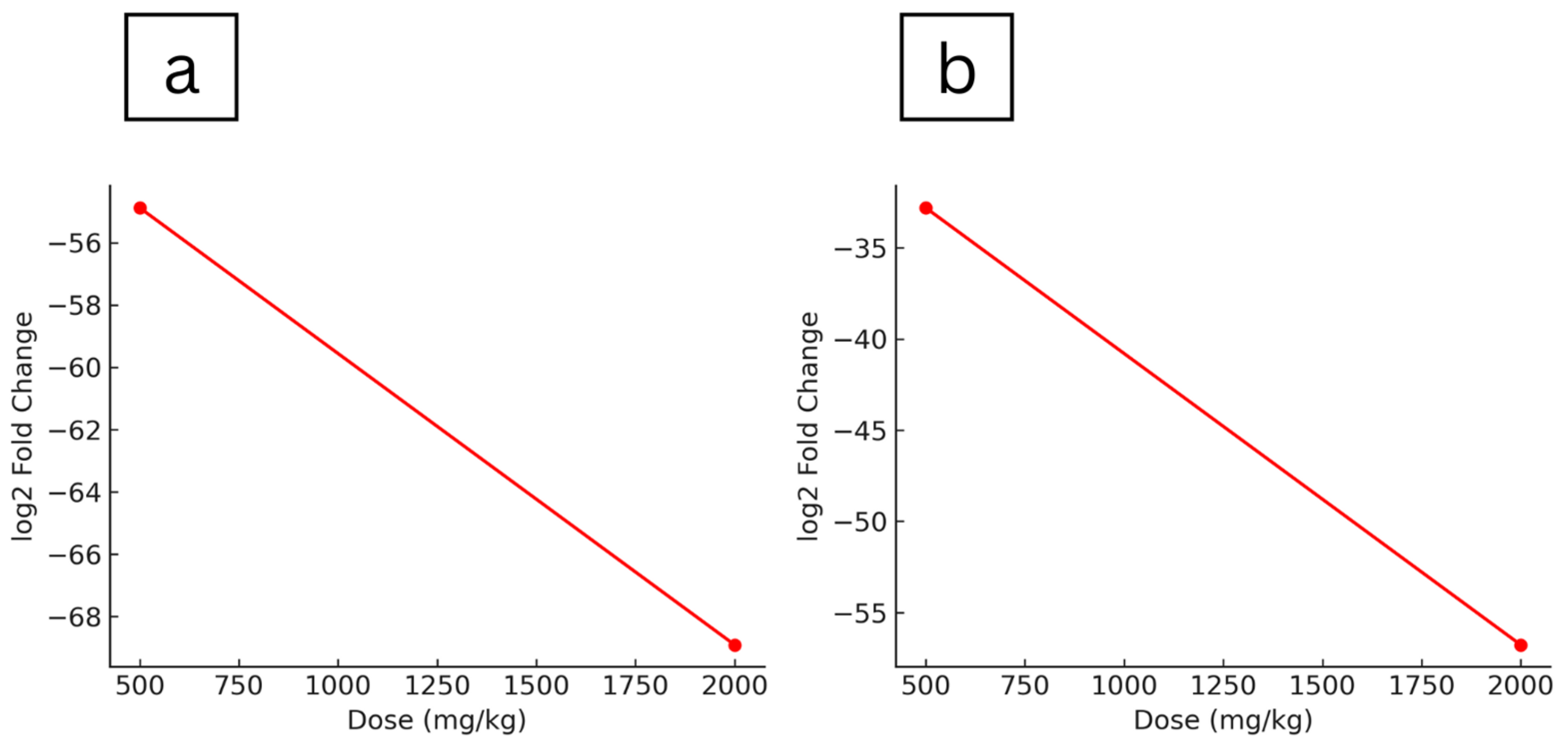
2.5. Gene Expression Analysis of Hepatic Drug Metabolism and Detoxification Pathways
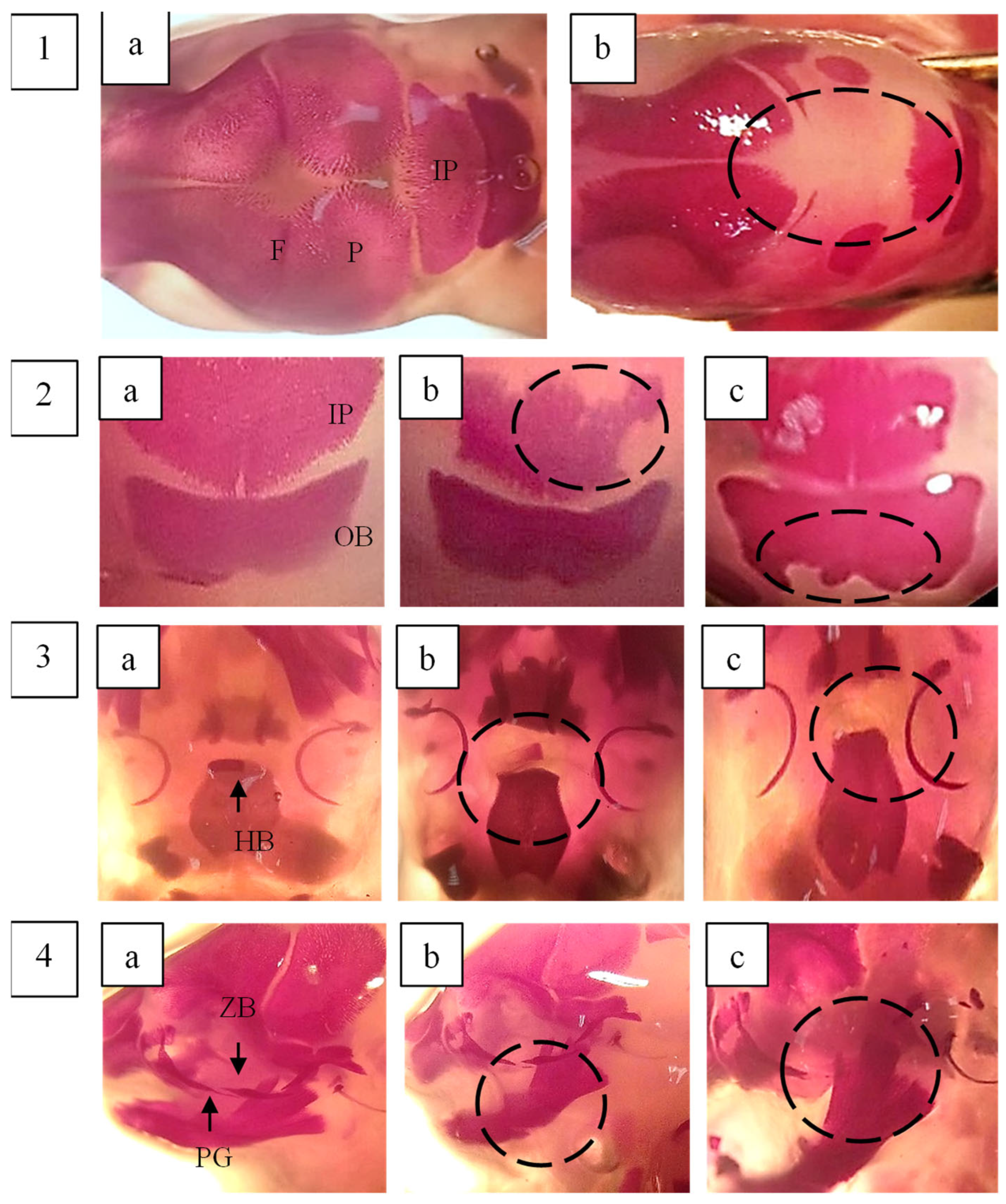
2.6. Foetal Examination
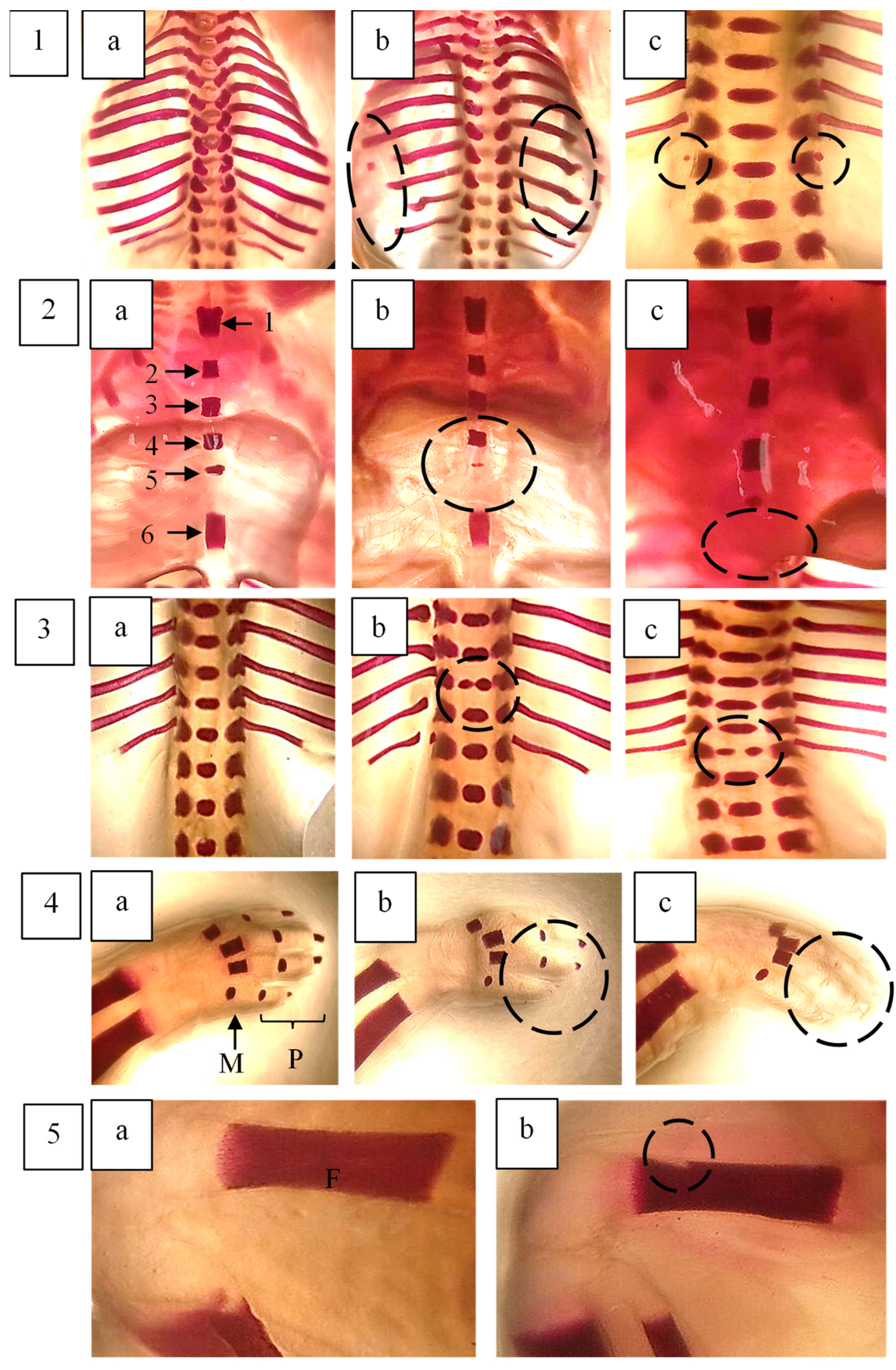
2.7. Skeletal Examination of Foetuses
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. F. deltoidea Aqueous Extraction Preparation
4.3. Animal
4.4. Study Design
4.5. Skeletal Examination of Foetuses
4.6. Gene Expression Profiles of Maternal Rat Liver
4.7. PARN-002Z RT2 ProfilerTM PCR Array Rat Drug Metabolism
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akerele, O. Summary of WHO guidelines for the assessment of herbal medicine. Herbalgram 1993, 28, 13–19. [Google Scholar]
- Bunawan, H.; Amin, N.M.; Bunawan, S.N.; Baharum, S.N.; Mohd Noor, N. Ficus deltoidea Jack: A Review on its phytochemical and pharmacological importance. Evid. Based Complement. Altern. Med. 2014, 2014, 902734. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, N.; Mohd Dom, S.N.; Adam, Z.; Hamid, M. Insulinotropic activity of standardized methanolic extracts of Ficus deltoidea from seven varieties. Evid. Based Complement. Altern. Med. 2018, 2018, 3769874. [Google Scholar] [CrossRef] [PubMed]
- Soib, H.H.; Ware, I.; Yaakob, H.; Mukrish, H.; Sarmidi, M.R. Antioxidant and anti-cancer actvity of standardized extracts of three varieties of Ficus deltoidea’s leaves. J. Teknol. 2015, 77, 19–25. [Google Scholar] [CrossRef]
- Abdulla, M.A.; Ahmed, K.A.-A.; Abu-Luhoom, F.M.; Muhanid, M. Role of Ficus deltoidea extract in the enhancement of wound healing in experimental rats. Biomed. Res. 2010, 21, 241–245. [Google Scholar]
- Zakaria, Z.A.; Hussain, M.K.; Mohamad, A.S.; Abdullah, F.C.; Sulaiman, M.R. Anti-inflammatory activity of the aqueous extract of Ficus deltoidea. Biol. Res. Nurs. 2012, 14, 90–97. [Google Scholar] [CrossRef]
- Amiera, Z.U.; Nihayah, M.; Wahida, I.F.; Rajab, N.F. Phytochemical characteristic and uterotonic effect of aqueous extract of Ficus deltoidea leaves in rats uterus. Pak. J. Biol. Sci. 2014, 17, 1046–1051. [Google Scholar] [CrossRef]
- Adam, Z.; Hamid, M.; Ismail, A.; Khamis, S. Effect of Ficus deltoidea aqueous extract on blood glucose level in normal and mild diabetic rats. J. Sains Kesihat. Malays. 2007, 5, 9–16. [Google Scholar]
- Hasni, M.; Azlina, A.A.; Norhaniza, A. Antidiabetic and antioxidant properties of Ficus deltoidea fruit extracts and fractions. BMC Complement. Altern. Med. 2013, 13, 118. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Hussain, M.K.; Zakaria, Z.A.; Somchit, M.N.; Moin, S.; Mohamad, A.S.; Israf, D.A. Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia 2008, 79, 557–561. [Google Scholar] [CrossRef]
- Farsi, E.; Shafaei, A.; Hor, S.Y.; Ahamed, M.B.K.; Yam, M.F.; Asmawi, M.Z.; Ismail, Z. Genotoxicity and acute and subchronic toxicity studies of a standardized methanolic extract of Ficus deltoidea leaves. Clinics 2013, 68, 865–875. [Google Scholar] [CrossRef]
- Muhammad, H.; Omar, M.H.; Rasid, E.N.I.; Suhaimi, S.N.; Mohkiar, F.H.; Siu, L.M.; Awang, N. Phytochemical and in vitro genotoxicity studies of standardized Ficus deltoidea var. kunstleri aqueous extract. Plants 2021, 10, 343. [Google Scholar]
- Chahoud, I.; Ligensa, A.; Dietzel, L.; Faqi, A.S. Correlation between maternal toxicity and embryo/fetal effects. Reprod. Toxicol. 1999, 13, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Tyl, R.W. Commentary on the Role of Maternal Toxicity on Developmental Toxicity. Birth Defects Res. B Dev. Reprod. Toxicol. 2012, 95, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Pierre, W.; Esther, N.; Nkeng-Efouet, P.A.; Nguelefack, T.B.; Albert, K. Reproductive effects of Ficus asperifolia (Moraceae) in female rats. Afr. Health Sci. 2009, 9, 49–53. [Google Scholar]
- Yoon, K.C.; Kwon, H.D.; Jo, H.S.; Choi, Y.Y.; Seok, J.I.; Kang, Y.; Lee, D.Y.; Kim, D.S. Explorative study of serum biomarkers of liver failure after liver resection. Sci. Rep. 2020, 10, 9960. [Google Scholar] [CrossRef]
- Alexandra, M.; Bryn, M.O.; Saskia, V.M.; Dirk, D.; Chikage, M.; Mohamed, B.; William, C.; Kristina, S.; Stuart, M.; Malcolm, P.; et al. The normal mechanisms of pregnancy-induced liver growth are not maintained in mice lacking the bile acid sensor Fxr. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G151–G158. [Google Scholar]
- Farsi, E.; Ahmad, M.; Hor, S.Y.; Ahamed, M.B.K.; Yam, M.F.; Asmawi, M.Z. Standardized extract of Ficus deltoidea stimulates insulin secretion and blocks hepatic glucose production by regulating the expression of glucose-metabolic genes in streptozitocin-induced diabetic rats. BMC Complement. Altern. Med. 2014, 14, 220. [Google Scholar] [CrossRef]
- Ham, C.P.; Uzar, F.; Abdullah, N.; Aminudin, N.; Abdul-Rahman, P.S. Alterations of cholesterol lowering-related proteins in the serum of hypercholesterolemic-induced rats treated with Ficus deltoidea. Sains Malays. 2020, 49, 1055–1066. [Google Scholar]
- Dai, G.; Bustamante, J.J.; Zou, Y.; Myronovych, A.; Bao, Q.; Kumar, S.; Soares, M.J. Maternal hepatic growth response to pregnancy in the mouse. Exp. Biol. Med. 2011, 236, 1322–1332. [Google Scholar] [CrossRef]
- Martignoni, M.; Groothuis, G.M.; de Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert. Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef] [PubMed]
- He, X.J.; Ejiri, N.; Nakayama, H.; Doi, K. Effects of pregnancy on CYPs protein expression in rat liver. Exp. Mol. Pathol. 2005, 78, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Jiang, Y.; Qu, C.; Yuan, H.; Hu, K.; He, L.; Chen, P.; Li, J.; Tu, M.; Lin, L.; et al. Pyruvate kinase M2 tetramerization protects against hepatic stellate cell activation and liver fibrosis. Am. J. Path 2020, 190, 2267–2281. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, H.; Thompson, D.C.; Shertzer, H.G.; Nebert, D.W.; Vasiliou, V. Glutathione defense mechanism in liver injury: Insights from animal models. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 60, 38–44. [Google Scholar] [CrossRef]
- Schardein, R.L. Chemically Induced Birth Defects; Marcel Dekker Inc.: New York, NY, USA; Basel, Switzerland, 2000. [Google Scholar]
- Maganha, J.; Rocha, E.D.S.; Brandão, M.A.F.; Peters, V.M.; Guerra, M.D.O. Embryo development alteration in rats treated with lapachol. Braz. Arch. Biol. Technol. 2006, 49, 927–934. [Google Scholar] [CrossRef]
- Neetu, J.J.; Charles, E.D.; Lakota, K.K.; Dandolu, V. Maternal obesity: Can pregnancy weight gain modify risk of selected adverse pregnancy outcomes? Am. J. Perinatol. 2007, 24, 291–298. [Google Scholar] [CrossRef]
- Hacker, A.; Fung, E.B.; Ellen, B.; King, F. Role of calcium during pregnancy: Maternal and fetal needs. Nutr. Rev. 2012, 70, 397–409. [Google Scholar] [CrossRef]
- Comar, C.L. Radiocalcium studies in pregnancy. Ann. New York Acad. Sci. 1956, 64, 281–298. [Google Scholar] [CrossRef]
- Marques, N.F.Q.; Marques, A.P.B.M.; Iwano, A.L.; Golin, M.; De-Carvalho, R.R.; Paumgartten, F.J.R.; Dalsenter, P.R. Delayed ossification in Wistar rats induced by Morinda citrifolia L. exposure during pregnancy. J. Ethnopharmacol. 2010, 128, 85–91. [Google Scholar] [CrossRef]
- Solecki, R.; Bürgin, H.; Buschmann, J.; Clark, R.; Duverger, M.; Fialkowski, O.; Hofmann, T. Harmonisation of rat fetal skeletal terminology and classification. Report of the third workshop on the terminology in developmental toxicology: Berlin, 14–16 September 2000. Reprod. Toxicol. 2001, 15, 713–721. [Google Scholar] [CrossRef]
- Mohammad, N.; Wei, Y.K.; Bakar, N.F.A. Determination of mineral content in the Ficus deltoidea leaves. J. Sains Kesihat. Malays. 2012, 10, 25–29. [Google Scholar]
- Adlin, A.; Kasim, N.; Ismail, N.H.; Azmi, N.; Ali, A.M.; Mat, N.; Wolfender, J.-L. Differentiation of Ficus deltoidea varieties and chemical marker determination by UHPLC-TOFMS metabolomics for establishing quality control criteria of this popular Malaysian medicinal herb. Metabolomics 2019, 15, 35. [Google Scholar] [CrossRef]
- DeSesso, J.M.; Scialli, A.R. Bone development in laboratory mammals used in developmental toxicity studies. Birth Defects Res. 2018, 110, 1157–1187. [Google Scholar] [CrossRef]
- Chernoffa, N.; Rogersa, E.H.; Gageb, M.I.; Francisc, B.M. The relationship of maternal and fetal toxicity in developmental toxicology bioassays with notes on the biological significance of the “no observed adverse effect level”. Reprod. Toxicol. 2008, 25, 192–202. [Google Scholar] [CrossRef]
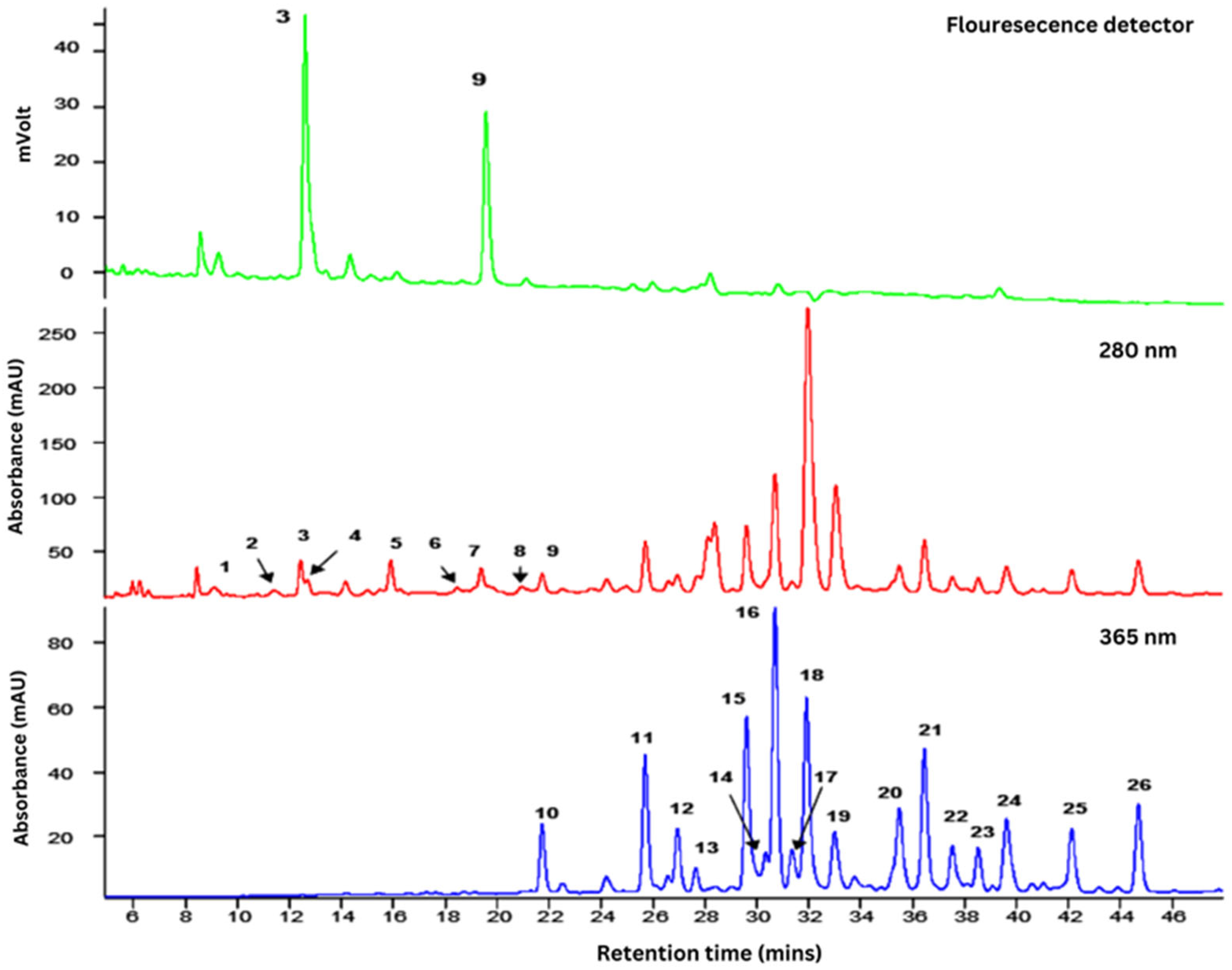
| Peak | tR (min) | λmax | Compound | [M−H]− (m/z) | MS2 Fragment Ions (m/z) |
|---|---|---|---|---|---|
| 1 | 6.6 | 270 | (+)-Gallocatechin | 305 | 261, 221, 179 |
| 2 | 11.4 | 275 | (–)-Epigallocatechin | 305 | 261, 221, 179 |
| 3 | 12.5 | 280 | (+)-Catechin | 289 | 245, 205, 179 |
| 4 | 12.7 | 280 | (Epi)catechin-(Epi)afzelechin | 561 | 435, 289, 273 |
| 5 | 14.2 | 280 | (Epi)catechin-(Epi)afzelechin | 561 | 435, 289, 273 |
| 6 | 15.1 | 275 | (Epi)catechin-(Epi)afzelechin-(Epi)afzelechin | 833 | 561, 543, 289 |
| 7 | 15.9 | 280 | (Epi)catechin-(Epi)afzelechin | 561 | 435, 289, 271 |
| 8 | 18.5 | 275 | (Epi)catechin-(Epi)afzelechin-(Epi)afzelechin | 833 | 561, 289, 271 |
| 9 | 19.4 | 280 | (–)-Epicatechin | 289 | 245, 205, 179 |
| 10 | 21.8 | 350 | Luteolin-6-8-C-diglucoside (Lucenin-2) | 609 | 519, 489, 399 |
| 11 | 25.7 | 340 | Apigenin-6, 8-C-diglucoside (Vicenin-2) | 593 | 503, 473, 353 |
| 12 | 27.0 | 345 | Luteolin-6-C-hexosyl-8-C-pentoside | 579 | 489, 459, 399 |
| 13 | 27.8 | 345 | Luteolin-6-C-glucosyl-8-C-arabinoside | 579 | 489, 459, 399 |
| 14 | 29.6 | 335 | Apigenin-6-C-arabinosyl-8-C-glucoside (Isoschaftoside) | 563 | 503, 473, 443 |
| 15 | 30.4 | 345 | Luteolin-6-C-arabinosyl-8-C-glucoside | 579 | 489, 459, 399 |
| 16 | 30.7 | 335 | Apigenin-6-C-glucoside-8-C-arabinoside (Schaftoside) | 563 | 503, 473, 443 |
| 17 | 31.4 | 335 | Luteolin-8-C-glucoside (Orientin) | 447 | 369, 357, 327 |
| 18 | 31.9 | 320 | Apigenin-6-C-pentosyl-8-C-glucoside | 563 | 473, 443, 353 |
| 19 | 33.0 | 310 | 4-p-Coumaroylquinic acid | 337 | 191, 173, 163 |
| 20 | 35.5 | 335 | Apigenin-8-C-glucoside (Vitexin) | 431 | 413, 341, 311 |
| 21 | 36.5 | 335 | Apigenin-6-C-glucosyl-8-C-pentoside | 563 | 473, 443, 353 |
| 22 | 37.5 | 335 | Apigenin-6,8-C-dipentoside | 533 | 515, 473, 443 |
| 23 | 38.5 | 335 | Apigenin-6,8-C-dipentoside | 533 | 515, 473, 443 |
| 24 | 39.6 | 335 | Apigenin-6-C-glucoside (Isovitexin) | 431 | 413, 341, 311 |
| 25 | 42.1 | 335 | Apigenin-6,8-C-dipentoside | 533 | 515, 473, 443 |
| 26 | 44.7 | 335 | Apigenin-6,8-C-dipentoside | 533 | 515, 473, 443 |
| Organs | Relative Organ Weight (Organ Weight/Body Weight at Necropsy) | ||||
|---|---|---|---|---|---|
| Control | 250 | 500 | 1000 | 2000 | |
| Liver | 3.03 ± 0.320 | 3.34 ± 0.196 * | 3.30 ± 0.310 * | 3.20 ± 0.222 * | 3.42 ± 0.267 * |
| Kidney (R) | 0.20 ± 0.014 | 0.21 ± 0.005 | 0.22 ± 0.013 | 0.21 ± 0.013 | 0.22 ± 0.024 |
| Kidney (L) | 0.20 ± 0.008 | 0.21 ± 0.008 | 0.21 ± 0.018 | 0.21 ± 0.019 | 0.22 ± 0.031 |
| Heart | 0.22 ± 0.018 | 0.24 ± 0.013 | 0.22 ± 0.021 | 0.23 ± 0.020 | 0.21 ± 0.006 |
| Lung | 0.35 ± 0.031 | 0.33 ± 0.021 | 0.37 ± 0.073 | 0.32 ± 0.048 | 0.36 ± 0.042 |
| Ovary (right) | 0.016 ± 0.005 | 0.015 ± 0.004 | 0.018 ± 0.007 | 0.016 ± 0.006 | 0.016 ± 0.003 |
| Ovary (left) | 0.017 ± 0.004 | 0.016 ± 0.007 | 0.020 ± 0.006 | 0.016 ± 0.007 | 0.017 ± 0.004 |
| F. deltoidea Aqueous Extract (mg/kg Body Weight) | |||||||
|---|---|---|---|---|---|---|---|
| Gene Symbol | Gene Name | 250 | 500 | 2000 | |||
| Fold Regulation | p-Value | Fold Regulation | p-Value | Fold Regulation | p-Value | ||
| Abcb1a | ATP-binding cassette, sub-family B (MDR/TAP), member 1A | −61.45 | 0.0047 | −10.16 | 0.0423 | ||
| Abcb1b | ATP-binding cassette, sub-family B (MDR/TAP), member 1B | −2.59 | 0.00002 | ||||
| Abcb4 | ATP binding cassette subfamily B member 4 | 3.94 | 0.0065 | −76.40 | 0.0034 | ||
| Ahr | aryl hydrocarbon receptor | 5.20 | 0.0238 | ||||
| Arnt | aryl hydrocarbon receptor nuclear translocator | −52.80 | 0.0240 | −40.75 | 0.0175 | ||
| Blvra | biliverdin reductase A | −12.00 | 0.0332 | ||||
| Ces2c | carboxylesterase 2C | −49.43 | 0.0481 | ||||
| Cyb5r3 | cytochrome b5 reductase 3 | −116.62 | 0.0300 | ||||
| Cyp19a1 | cytochrome P450 family 19 subfamily A member 1 | −2.59 | 0.00002 | ||||
| Cyp27b1 | cytochrome P450 family 27 subfamily B member 1 | −5.95 | 0.0101 | −36.33 | 0.0067 | ||
| Cyp4b1 | cytochrome P450 family 4 subfamily B member 1 | −52.57 | 0.0086 | ||||
| Faah | fatty acid amide hydrolase | −158.27 | 0.0050 | −166.92 | 0.0051 | ||
| Gad1 | glutamate decarboxylase 1 | −2.59 | 0.00002 | ||||
| Gckr | glucokinase regulator | 7.97 | 0.0062 | ||||
| Gpi | glucose-6-phosphate isomerase | −14.07 | 0.0450 | ||||
| Gpx3 | glutathione peroxidase 3 | −231.63 | 0.0044 | ||||
| Gpx5 | glutathione peroxidase 5 | −32.81 | 0.0413 | −56.78 | 0.0217 | ||
| Gstm1 | glutathione S-transferase mu 1 | 3.27 | 0.0322 | ||||
| Gstt1 | glutathione S-transferase theta 1 | −796.33 | 0.0405 | −28.04 | 0.0476 | −162.61 | 0.0449 |
| Nat1 | N-acetyltransferase 1 | 5.36 | 0.0170 | ||||
| Pkm | Pyruvate Kinase M1/2) | −54.88 | 0.0110 | −68.91 | 0.0115 | ||
| Snn | stannin | −2.59 | 0.00002 | ||||
| Treatment | F. deltoidea Aqueous Extract (mg/kg Body Weight/Day) | ||||
|---|---|---|---|---|---|
| 0 | 250 | 500 | 1000 | 2000 | |
| Foetuses examined (n) | 30 | 30 | 30 | 30 | 30 |
| Litters examined (n) | 5 | 5 | 5 | 5 | 5 |
| Percentage of foetuses showing anomalies (%) and litters affected (%) | |||||
| SKULL | |||||
| Os.Parietale (incpl.ossif.) | 13.33 (55.56) | 12.67 (52.78) | 14.67 (61.11) | 17.33 (72.22) | 16 (66.67) |
| Os.Frontale (incpl.ossif.) | 1.33 (5.56) | 2 (8.33) | 8.67 * (36.11) | 12.67 * (52.78) | 6.67 * (27.78) |
| Os.Occipitale (incpl.ossif.) | 2.67 (11.11) | 7.33 * (30.56) | 6.67 (27.78) | 4.67 (19.44) | 6.67 (27.78) |
| Os. Interparietale (ad.ossif.) | 7.33 (30.56) | 8.67 (36.11) | 15.33 * (63.89) | 16.67 * (69.44) | 17.33 * (72.22) |
| Os hyoid (absent) (incpl.ossif) | 0.67 (2.78) | 8.67 * (36.11) | 3.33 (13.89) | 10 * (41.67) | 2 (8.33) |
| 4.67 (19.44) | 2 (8.33) | 2.67 (11.11) | 1.33 (5.56) | 3.33 (13.89) | |
| Proc. Jugalis maxilla (incpl.ossif.) | 2 (8.33) | 3.33 (13.89) | 3.33 (13.89) | 4.67 (19.44) | 2 (8.33) |
| Os. Zygomatic (incpl.ossif.) | 3.33 (13.89) | 4 (16.67) | 4.67 (19.44) | 1.33 (5.56) | 5.33 (22.22) |
| STERNUM | |||||
| All stenerbrae (split) | 0 | 0 | 0 | 0 | 0 |
| (misaligned) | 0 | 0 | 0.67 (2.78) | 0 | 0 |
| Sternebra 1 (split) | 0 | 0 | 0 | 0 | 0 |
| (incpl.ossif.) | 0 | 0.67 (2.78) | 0 | 0 | 0 |
| (misaligned) | 0 | 0 | 0 | 0 | 0 |
| Sternebra 2 (misshap.) | 0 | 0 | 0 | 0 | 0 |
| (smaller) | 0 | 0 | 0.67 (2.78) | 0 | 0 |
| (incpl.ossif.) | 0 | 0.67 (2.78) | 0 | 0 | 0.67 (2.78) |
| (misaligned) | 0 | 0 | 0 | 1.33 (5.56) | 0.67 (2.78) |
| Sternebra 3 (misshap.) | 0 | 0 | 0 | 0 | 0 |
| (smaller) | 0 | 0 | 0 | 0 | 0 |
| (incpl.ossif.) | 0.67 (2.78) | 0 | 0 | 0 | 2 (8.33) |
| (misaligned) | 0 | 0 | 0.67 (2.78) | 2 (8.33) | 2.67 (11.11) |
| Sternebra 4 (misshap.) | 0 | 0 | 0 | 0 | 0 |
| (incpl.ossif) | 0.67 (2.78) | 0 | 0 | 0 | 0 |
| (misaligned) | 0 | 0 | 0.67 (2.78) | 2 (8.33) | 2 (8.33) |
| Sternebra 5 (misshap.) | 0.67 (2.78) | 0 | 0 | 4 (16.67) | 4 (16.67) |
| (smaller) | 0 | 0 | 0 | 0 | 0 |
| (incpl.ossif.) | 3.33 (13.89) | 5.33 (22.22) | 0.67 (2.78) | 4.67 (19.44) | 5.33 (22.22) |
| (absent) | 0.67 (2.78) | 0.67 (2.78) | 0 | 0.67 (2.78) | 1.33 (5.56) |
| (misaligned) | 0.67 (2.78) | 0 | 0.67 (2.78) | 1.33 (5.56) | 0.67 (2.78) |
| Xiphisternum (split) | 0 | 0 | 0 | 0 | 0 |
| (incpl.ossif.) | 1.33 (5.56) | 1.33 (5.56) | 0.67 (2.78) | 0 | 8 * (33.33) |
| (absent) | 0 | 0 | 0 | 1.33 (5.56) | 0 |
| RIBS | |||||
| (fused) | 0 | 0 | 0 | 0 | 0 |
| (wavy) | 4 (16.67) | 2.67 (11.11) | 4.67 (19.44) | 2.67 (11.11) | 6.67 (27.78) |
| (incpl.ossif) | 1.33 (5.56) | 1.33 (5.56) | 3.33 (13.89) | 1.33 (5.56) | 2 (8.33) |
| 13th rib (short) | 0 | 0 | 0 | 0 | 0 |
| Supernumery ribs (short) | 0 | 0 | 0 | 0 | 0 |
| (both sides) | 0 | 0 | 0 | 0 | 0 |
| (one sides) | 0 | 0 | 0 | 0 | 0 |
| 14th Rib (rudimentary) | 0 | 0 | 0 | 0 | 0 |
| (both sides) | 0.67 (2.78) | 0.67 (2.78) | 1.33 (5.56) | 0.67 (2.78) | 0 |
| (one side) | 0.67 (2.78) | 0.67 (2.78) | 0 | 1.33 (5.56) | 0.67 (2.78) |
| VERTEBRAL COLUMN | |||||
| Atlas (misshap) | 0 | 0 | 0 | 0 | 0.67 (2.78) |
| (incpl.ossif.) | 0.67 (2.78) | 1.33 (5.56) | 2.67 (11.11) | 0.67 (2.78) | 2 (8.33) |
| Thoracic verto.c. (dumbbell) (bipartite) (hemicentric) (split) | 4 (16.67) | 2.67 (11.11) | 11.33 * (47.22) | 11.3 * (47.22) | 8.67 (36.11) |
| 0 | 0 | 0 | 0 | 0.67 (2.78) | |
| 0 | 0 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 4 (16.67) | 4 (16.67) | |
| Lumbar vert (dumbbell) | 0 | 0 | 0.67 (2.78) | 0 | 0 |
| (bipartite) | 0 | 0 | 0 | 1.33 (5.56) | 0.67 (2.78) |
| (split) | 0 | 0 | 0 | ||
| FORELIMBS | |||||
| Fingers (poorly ossified) | 19.33 (80.56) | 18.67 (77.78) | 16.67 (69.44) | 18 (75.00) | 18 (75.00) |
| Os humerus (incpl.ossif.) | 0 | 2 (8.33) | 2 (8.33) | 0 | 0.67 (2.78) |
| HINDLIMBS | |||||
| Os femur (misshap.) (incpl.ossif) | 1.33 (5.56) | 7.33 * (30.56) | 5.33 (22.22) | 7.33 * (30.56) | 8 * (33.33) |
| 0 | 2 (8.33) | 0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, H.; Nik Zainuddin, N.A.S.; Md Saad, W.M.; Omar, M.H.; Lokman, E.F. Impact of Ficus deltoidea Aqueous Extract on Maternal Hepatic Drug Metabolism and Foetal Development in Rats. Plants 2025, 14, 3623. https://doi.org/10.3390/plants14233623
Muhammad H, Nik Zainuddin NAS, Md Saad WM, Omar MH, Lokman EF. Impact of Ficus deltoidea Aqueous Extract on Maternal Hepatic Drug Metabolism and Foetal Development in Rats. Plants. 2025; 14(23):3623. https://doi.org/10.3390/plants14233623
Chicago/Turabian StyleMuhammad, Hussin, Nik Aina Syazana Nik Zainuddin, Wan Mazlina Md Saad, Maizatul Hasyima Omar, and Ezarul Faradianna Lokman. 2025. "Impact of Ficus deltoidea Aqueous Extract on Maternal Hepatic Drug Metabolism and Foetal Development in Rats" Plants 14, no. 23: 3623. https://doi.org/10.3390/plants14233623
APA StyleMuhammad, H., Nik Zainuddin, N. A. S., Md Saad, W. M., Omar, M. H., & Lokman, E. F. (2025). Impact of Ficus deltoidea Aqueous Extract on Maternal Hepatic Drug Metabolism and Foetal Development in Rats. Plants, 14(23), 3623. https://doi.org/10.3390/plants14233623







