The Potential of Pontederia crassipes to Remediate Heavy Metals in Water
Abstract
1. Introduction
2. Research Progress and Current Status
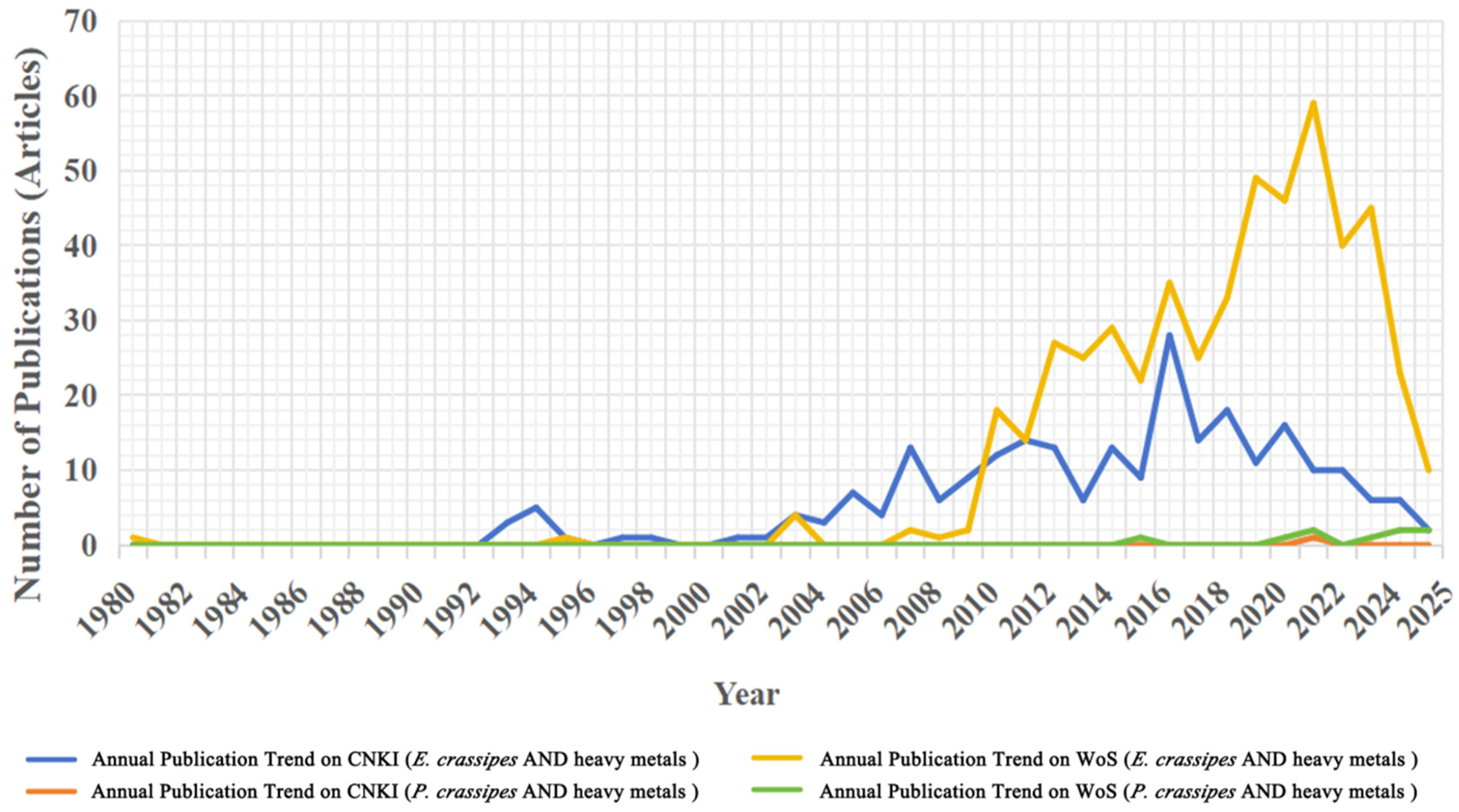
2.1. Bibliometric Data Integration Strategy and Research Progress
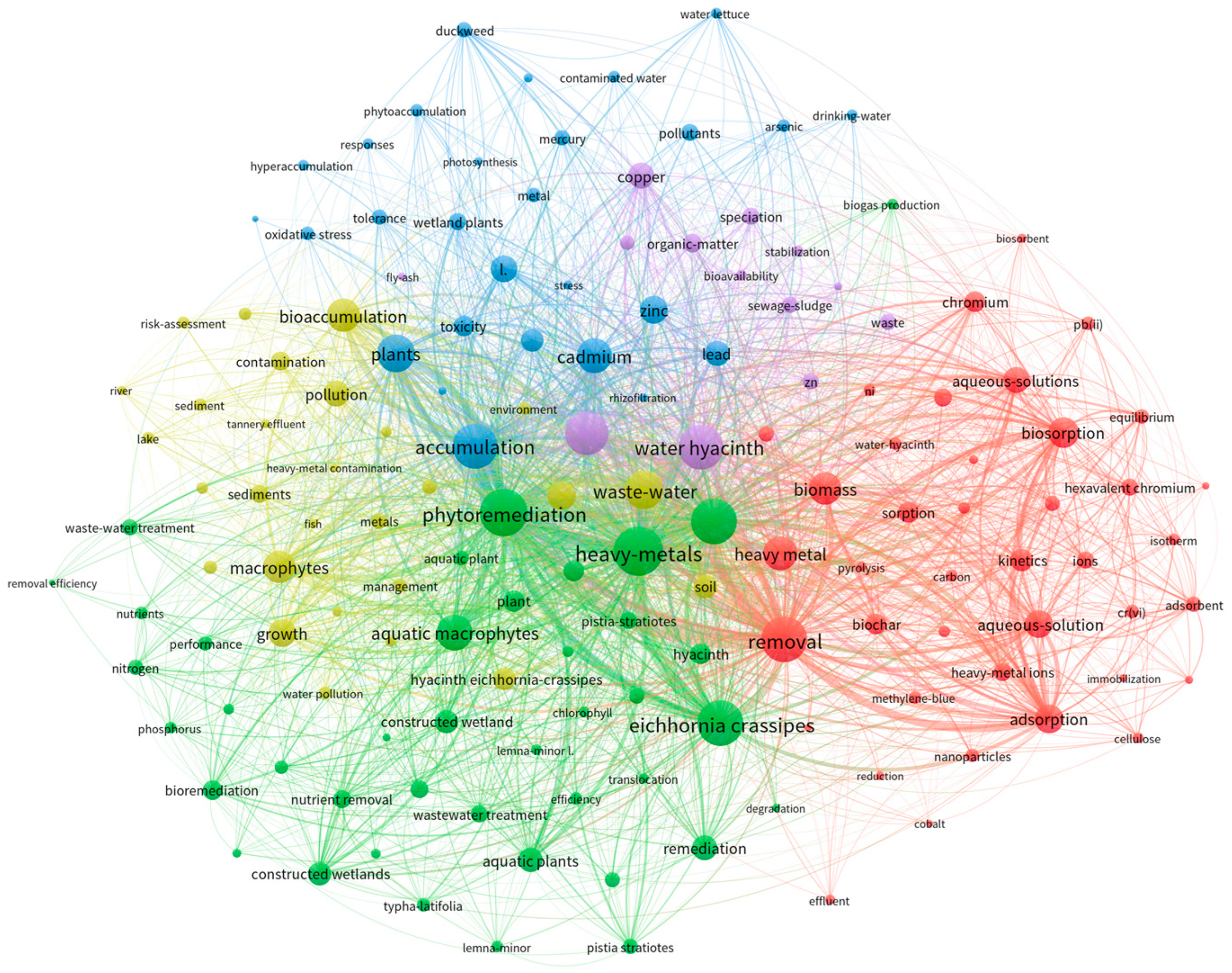
2.2. Bibliometric Analysis and Publication Statistics
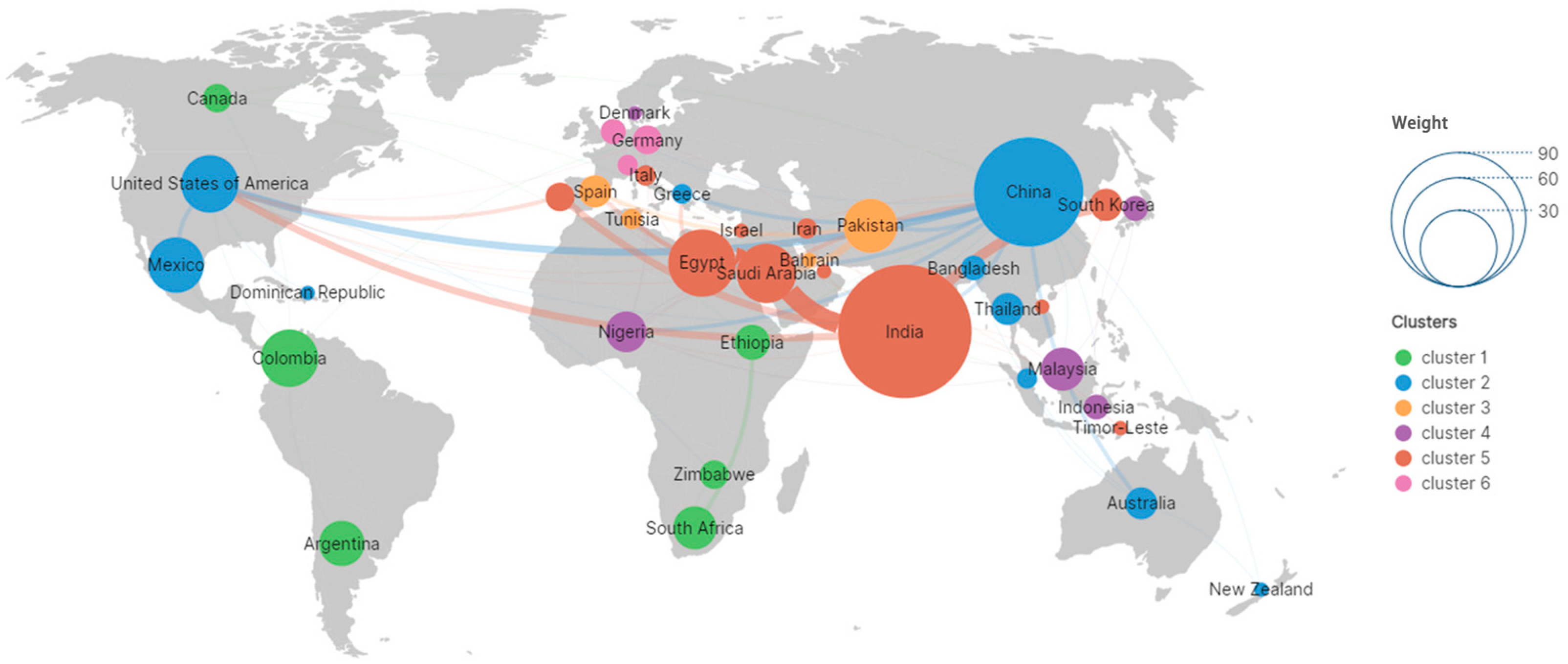
2.3. International Collaboration and Linkages
3. Remediation Efficacy of Heavy Metals by P. crassipes
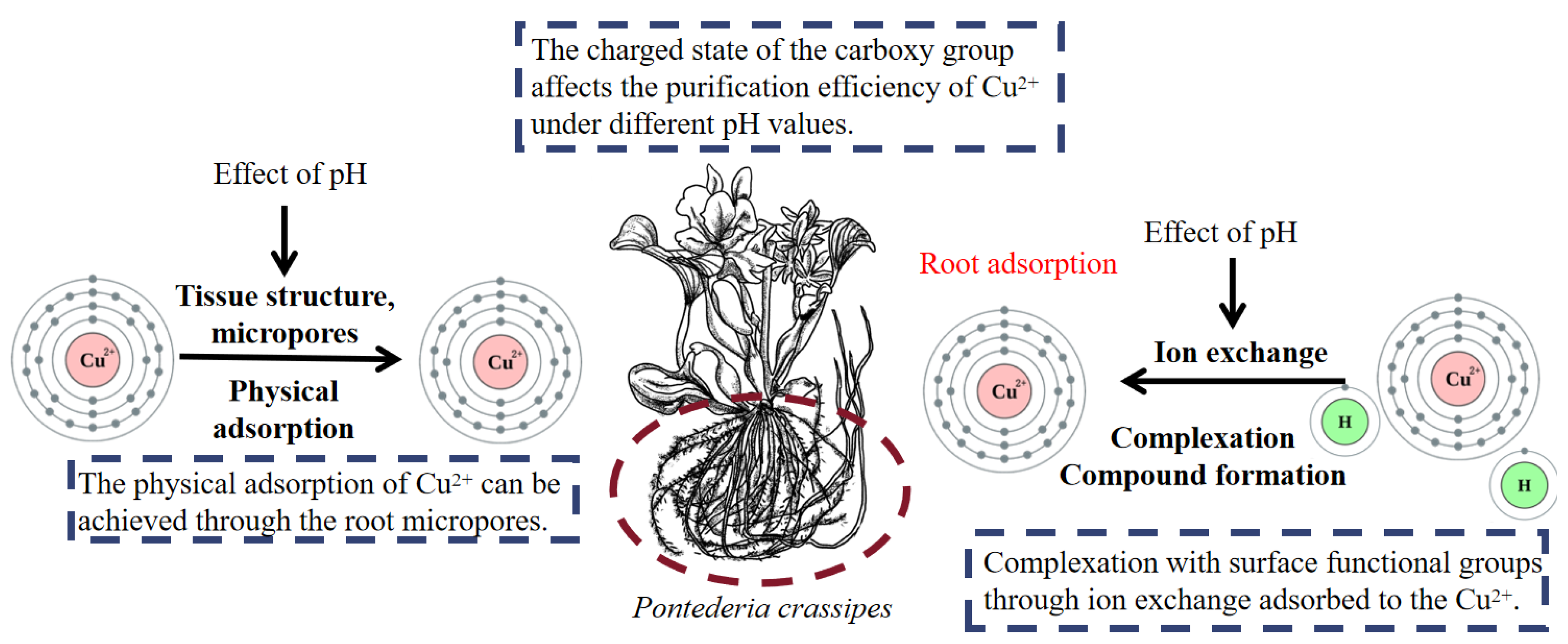
3.1. Copper (Cu)
3.2. Chromium (Cr)
3.3. Lead (Pb)
3.4. Zinc (Zn)
3.5. Remediation Efficacy for Multi-Metal Contamination
4. Advantages and Limitations of P. crassipes in Remediation of Heavy Metal-Contaminated Water
4.1. Advantages of P. crassipes in Heavy Metal Remediation
4.1.1. Ecological Adaptability and Remediation Capacity of P. crassipes
4.1.2. Ecosystem Service Functions and Ecological Risks of P. crassipes
4.1.3. Enhanced Remediation Efficiency Using P. crassipes-Derived Biochar
| Pyrolysis Feedstock | Pyrolysis Temperature (°C) | Adsorption Mechanism | Solution pH | Target Heavy Metal | Maximum Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|---|---|
| P. crassipes, iron salts, K2CO3 | 300~500 | Monolayer chemisorption | 2.0 | Cr | 18.50 | [39] |
| P. crassipes | 393 | Electrostatic attraction | 7.0 | Cu | 177.66 | [62] |
| 5.0 | Pb | 195.24 | ||||
| 9.0 | Cd | 142.59 | ||||
| 6.0 | Zn | 146.14 | ||||
| P. crassipes, sludge | 300~500 | Electron donor–acceptor interaction | ---- | Cr | 44.96 | [63] |
4.2. Limitations of P. Crassipes in Remediation of Heavy Metal-Contaminated Water
4.2.1. Ecological Risks of P. crassipes
4.2.2. Secondary Pollution Risk of P. crassipes
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Kumar, V.; Pandita, S.; Singh, S.; Bhardwaj, R.; Varol, M.; Rodrigo-Comino, J. A Global Meta-Analysis of Toxic Metals in Continental Surface Water Bodies. J. Environ. Chem. Eng. 2023, 11, 109964. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO Press: Geneva, Switzerland, 2017. [Google Scholar]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Anglana, C.; Barozzi, F.; Capaci, P.; Migoni, D.; Rojas, M.; Fanizzi, F.P.; Di Sansebastiano, G.P. Characterization of three species of aquatic mosses in axenic culture for biomonitoring and biotechnological applications. Aquat. Bot. 2024, 193, 103762. [Google Scholar] [CrossRef]
- Anglana, C.; Capaci, P.; Barozzi, F.; Migoni, D.; Rojas, M.; Stigliano, E.; Fanizzi, F.P.; Di Sansebastiano, G.P.; Papadia, P. Dittrichia viscosa Selection Strategy Based on Stress Produces Stable Clonal Lines for Phytoremediation Applications. Plants 2023, 12, 2499. [Google Scholar] [CrossRef]
- Papadia, P.; Barozzi, F.; Angilé, F.; Migoni, D.; Piro, G.; Fanizzi, F.P.; Di Sansebastiano, G.P. Evaluation of Dittrichia viscosa performance in substrates with moderately low levels of As and Cd contamination. Plant Biosyst. 2020, 154, 983–989. [Google Scholar] [CrossRef]
- Aida Abdali, D.; Alaei, E.; Azadi, P.; Shavandi, M.; Amir Mousavi, S. Testing the ability of Vetiveria zizanioides plants to bind cadmium and its influence on soil microbial diversity. Asian J. Water Environ. Pollut. 2025, 22, 32–42. [Google Scholar] [CrossRef]
- Shi, H.; Luo, S.; Liang, Y.; Qin, L.; Zeng, H.; Song, X. Synergistic Removal of β-Hexachlorocyclohexane from Water via Microorganism–Plant Technology and Analysis of Bacterial Community Characteristics. Water 2023, 15, 2328. [Google Scholar] [CrossRef]
- López Arias, T.R.; Franco, D.; Medina, L.; Benítez, C.; Villagra, V.; McGahan, S.; Duré, G.M.; Kurita-Oyamada, H.G. Removal of Chromium (III) and Reduction in Toxicity in a Primary Tannery Effluent Using Two Floating Macrophytes. Toxics 2024, 12, 152. [Google Scholar] [CrossRef]
- Martins, A.S.D.; Huarancca Reyes, T.; Guglielminetti, L.; Damiani, C.R. Screening of In Vitro Heavy Metal Tolerance in Tocoyena brasiliensis Mart. (Rubiaceae). Plants 2025, 14, 1331. [Google Scholar] [CrossRef]
- Wang, T.J.; Ding, Z.Y.; Hua, Z.W.; Yuan, Z.W.; Niu, Q.H.; Zhang, H. Investigation into the Enhancement Effects of Combined Bioremediation of Petroleum-Contaminated Soil Utilizing Immobilized Microbial Consortium and Sudan Grass. Toxics 2025, 13, 599. [Google Scholar] [CrossRef]
- Mishra, V.K.; Tripathi, B.D. Concurrent Removal and Accumulation of Heavy Metals by the Three Aquatic Macrophytes. Bioresour. Technol. 2008, 99, 7091–7097. [Google Scholar] [CrossRef]
- De Laet, C.; Matringe, T.; Petit, E.; Grison, C. Eichhornia crassipes: A Powerful Bio-Indicator for Water Pollution by Emerging Pollutants. Sci. Rep. 2019, 9, 7326. [Google Scholar] [CrossRef]
- Strotmann, U.; Pastor Flores, D.; Konrad, O.; Gendig, C. Bacterial Toxicity Testing: Modification and Evaluation of the Luminescent Bacteria Test and the Respiration Inhibition Test. Processes 2020, 8, 1349. [Google Scholar] [CrossRef]
- Strotmann, U.; Durand, M.; Thouand, G.; Eberlein, C.; Heipieper, H.J.; Gartiser, S.; Pagga, U. Microbiological Toxicity Tests Using Standardized ISO/OECD Methods—Current State and Outlook. Appl. Microbiol. Biotechnol. 2024, 108, 454. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Che, G. Remediation of Heavy Metals in Wastewater by Eichhornia crassipes. J. Environ. Chem. 1987, 2, 43–50. (In Chinese) [Google Scholar]
- Lu, X.; Kruatrachue, M.; Pokethitiyook, P.; Homyok, K. Removal of Cadmium and Zinc by Water Hyacinth, Eichhornia crassipes. ScienceAsia 2004, 30, 93–103. [Google Scholar] [CrossRef]
- Rezania, S.; Ponraj, M.; Talaiekhozani, A.; Mohamad, S.E.; Din, M.F.M.; Taib, S.M.; Sabbagh, F.; Sairan, F.M. Perspectives of Phytoremediation Using Water Hyacinth for Removal of Heavy Metals, Organic and Inorganic Pollutants in Wastewater. J. Environ. Manag. 2015, 163, 125–133. [Google Scholar] [CrossRef]
- Ma, F.; Wang, H.; Tzachor, A.; Hidalgo, C.A.; Schandl, H.; Zhang, Y.; Zhang, J.; Chen, W.Q.; Zhao, Y.; Zhu, Y.G.; et al. The Disparities and Development Trajectories of Nations in Achieving the Sustainable Development Goals. Nat. Commun. 2025, 16, 1107. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Wang, Y.; Cui, X.; Dong, J.; Gu, P.; Hao, Y.; Xue, K.; Duan, H.; Xia, A.; et al. Overlooked Uneven Progress Across Sustainable Development Goals at the Global Scale: Challenges and Opportunities. Innovation 2024, 5, 100573. [Google Scholar] [CrossRef]
- Pang, Y.L.; Quek, Y.Y.; Lim, S.; Shuit, S.H. Review on Phytoremediation Potential of Floating Aquatic Plants for Heavy Metals: A Promising Approach. Sustainability 2023, 15, 1290. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Z.; Song, R.; Fang, C.; Sindersberger, D.; Monkman, G.J.; Guo, J. Time-Dependent Electroadhesive Force Degradation. Smart Mater. Struct. 2020, 29, 055009. [Google Scholar] [CrossRef]
- Bartlett, D.F.; Goldhagen, P.E.; Phillips, E.A. Experimental Test of Coulomb’s Law. Phys. Rev. D 1970, 2, 483. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Y.; Zhang, J. Adsorption of Cu2+ and Pb2+ from Aqueous Solution with Water Hyacinth. Technol. Water Treat. 2015, 41, 56–61, (In Chinese with English Abstract). [Google Scholar]
- Wang, Z.; Wen, Y.; Huang, Z.; Li, H. Adaptability of Several Plants to Heavy Metal Wastewater Treatment. J. Ecol. Environ. Sci. 2005, 14, 540–544, (In Chinese with English Abstract). [Google Scholar]
- Chen, X.; Wei, Z.Y.; Zhao, H.B.; Wu, G.Z.; Liu, L.L. Absorption Characteristics and Mechanisms of Ofloxacin-Cu by Activated Carbon Based on Long-Root Eichhornia crassipes. Agric. Resour. Environ. 2020, 37, 59–65, (In Chinese with English Abstract). [Google Scholar]
- Yang, J.; Zheng, J.; Tang, M. Removal of Cu2+ in Aqueous Media by Biosorption Using Water Hyacinth Roots. Ningxia Agric. For. Sci. Technol. 2012, 53, 110–114, (In Chinese with English Abstract). [Google Scholar]
- Sharma, K.; Saxena, P.; Kumari, A. Comparative Study of Chromium Phytoremediation by Two Aquatic Macrophytes. Bull. Environ. Contam. Toxicol. 2023, 111, 16. [Google Scholar] [CrossRef]
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A Critical Assessment of Chromium in the Environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Jin, L.F.; Yuan, Y.M.; Hu, Y.R.; Ni, J. Research Progress in Cytotoxicity and Mechanism of Hexavalent Chromium. Chin. J. Cell Biol. 2013, 35, 142–147, (In Chinese with English Abstract). [Google Scholar]
- Mondal, N.K.; Nayek, P. Hexavalent Chromium Accumulation Kinetics and Physiological Responses Exhibited by Eichhornia sp. and Pistia sp. Int. J. Environ. Sci. Technol. 2020, 17, 1397–1410. [Google Scholar] [CrossRef]
- Li, W.; Du, N.; Zhou, J.; Qin, J.; Li, H.; Chen, G. Simulation of Ecotoxicological Effects of Perchlorate and Hexavalent Chromium Combined Pollution in the Aquatic Ecosystem. J. South China Agric. Univ. Nat. Sci. Ed. 2024, 45, 52–59, (In Chinese with English Abstract). [Google Scholar]
- Seguil, Y.P.; Garay, L.V.; Cortez, C.M. Synthesis of Eichhornia crassipes Biochar: Sustainable Efficient Adsorbent for Reducing Cr (VI) Metal Ion. J. Phys. Conf. Ser. 2020, 1539, 012003. [Google Scholar] [CrossRef]
- Tan, Y.; Xing, Y.; Li, M.; Li, F.; Shi, X.; Yan, X. Research Progress of Common Methods for Degradation of Hexavalent Chromium and Its Adsorption Method. J. Mater. Sci. 2023, 13, 794, (In Chinese with English Abstract). [Google Scholar]
- Hayyat, M.U.; Nawaz, R.; Irfan, A.; Al-Hussain, S.A.; Aziz, M.; Siddiq, Z.; Ahmad, S.; Zaki, M.E.A. Evaluating the Phytoremediation Potential of Eichhornia crassipes for the Removal of Cr and Li from Synthetic Polluted Water. Int. J. Environ. Res. Public Health 2023, 20, 3512. [Google Scholar] [CrossRef]
- Mishra, V.K.; Tripathi, B.D. Accumulation of Chromium and Zinc from Aqueous Solutions Using Water Hyacinth (Eichhornia crassipes). J. Hazard. Mater. 2009, 164, 1059–1063. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, X.; Zhang, H.; Zeng, H.; Lu, Y.; Shi, Q. Adsorption Performance of Magnetic Eichhornia crassipes-Derived Biochar for Hexavalent Chromium in Wastewater. J. Guilin Univ. Technol. 2020, 40, 193–200, (In Chinese with English Abstract). [Google Scholar]
- Tan, C.; Lin, Y.; Chen, Z. The Use of Eichhornia crassipes for the Removal of Heavy Metals from Aqueous Solutions. J. Subtrop. Resour. Environ. 2009, 5, 123–128, (In Chinese with English Abstract). [Google Scholar]
- Santana, C.S.; de Almeida, O.N.; Luzardo, F.H.; Tokumoto, M.S.; Velasco, F.G. Application of the KIM Equation for Direct Analysis of Pb and Ni by EDXRF in Lignocellulosic Fibers Used as Adsorbents of Metals. Environ. Technol. Innov. 2020, 17, 100534. [Google Scholar] [CrossRef]
- Manna, S.; Roy, D.; Saha, P.; Adhikari, B. Defluoridation of Aqueous Solution Using Alkali-Steam Treated Water Hyacinth and Elephant Grass. J. Taiwan Inst. Chem. Eng. 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Kong, L.J.; Hu, X.L.; Xie, Z.Y.; Ren, X.Y.; Long, J.Y.; Su, M.H.; Diao, Z.H.; Chen, D.Y.; Shih, K.M.; Hou, L.A. Accelerated Phosphorus Recovery from Aqueous Solution onto Decorated Sewage Sludge Carbon. Sci. Rep. 2018, 8, 13421. [Google Scholar] [CrossRef]
- Zhou, J.M.; Jiang, Z.C.; Qin, X.Q.; Zhang, L.K.; Huang, Q.B.; Xu, G.L.; Dionysiou, D.D. Efficiency of Pb, Zn, Cd, and Mn Removal from Karst Water by Eichhornia crassipes. Int. J. Environ. Res. Public Health 2020, 17, 5329. [Google Scholar] [CrossRef]
- Malar, S.; Shivendra Vikram, S.; Favas, P.J.C.; Perumal, V. Lead Heavy Metal Toxicity Induced Changes on Growth and Antioxidative Enzymes Level in Water Hyacinths [Eichhornia crassipes (Mart.)]. Bot. Stud. 2014, 55, 54. [Google Scholar] [CrossRef]
- Chen, W.P.; Xu, S.Y.; Zu, Z.; Yang, H.J.; Huang, B.Y.; Wang, Y. Effect of Purple Root Water Hyacinth (Eichhornia crassipes) on Purification of Water Containing Heavy Metals. Chin. J. Environ. Eng. 2016, 10, 2284–2290, (In Chinese with English Abstract). [Google Scholar]
- Mahamadi, C.; Nharingo, T. Utilization of Water Hyacinth Weed (Eichhornia crassipes) for the Removal of Pb(II), Cd(II) and Zn(II) from Aquatic Environments: An Adsorption Isotherm Study. Environ. Technol. 2010, 31, 1221–1228. [Google Scholar] [CrossRef]
- Cheng, B.; Ma, M.; Wang, Z.; Chen, J.; Hong, S. Studies on Acute Toxicity Effects of Heavy Metal Pollutants Collected from the Yangtze and Yellow Rivers. Acta Sci. Nat. Univ. Pekin. 2004, 40, 950–956. (In Chinese) [Google Scholar]
- Yang, Y.; Jia, X. Joint Toxicity of Cu2+, Zn2+ and Cd2+ to Tadpole of Bufo bufo gargarizans. Chin. J. Appl. Environ. Biol. 2006, 12, 356–359, (In Chinese with English Abstract). [Google Scholar]
- Zheng, J. The Performance and Mechanism of Removal of Heavy Metals from Water by Water Hyacinth Roots as a Biosorbent Material. Ph.D. Thesis, University of Science and Technology of China, Hefei, China, 2010. (In Chinese with English Abstract). [Google Scholar]
- Eid, E.M.; Shaltout, K.H.; Almuqrin, A.H.; Aloraini, D.A.; Khedher, K.M.; Taher, M.A.; Alfarhan, A.H.; Picó, Y.; Barcelo, D. Uptake Prediction of Nine Heavy Metals by Eichhornia crassipes Grown in Irrigation Canals: A Biomonitoring Approach. Sci. Total Environ. 2021, 782, 146887. [Google Scholar] [CrossRef]
- Yue, L.; Luo, C.; Wang, J.; Ni, M.; Yang, T.; Huang, X. Effects of Cadmium Stress and Temperature Stress on Several Physiological Indicators of Eichhornia crassipes. J. North. Hortic. 2020, 9, 89–96, (In Chinese with English Abstract). [Google Scholar]
- Lin, R.; Chen, T.; Wang, B.; Lin, W. Adaptation of the Invasive Plant Water Hyacinth (Eichhornia crassipes) to Different Acidic Conditions. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2007, 36, 525–531. [Google Scholar]
- Chen, L.; Li, C.; Li, F.; Chong, Y.; Hu, H.; Gao, S.; Zhou, W.; Sun, Z. Review on Water Purification Ability of Aquatic Ecological Restoration Plants. Environ. Pollut. Control 2022, 44, 1079–1084, (In Chinese with English Abstract). [Google Scholar]
- Cao, W.; Chen, Y.; Yang, B.; Ke, Y.; Li, X.; Wu, R. Experimental Study on Zn Residue Wastewater Purification by Plant-Substrate Constructed Wetland System. J. Lanzhou Jiaotong Univ. 2021, 40, 112–119, (In Chinese with English Abstract). [Google Scholar]
- Mitan, N.M.M. Water Hyacinth: Potential and Threat. Mater. Today Proc. 2019, 19, 1408–1412. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, J.; Liu, H.; Chang, Z.; Chen, L.; Yan, S. Role of Eichhornia crassipes Uptake in the Removal of Nitrogen and Phosphorus from Eutrophic Waters. Jiangsu Agric. Sci. 2010, 3, 251–253, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Dong, Z.; Shi, L.; Zhou, L.; Xie, T.; Shi, G. Organic Fertilizer Composting Technology Using Eichhornia crassipes and Its Application. J. South. Agric. 2010, 41, 1205–1207, (In Chinese with English Abstract). [Google Scholar]
- Sundari, M.T.; Ramesh, A. Isolation and Characterization of Cellulose Nanofibers from the Aquatic Weed Water Hyacinth—Eichhornia crassipes. Carbohydr. Polym. 2012, 87, 1701–1705. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, B.; Chen, M.; Wu, P.; Lee, X.; Xing, Y. Invasive Plants as Potential Sustainable Feedstocks for Biochar Production and Multiple Applications: A Review. Resour. Conserv. Recycl. 2021, 164, 105204. [Google Scholar] [CrossRef]
- Wu, J. Adsorption Properties of Modified Biochar Microspheres on Cd2+ in Water. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2018. (In Chinese with English Abstract). [Google Scholar]
- Zhou, R.; Zhang, M. Adsorption Characteristics of Heavy Metal Ions in Water by Water Hyacinth Biochar. Saf. Environ. Eng. 2022, 29, 168–177, (In Chinese with English Abstract). [Google Scholar]
- Hong, Y.J.; Xu, Z.X.; Feng, C.L.; Xu, D.Y. Co-Pyrolysis of Water Hyacinth and Sewage Sludge for Preparation of Biochar Particles and Its Adsorption Properties for Cr3+. Res. Environ. Sci. 2020, 33, 1052–1061, (In Chinese with English Abstract). [Google Scholar]
- Villamagna, A.M.; Murphy, B.R. Ecological and Socio-Economic Impacts of Invasive Water Hyacinth (Eichhornia crassipes): A Review. Freshw. Biol. 2010, 55, 282–298. [Google Scholar] [CrossRef]
- Jha, R.R.; Li, D. Quantifying the Effects of Water Hyacinth (Pontederia crassipes) on Freshwater Ecosystems: A Meta-Analysis. Biol. Invasions 2025, 27, 1–11. [Google Scholar] [CrossRef]
- Zhou, X.S.; Lou, S.; Radnaeva, L.D.; Nikitina, E.; Wang, H. Advances in Heavy Metal Accumulation Characteristics of Plants in Soil. Asian J. Ecol. Toxicol. 2022, 17, 400–410. [Google Scholar] [CrossRef]
- Chang, Z.; Zheng, J. Ecological Risks and Control Strategies of Eichhornia crassipes (Water Hyacinth) Introduction. J. Jiangsu Agric. Sci. 2008, 3, 251–253. [Google Scholar]
- Degaga, A.H. Water Hyacinth (Eichhornia crassipes) Biology and Its Impacts on Ecosystem, Biodiversity, Economy and Human Well-Being. J. Life Sci. Biomed. 2018, 8, 94–100. [Google Scholar]
- Zhang, W.; Yu, C.; Jin, H.; Huang, H.; Zhao, L. Advanced Progress in Treatment and Resource Utilization of Water Hyacinth. Ecol. Environ. Monit. Three Gorges 2023, 8, 11–16. [Google Scholar] [CrossRef]
- Abba, A.; Sankarannair, S. Global Impact of Water Hyacinth (Eichhornia crassipes) on Rural Communities and Mitigation Strategies: A Systematic Review. Environ. Sci. Pollut. Res. 2024, 31, 43616–43632. [Google Scholar] [CrossRef]
- Faltlhauser, A.C.; Jiménez, N.L.; Righetti, T.; Visintin, A.M.; Torrens, J.; Salinas, N.A.; McKay, F.; Hill, M.; Cordo, H.A.; Sosa, A.J. The Importance of Long-Term Post-Release Studies in Classical Biological Control: Insect-Plant Monitoring and Public Awareness of Water Hyacinth Management (Pontederia crassipes) in Dique Los Sauces, Argentina. Entomol. Exp. Appl. 2023, 171, 965–977. [Google Scholar] [CrossRef]
- Tipping, P.W.; Martin, M.R.; Pokorny, E.N.; Nimmo, K.R.; Fitzgerald, D.L.; Dray, F.A., Jr.; Center, T.D. Current Levels of Suppression of Water Hyacinth in Florida USA by Classical Biological Control Agents. Biol. Control 2014, 71, 65–69. [Google Scholar] [CrossRef]
- Su, W.; Sun, Q.; Xia, M.; Wen, Z.; Yao, Z. The Resource Utilization of Water Hyacinth (Eichhornia crassipes [Mart.] Solms) and Its Challenges. Resources 2018, 7, 46. [Google Scholar] [CrossRef]
- Bergen, S.D.; Bolton, S.M.; Fridley, J.L. Design Principles for Ecological Engineering. Ecol. Eng. 2001, 18, 201–210. [Google Scholar] [CrossRef]
- Jørgensen, S.E. Ecological Engineering: Overview. In Applied Ecological Engineering; Jørgensen, S.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Vaz, A.S.; Kueffer, C.; Kull, C.A.; Richardson, D.M.; Schindler, S.A.; Muñoz-Pajares, J.; Martins, J.V.R.; Hui, C.; Kühn, I.; Honrado, J.P. The Progress of Interdisciplinarity in Invasion Science. Ambio 2017, 46, 428–442. [Google Scholar] [CrossRef] [PubMed]
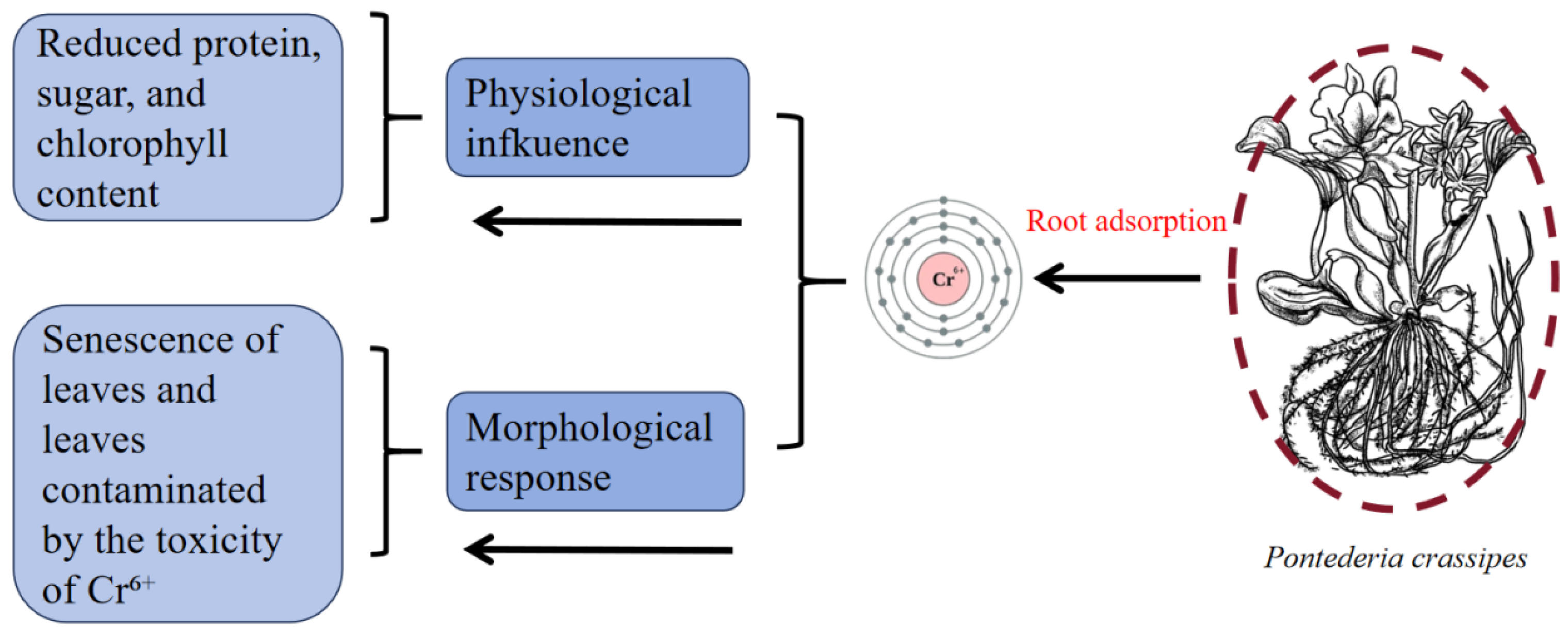
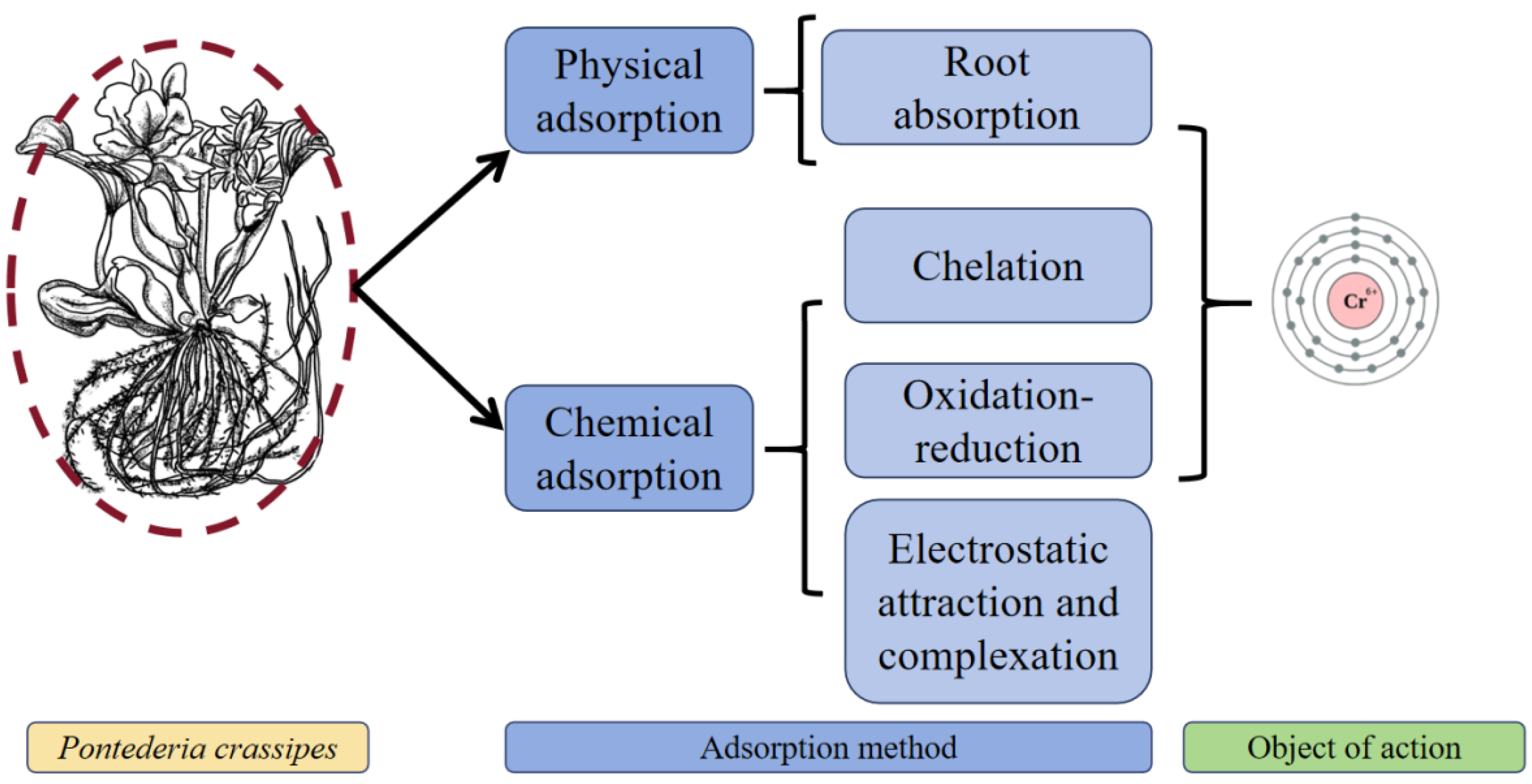
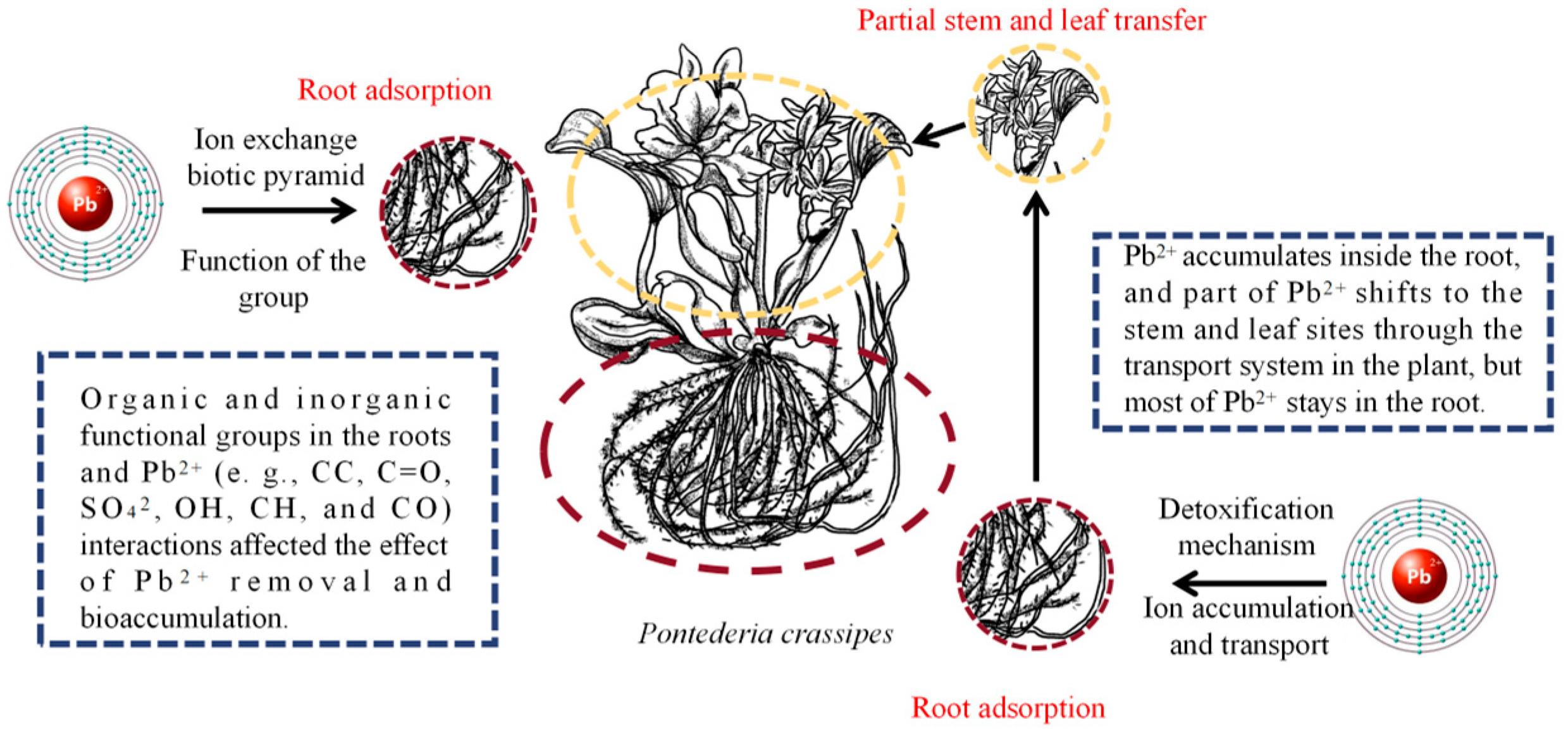
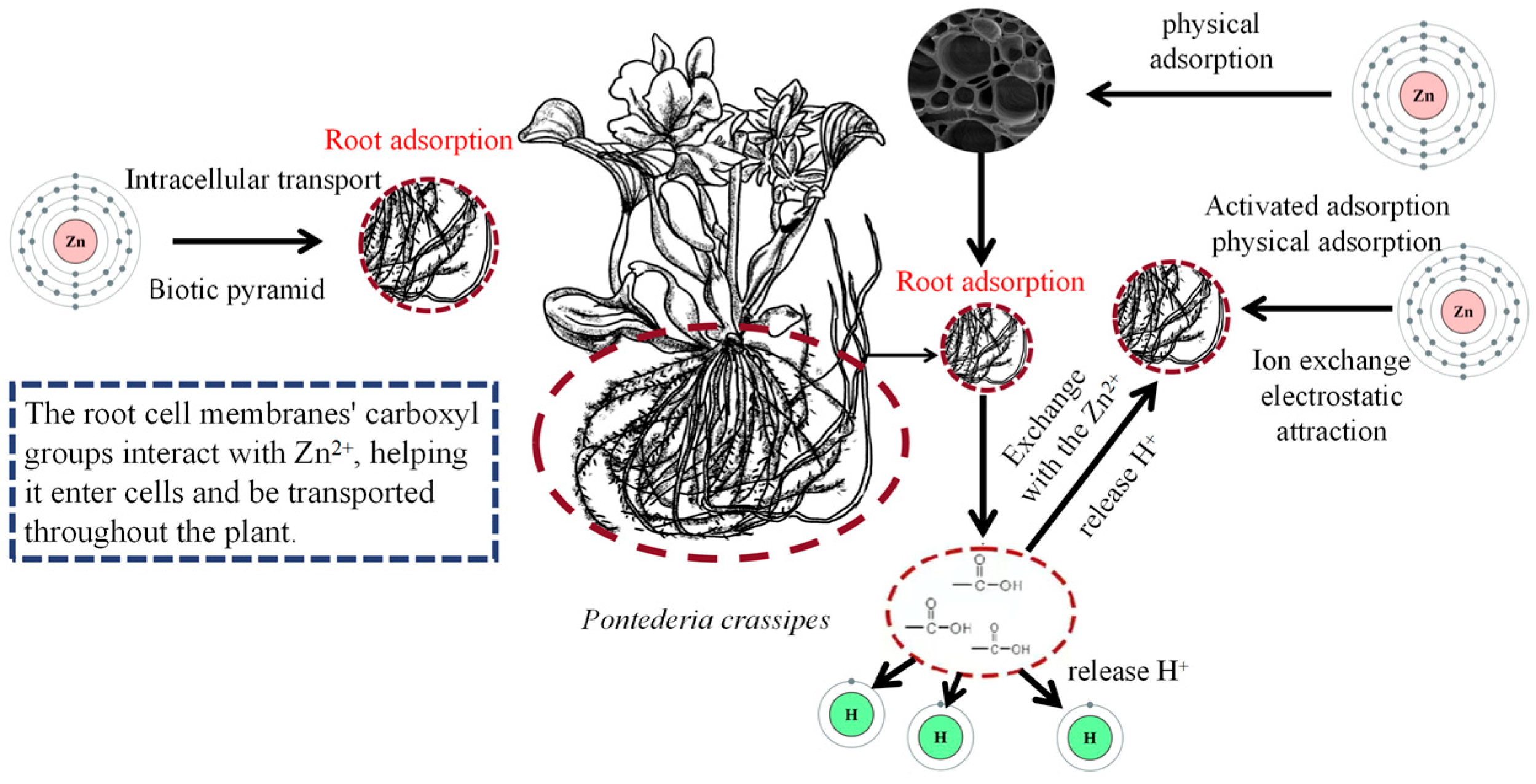
| Heavy Metal | Ionic Form/Complex Compound | Species/Subject | Toxicity Specific | Heavy Metal Treatment Conditions |
|---|---|---|---|---|
| Cr6+ | Cr2O72− (from K2Cr2O7), CrO42−, Phosphate complex | Vibrio fischeri | EC50: 2.65 mg/L | 30 min, luminescence inhibition |
| Activated sludge | EC50: 5.84 mg/L | 30 min, respiration inhibition | ||
| Oryzias latipes | LC50: 2.30 mg/L | 96 h exposure, lethality test | ||
| Daphnia magna | EC50: 3.20 mg/L | 24 h exposure, reproduction inhibition | ||
| Pseudokirchneriella subcapitata | EC50: 0.30 mg/L | 72 h exposure, growth inhibition | ||
| Zn2+ | Zn2+ (from ZnSO4⋅7H2O), Bovine serum albumin complex | Vibrio fischeri | EC50: 22.74 mg/L | 30 min, luminescence inhibition |
| EC50: 20.93 mg/L | 60 min, luminescence inhibition | |||
| Activated sludge | EC50: 30.0 mg/L | 24 h exposure, enzyme activity inhibition | ||
| Brachydanio rerio | LC50: 2.50 mg/L | 96 h exposure, lethality test | ||
| Daphnia magna | EC50: 1.00 mg/L | 24 h exposure, survival inhibition | ||
| Desmodesmus subspicatus | EC50: 0.50 mg/L | 72 h exposure, growth inhibition | ||
| Cu2+ | Cu2+, Complex with metal sites at dehydrogenase active center | Pseudomonas putida | EC50: 21.4 mg/L | 16 h, growth inhibition |
| Activated sludge | EC50: 0.50 mg/L | 24 h exposure, enzyme activity inhibition | ||
| Crassius auratus | LC50: 0.30 mg/L | 24 h exposure, lethality test | ||
| Daphnia magna | EC50: 0.05 mg/L | 24 h exposure, reproduction inhibition | ||
| Pseudokirchneriella subcapitata | EC50: 0.03 mg/L | 72 h exposure, growth inhibition | ||
| Cd2+ | Cd2+, Complex with sludge organic matter | Activated sludge | EC50: 5.0 mg/L | 30 min, respiration inhibition |
| Vibrio fischeri | LC50: 10.0 mg/L | 30 min, luminescence inhibition | ||
| Oryzias latipes | EC50: 0.50 mg/L | 96 h exposure, lethality test | ||
| Daphnia magna | EC50: 0.10 mg/L | 24 h exposure, survival inhibition | ||
| Desmodesmus subspicatus | EC50: 0.08 mg/L | 72 h exposure, growth inhibition | ||
| Ni2+ | Ni2+, Phosphate complex in medium | Activated sludge | EC50: 2.0 mg/L | 24 h exposure, enzyme activity inhibition |
| Vibrio fischeri | EC50: 15.0 mg/L | 30 min, luminescence inhibition | ||
| Brachydanio rerio | LC50: 3.0 mg/L | 96 h exposure, lethality test | ||
| Daphnia magna | EC50: 1.0 mg/L | 24 h exposure, reproduction inhibition | ||
| Pseudokirchneriella subcapitata | EC50: 0.20 mg/L | 72 h exposure, growth inhibition |
| Ranking | Country and Institution | Total Number of Publications | Total Citation Count | Average Citations per Paper |
|---|---|---|---|---|
| C1 | India | 100 | 2941 | 29.41 |
| C2 | China | 68 | 2071 | 30.46 |
| C3 | Egypt | 23 | 792 | 34.43 |
| C4 | Pakistan | 19 | 545 | 26.45 |
| C5 | Saudi Arabia | 19 | 507 | 26.31 |
| I1 | King Khalid University(Saudi Arabia) | 6 | 133 | 22.17 |
| I2 | National Institute of Oceanography and Fisheries (Egypt) | 6 | 113 | 18.83 |
| I3 | King Saud University (Saudi Arabia) | 5 | 55 | 11.00 |
| I4 | Tanta University (Egypt) | 4 | 152 | 38.00 |
| I5 | Government College University (Pakistan) | 4 | 100 | 25.00 |
| Species | Target Site | Purification Mechanism | Cu Solution pH | Peak Purification Efficiency (%) | Reference |
|---|---|---|---|---|---|
| P. crassipes | Root | Ion exchange and complexation | 4.0–6.5 | 75% | [23] |
| P. crassipes | Stem | Electrostatic attraction | 4.5 | 97% | [26] |
| Long-root P. crassipes | Whole plant | Electron donor–acceptor interactions | 6.0 | ---- | [28] |
| P. crassipes | Root | Ion exchange | 4.0–6.5 | 75% | [29] |
| Chromium Concentration in Solution (mg/L) | Root Removal Rate (%) | Stem Removal Rate (%) | Leaf Removal Rate (%) |
|---|---|---|---|
| 2 | 65.0% | 27.4% | 16.4% |
| 4 | 56.0% | --- | --- |
| 6 | 57.0% | --- | --- |
| 8 | 58.0% | 34.0% | 11.0% |
| Material | Purification Mechanism | Peak Efficiency (%) | Cr Solution pH | Adsorption Type | Reference |
|---|---|---|---|---|---|
| Root | Biosorption and phytostabilization | 55% | ---- | Physical adsorption | [37] |
| Root | Root accumulation | 84% | ---- | Physicochemical synergistic adsorption | [38] |
| Whole plant | Redox reaction, electrostatic attraction, and complexation | 85.7% | 2.0 | Physicochemical synergistic adsorption | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Zhang, S.; Wang, X.; Yang, L.; Li, H.; Gao, K. The Potential of Pontederia crassipes to Remediate Heavy Metals in Water. Plants 2025, 14, 3604. https://doi.org/10.3390/plants14233604
Fan Y, Zhang S, Wang X, Yang L, Li H, Gao K. The Potential of Pontederia crassipes to Remediate Heavy Metals in Water. Plants. 2025; 14(23):3604. https://doi.org/10.3390/plants14233604
Chicago/Turabian StyleFan, Yongming, Shilong Zhang, Xiaohua Wang, Lulu Yang, Haiying Li, and Kang Gao. 2025. "The Potential of Pontederia crassipes to Remediate Heavy Metals in Water" Plants 14, no. 23: 3604. https://doi.org/10.3390/plants14233604
APA StyleFan, Y., Zhang, S., Wang, X., Yang, L., Li, H., & Gao, K. (2025). The Potential of Pontederia crassipes to Remediate Heavy Metals in Water. Plants, 14(23), 3604. https://doi.org/10.3390/plants14233604






