Phenotypic and Metabolic Variations Induced by Autopolyploidization in Chinese Jujube Cultivars
Abstract
1. Introduction
2. Results
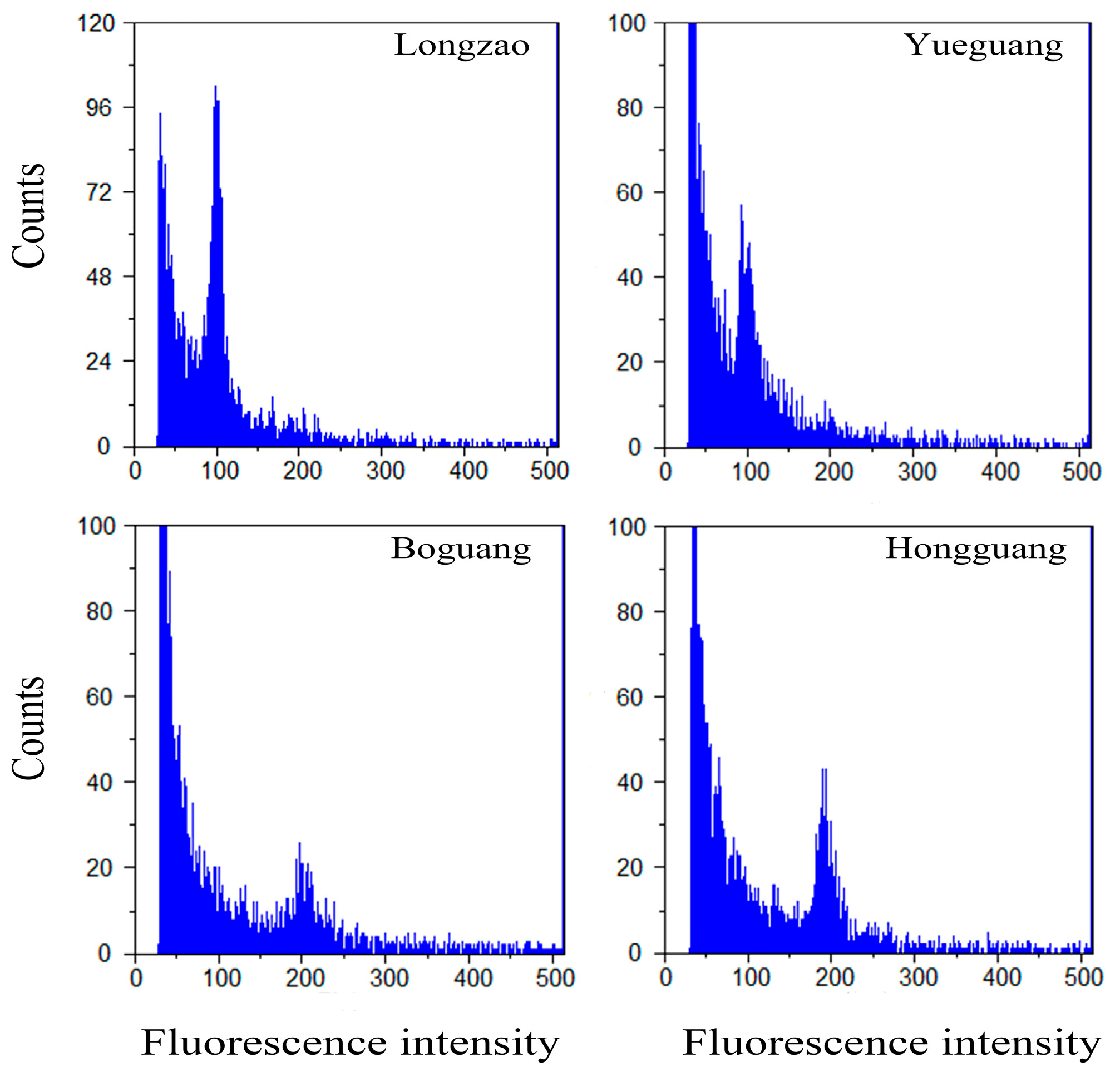
2.1. Ploidy Confirmation in Fruit by Flow Cytometry
2.2. Morphological Changes After Autopolyploidization
2.2.1. Changes in Tree Vigor
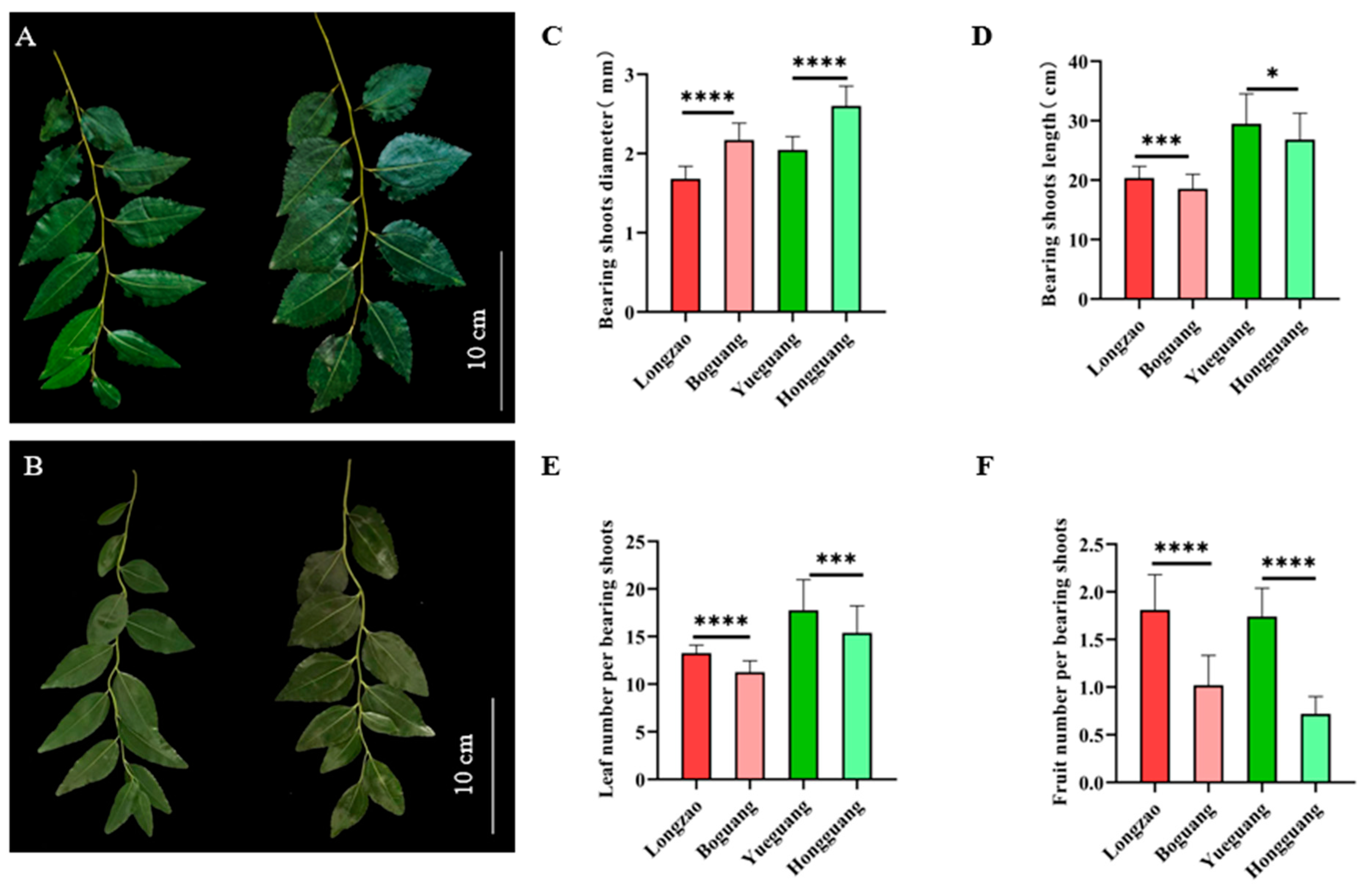
2.2.2. Changes in Bearing Shoots
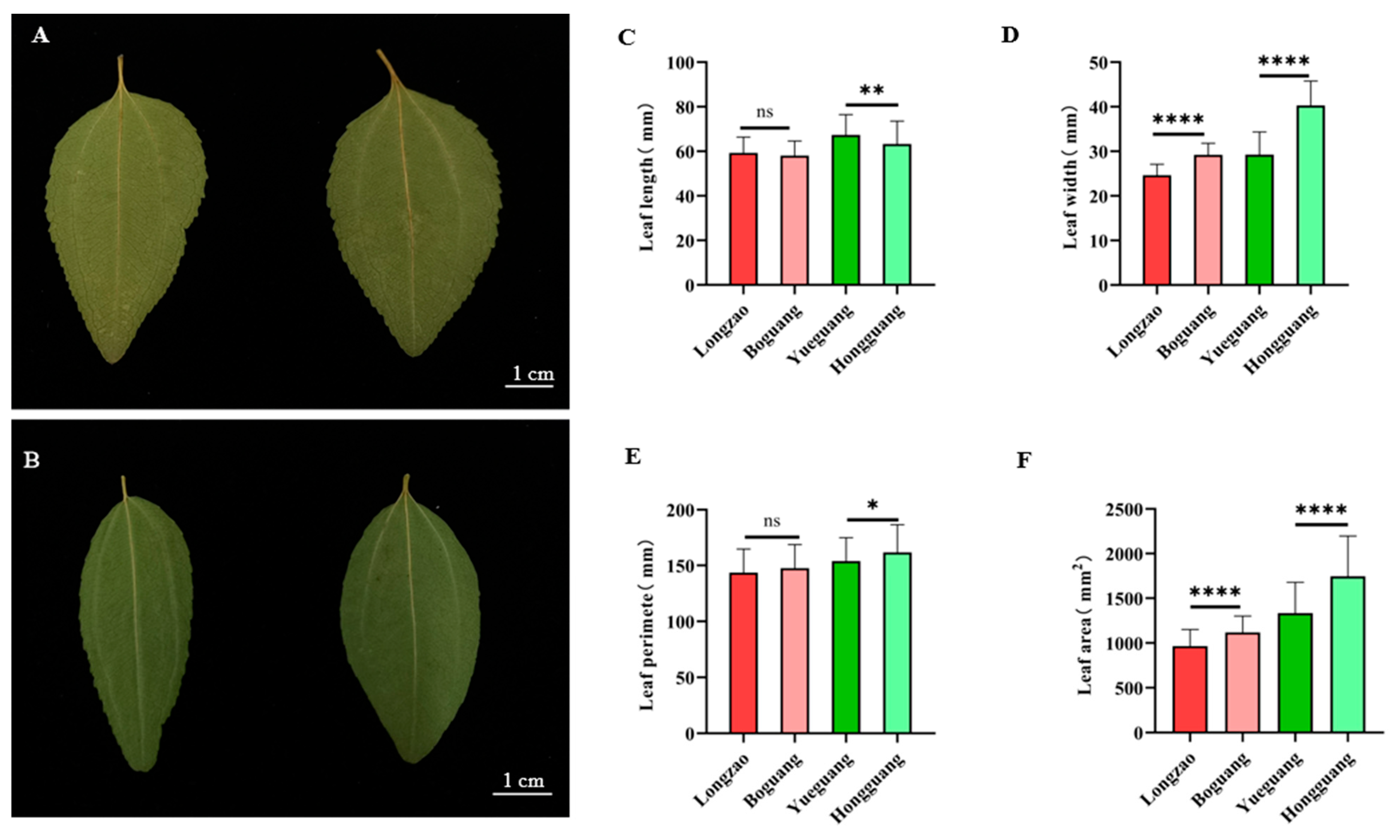
2.2.3. Changes in Leaf
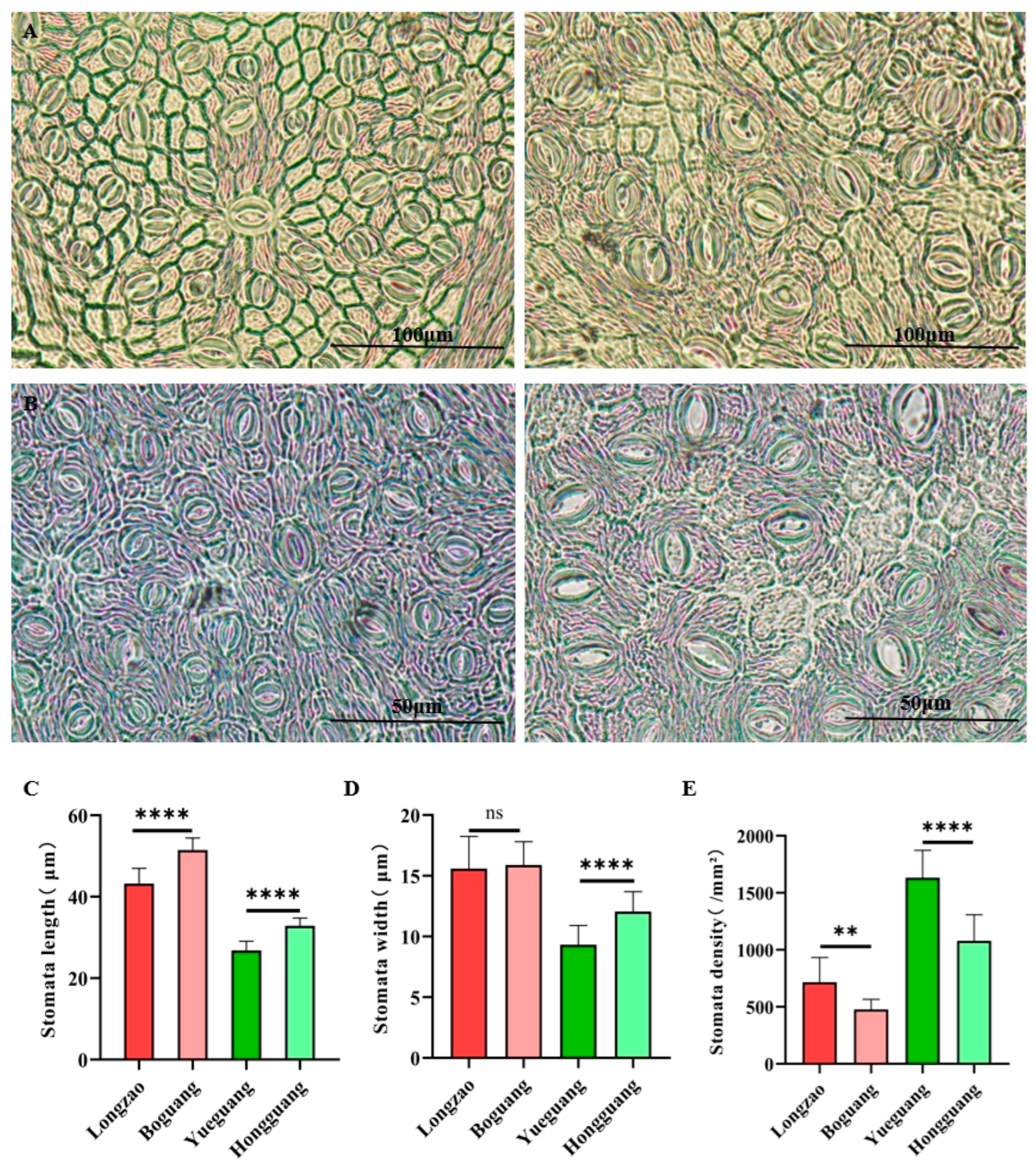
2.2.4. Changes in Stomata
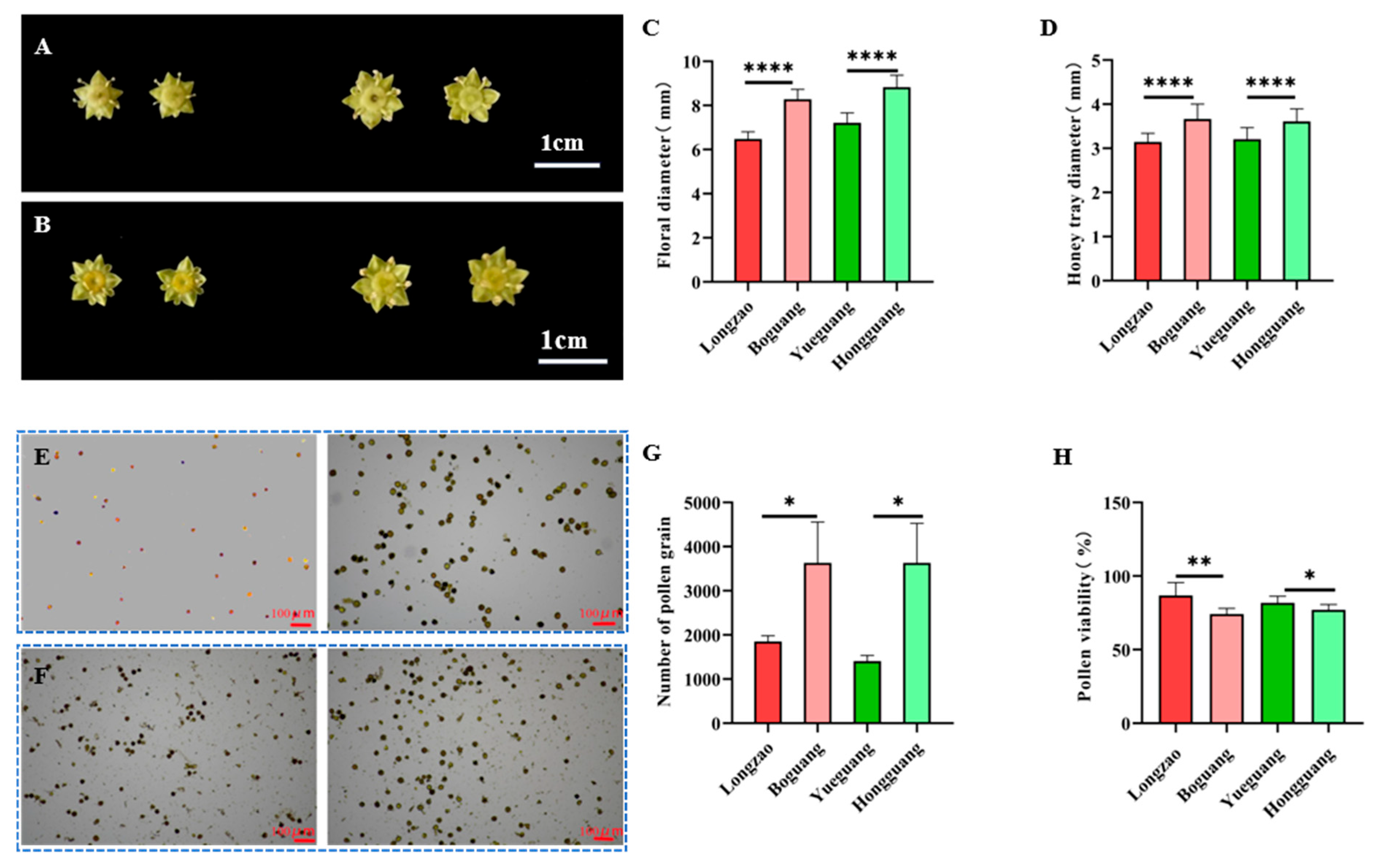
2.2.5. Changes in Flowers
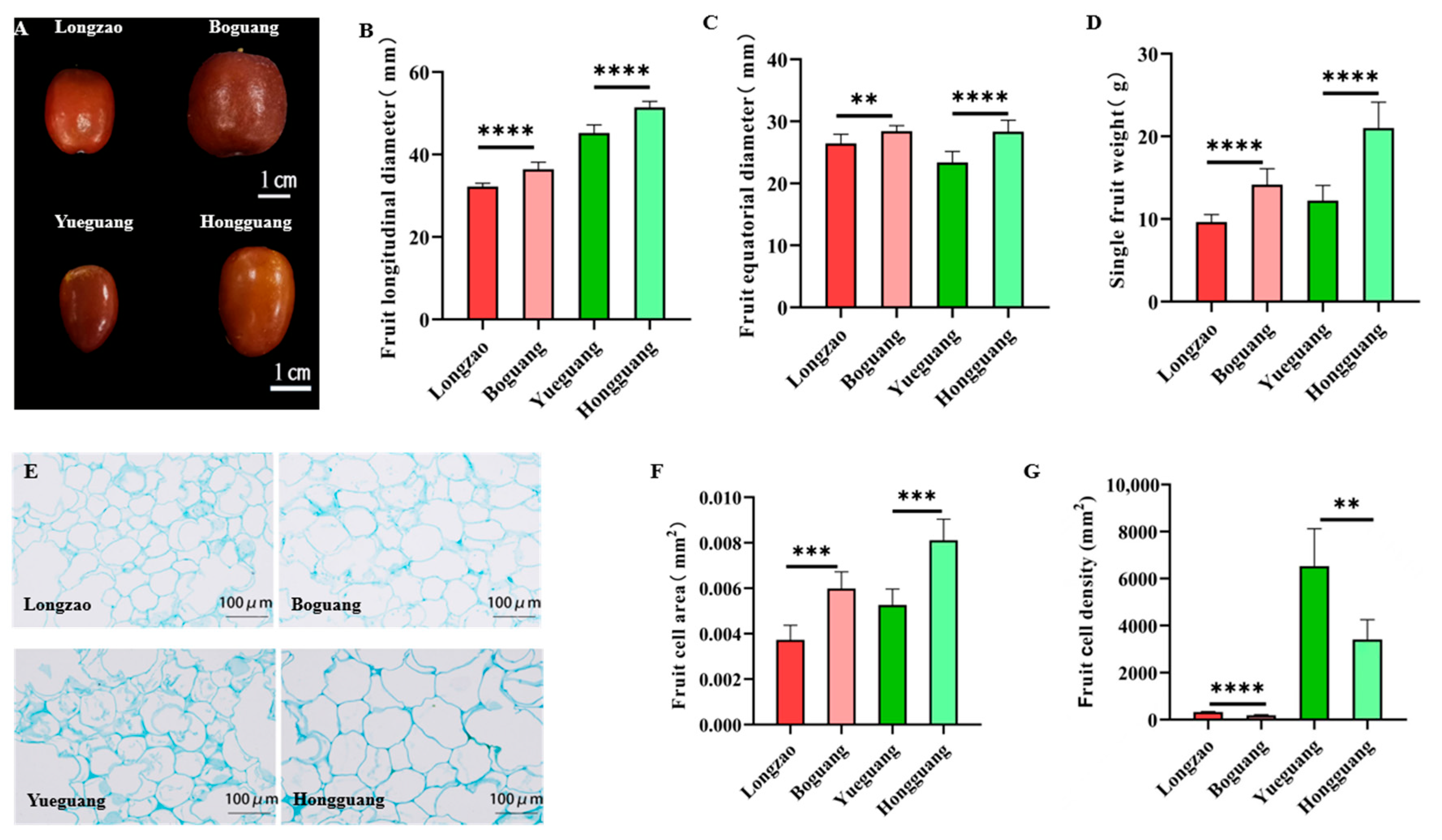
2.2.6. Changes in Fruits
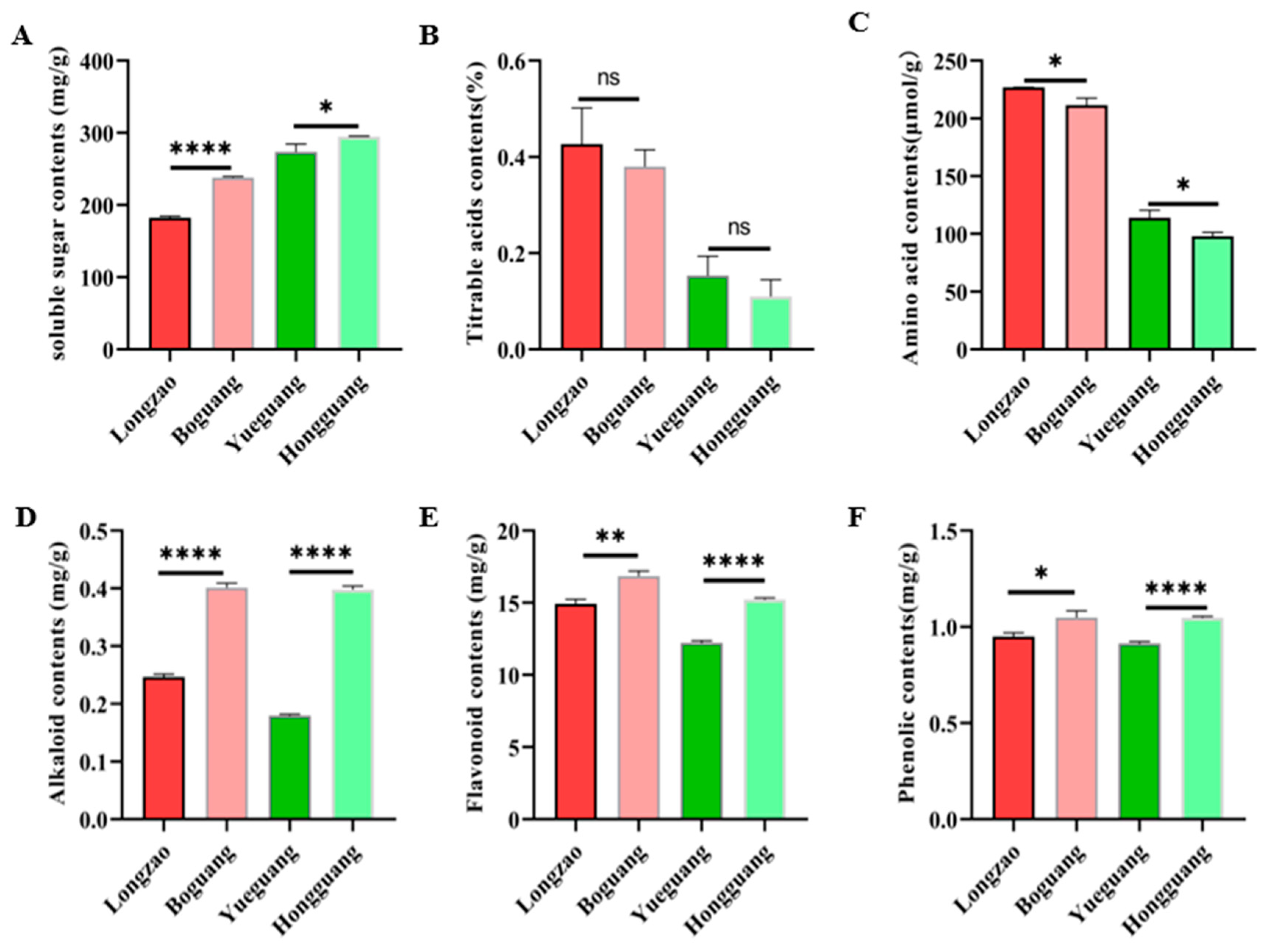
2.3. Comparison of Metabolite Characteristics Between Diploid and Autotetraploid Jujubes
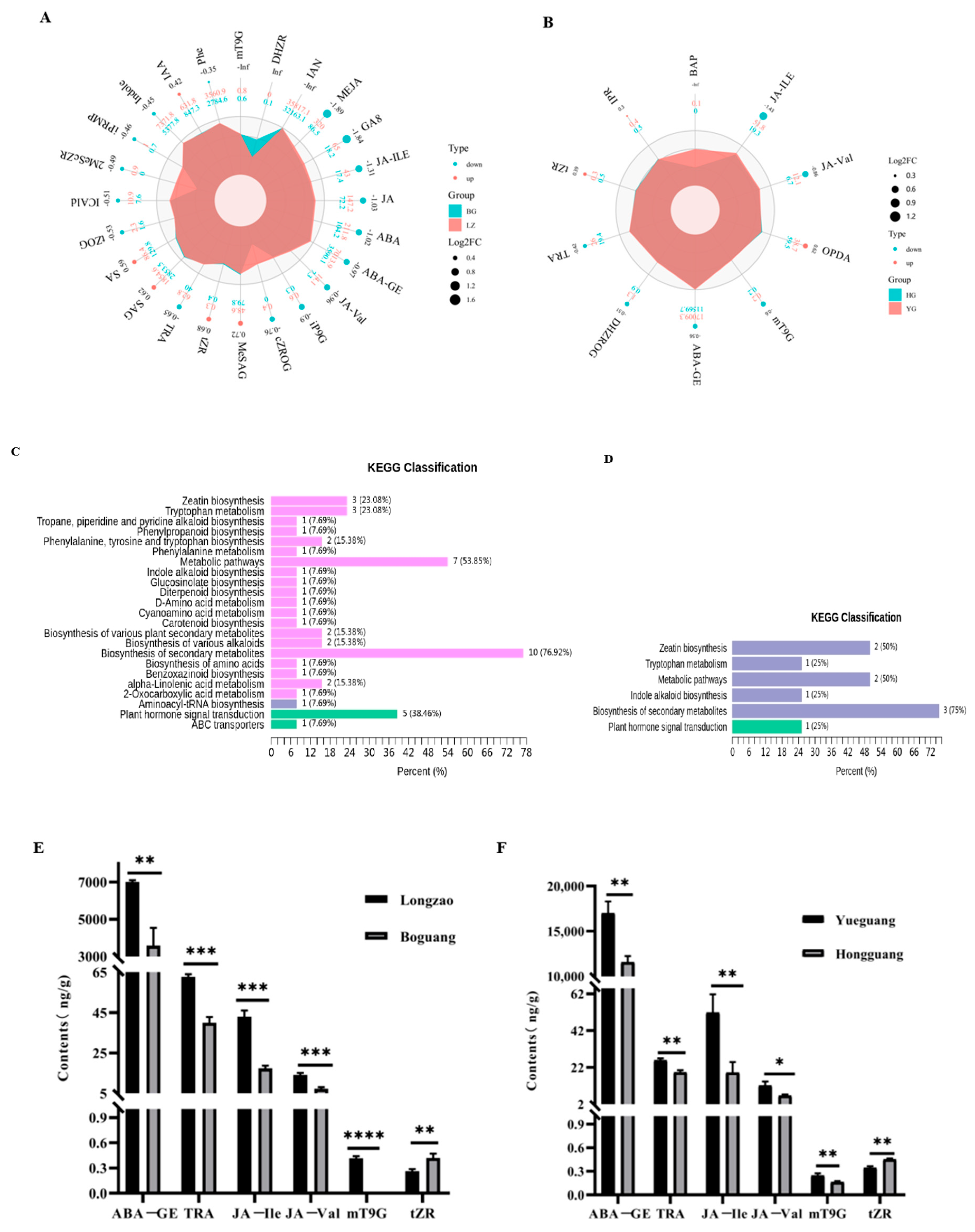
2.4. Comparison of Phytohormone Levels in Leaves Between Diploid and Autotetraploid Jujubes
3. Discussion
3.1. Common Phenotypic Variations Induced by Autopolyploidy and Their Implications for Jujube Cultivation
3.2. Mechanisms and Significance of Enhanced Secondary Metabolites in Autopolyploids
3.3. Reconstruction of Endogenous Hormone Homeostasis and Its Phenotypic Implications in Autotetraploid Jujube
4. Material and Methods
4.1. Plant Materials
4.2. Growth Conditions
4.3. Morphological Analysis
4.4. Cytological Observation of Stomata, Pollen and Fruit Pulp Cell
4.5. Extraction and Analysis of Metabolites
4.6. Determination of Endogenous Phytohormone Levels with LC-MS/MS
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parisod, C.; Holderegger, R.; Brochmann, C. Evolutionary consequences of autopolyploidy. New Phytol. 2010, 186, 5–17. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Ozkan, H.; Tuna, M.; Galbraith, D.W. No DNA loss in autotetraploids of Arabidopsis thaliana. Plant Breed. 2006, 125, 288–291. [Google Scholar] [CrossRef]
- Martelotto, L.G.; Ortiz, J.P.A.; Stein, J.; Espinoza, F.; Quarin, C.L.; Pessino, S.C. Genome rearrangements derived from autopolyploidization in Paspalum sp. Plant Sci. 2007, 172, 970–977. [Google Scholar] [CrossRef]
- Rivero-Guerra, A.O. Cytogenetics, geographical distribution, and pollen fertility of diploid and tetraploid cytotypes of Santolina pectinata Lag. (Asteraceae: Anthemideae). Bot. J. Linn. Soc. 2008, 156, 657–667. [Google Scholar] [CrossRef]
- Eilam, T.; Gustafson, P.; Anikster, Y.; Millet, E.; Manisterski, J.; Feldman, M. Genome size in natural and synthetic autopolyploids and in a natural segmental allopolyploid of several Triticeae species. Genome 2009, 52, 275–285. [Google Scholar] [CrossRef]
- Lavania, U.C.; Srivastava, S.; Lavania, S.; Basu, S.; Misra, N.K.; Mukai, Y. Autopolyploidy differentially influences body size in plants, but facilitates enhanced accumulation of secondary metabolites, causing increased cytosine methylation. Plant J. 2012, 71, 539–549. [Google Scholar] [CrossRef]
- Gao, R.; Wang, H.; Dong, B.; Yang, X.; Chen, S.; Jiang, J.; Zhang, Z.; Liu, C.; Zhao, N.; Chen, F. Morphological, genome and gene expression changes in newly induced autopolyploid Chrysanthemum lavandulifolium (Fisch. ex Trautv.) Makino. Int. J. Mol. Sci. 2016, 17, 1690. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Wang, Z.; Luo, G.; Tang, C. Phenotypic and transcriptomic analyses of autotetraploid and diploid mulberry (Morus alba L.). Int. J. Mol. Sci. 2015, 16, 22938–22956. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Fait, A.; Tel-Zur, N. Morphological, cytological and metabolic consequences of autopolyploidization in Hylocereus (Cactaceae) species. BMC Plant Biol. 2013, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, X.; Li, X.; Zhou, H.; Wang, S.; Yuan, Z.; Zhang, Y.; Li, S.; You, A.; Zhou, L.; et al. A genome doubling event reshapes rice morphology and products by modulating chromatin signatures and gene expression profiling. Rice 2021, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xue, H.; Zhang, L.; Zhang, F.; Ou, C.; Wang, F.; Zhang, Z. Involvement of auxin and brassinosteroid in dwarfism of autotetraploid apple (Malus × domestica). Sci. Rep. 2016, 6, 26719. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, H.; Meng, H.; Qiao, L.; Zhang, G.; Cheng, Z. In vitro Induction and Phenotypic Variations of Autotetraploid Garlic (Allium sativum L.) with Dwarfism. Front. Plant Sci. 2022, 13, 917910. [Google Scholar] [CrossRef]
- Tavan, M.; Mirjalili, M.H.; Karimzadeh, G. In vitro polyploidy induction: Changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult. 2015, 122, 573–583. [Google Scholar] [CrossRef]
- Hannweg, K.; Visser, G.; de Jager, K.; Bertling, I. In vitro-induced polyploidy and its effect on horticultural characteristics, essential oil composition and bioactivity of Tetradenia riparia. S. Afr. J. Bot. 2016, 106, 186–191. [Google Scholar] [CrossRef]
- Berkov, S.; Philipov, S. Alkaloid production in diploid and autotetraploid plants of Datura stramonium. Pharm. Biol. 2008, 40, 617–621. [Google Scholar] [CrossRef]
- Madani, H.; Hosseini, B.; Dehghan, E.; Rezaei-Chiyaneh, E. Enhanced production of scopolamine in induced autotetraploid plants of Hyoscyamus reticulatus L. Acta Physiol. Plant. 2015, 37, 55. [Google Scholar] [CrossRef]
- Xia, J.; Ma, Y.J.; Wang, Y.; Wang, J.W. Deciphering transcriptome profiles of tetraploid Artemisia annua plants with high artemisinin content. Plant Physiol. Biochem. 2018, 130, 112–126. [Google Scholar] [CrossRef]
- Zhu, W.; Li, R.; Guo, X.; Li, J.; Muhammad, N.; Qi, C.; Gao, M.; Wang, C.; Liu, M.; Tang, G.; et al. Integrated anatomical structure, physiological, and transcriptomic analyses to identify differential cold tolerance responses of Ziziphus jujuba mill. ‘Yueguang’ and its autotetraploid ‘Hongguang’. Plant Physiol. Biochem. 2024, 211, 108679. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xiao, G.-A.; Bao, Y.; Xia, Q.-M.; Xie, K.-D.; Wu, X.-M.; Guo, W.-W. Integrative DNA methylome and transcriptome analysis illuminate leaf phenotype alterations in tetraploid citrus. Hortic. Adv. 2024, 2, 28. [Google Scholar] [CrossRef]
- Yingying, Z.; Lei, K.; Shiying, L.; Qi, P.; Xianhong, G.; Zaiyun, L.; Jin-Song, Z. Transcriptomic analysis reveals differential gene expressions for cell growth and functional secondary metabolites in induced autotetraploid of Chinese Woad (Isatis indigotica Fort.). PLoS ONE 2015, 10, e0116392. [Google Scholar] [CrossRef]
- Fasano, C.; Diretto, G.; Aversano, R.; D’AGostino, N.; Di Matteo, A.; Frusciante, L.; Giuliano, G.; Carputo, D. Transcriptome and metabolome of synthetic Solanum autotetraploids reveal key genomic stress events following polyploidization. New Phytol. 2016, 210, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Vergara, F.; Kikuchi, J.; Breuer, C. Artificial autopolyploidization modifies the tricarboxylic acid cycle and GABA Shunt in Arabidopsis thaliana Col-0. Sci. Rep. 2016, 6, 26515. [Google Scholar] [CrossRef] [PubMed]
- Pozo, J.C.D.; Ramirez-Parra, E. Deciphering the molecular bases for drought tolerance in Arabidopsis autotetraploids. Plant Cell Environ. 2014, 37, 2722–2737. [Google Scholar] [CrossRef]
- Liu, M.J.; Liu, P.; Liu, G.N. Advances of research on germplasm resources of Chinese jujube. Acta Hortic. 2013, 993, 15–20. [Google Scholar] [CrossRef]
- Yanwei, L.; Meiyu, L.; Jingle, Z.; Liu, Y.; Jiao, G.; Decang, K.; Yingyue, L.; Xiaoming, P. Excavation and SSR Identification of Polyploid Germplasm Resources in Jujube Trees. Mol. Plant Breed. 2018, 16, 3395–3400. [Google Scholar] [CrossRef]
- Xue-Sheng, L.; Long, C.; Jin-Xin, W.; Li, L.I.; Peng, A. Discovery and identification of natural triploid ploidy of Chinese Jujube cultivar ‘Pingguozao’. Acta Hortic. Sin. 2013, 40, 426–432. [Google Scholar] [CrossRef]
- Meng-Jun, L.; Jiu-Rui, W.; Ping, L.; Min-Juan, L.; Jing, X.; Zhi-Guo, L.; Xue-Chao, S. Design and practice of emasculation-free cross breeding in Chinese Jujube. Acta Hortic. Sin. 2014, 41, 1495–1502. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Yuan, Y.; Zhang, Y.; Li, H.; Zhao, J.; Liu, M. The regulatory pathways of distinct flowering characteristics in Chinese jujube. Hortic. Res. 2020, 7, 123. [Google Scholar] [CrossRef]
- Liu, M.J.; Zhao, J.; Cai, Q.L.; Liu, G.C.; Wang, J.R.; Zhao, Z.H.; Liu, P.; Dai, L.; Yan, G.; Wang, W.J.; et al. The complex jujube genome provides insights into fruit tree biology. Nat. Commun. 2014, 5, 5315. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, P.; Wang, J.; Xu, J.; Ning, Q.; Liu, M. A novel in vivo shoot regeneration system via callus in woody fruit tree Chinese jujube (Ziziphus jujuba Mill.). Sci. Hortic. 2015, 188, 30–35. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, P.; Liu, M.; Wang, J.; Zhao, J.; Zhao, Z.; Dai, L. In vivo fast induction of homogeneous autopolyploids via callus in sour jujube (Ziziphus acidojujuba Cheng et Liu). Hortic. Plant J. 2016, 2, 147–153. [Google Scholar] [CrossRef]
- Li-Hu, W.; Lan, H.U.; Deng-Ke, L.I.; Ping, L.; Meng-Jun, L. In vivo bud regeneration ability and homogeneous polyploid induction via callus of different jujube genotypes. J. Plant Genet. Resour. 2017, 18, 164–170. [Google Scholar] [CrossRef]
- Ping, L.; Jiurui, W.; Zhiguo, L.; Lihu, W.; Jiabin, L.; Juan, X.U.; Qinghua, S.; Mengjun, L. A new excellent early-ripening tetraploid table cultivar of Chinese Jujube ‘Hongguang’. Acta Hortic. Sin. 2018, 45, 1423–1424. [Google Scholar] [CrossRef]
- Pei, Y.; Yao, N.; He, L.; Deng, D.; Li, W.; Zhang, W. Comparative study of the morphological, physiological and molecular characteristics between diploid and tetraploid radish (Raphunas sativus L.). Sci. Hortic. 2019, 257, 108739. [Google Scholar] [CrossRef]
- Xing, S.H.; Guo, X.B.; Wang, Q.; Pan, Q.F.; Tian, Y.S.; Liu, P.; Zhao, J.Y.; Wang, G.F.; Sun, X.F.; Tang, K.X. Induction and flow cytometry identification of tetraploids from seed-derived explants through colchicine treatments in Catharanthus roseus (L.) G. Don. J. Biomed. Biotechnol. 2011, 2011, 793198. [Google Scholar] [CrossRef] [PubMed]
- Ghotbi Ravandi, E.; Rezanejad, F.; Zolala, J.; Dehghan, E. The effects of chromosome-doubling on selected morphological and phytochemical characteristics of Cichorium intybus L. J. Hortic. Sci. Biotechnol. 2015, 88, 701–709. [Google Scholar] [CrossRef]
- Li, M.; Ding, B.; Huang, W.; Pan, J.; Ding, Z.; Jiang, F. Induction and characterization of tetraploids from seeds of Bletilla striata (Thunb.) Reichb.f. Biomed. Res. Int. 2018, 2018, 3246398. [Google Scholar] [CrossRef]
- Talei, D.; Fotokian, M.H. Improving growth indices and productivity of phytochemical compounds in Lemon balm (Melissa officinalis L.) via induced polyploidy. BioTechnologia 2020, 101, 215–226. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, Y.; Gao, F.; Xu, Y.; Cheng, C.; Deng, C.; Chen, J.; Yuan, X.; Zhang, X.; Su, J. Transcriptomic and metabolomic analyses reveal the differential accumulation of phenylpropanoids and terpenoids in hemp autotetraploid and its diploid progenitor. BMC Plant Biol. 2023, 23, 616. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Liu, Y.; Zhang, C.; Zhan, X.; Wang, Y.; Yu, X.; Zhang, C.; Wang, N.; Ma, J. Integrated transcriptomic, proteomic, and metabolomic analyses reveal mechanisms underlying day-night differences in carbohydrate metabolism between diploid and tetraploid Rice. Rice 2025, 18, 65. [Google Scholar] [CrossRef]
- Milosavljevic, S.; Kauai, F.; Mortier, F.; Van de Peer, Y.; Bonte, D. A metabolic perspective on polyploid invasion and the emergence of life histories: Insights from a mechanistic model. Am. J. Bot. 2024, 111, e16387. [Google Scholar] [CrossRef]
- Kuljarusnont, S.; Iwakami, S.; Iwashina, T.; Tungmunnithum, D. Flavonoids and other phenolic compounds for physiological roles, plant species delimitation, and medical benefits: A promising view. Molecules 2024, 29, 5351. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From biosynthesis to health benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.L.; Chuang, L.; Liu, S.; Seligmann, B.; Brendel, F.L.; Chavez, B.G.; Hoffie, R.E.; Hoffie, I.; Kumlehn, J.; Bultemeier, A.; et al. Biosynthesis of the allelopathic alkaloid gramine in barley by a cryptic oxidative rearrangement. Science 2024, 383, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Florea, S.; Pan, J.; Nagabhyru, P.; Bec, S.; Calie, P.J. The epichloae: Alkaloid diversity and roles in symbiosis with grasses. Curr. Opin. Plant. Biol. 2013, 16, 480–488. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X. A comprehensive review of phenolic compounds in horticultural plants. Int. J. Mol. Sci. 2025, 26, e202423654. [Google Scholar] [CrossRef]
- Chagas, M.d.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F.; Angeloni, C. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxidative Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356, Erratum in Int. J. Antimicrob. Agents 2006, 27, 181. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Zhang, T.J.; Qiu, Y.; Hua, Z. The emerging perspective of morphine tolerance: MicroRNAs. Pain Res. Manag. 2019, 2019, 9432965. [Google Scholar] [CrossRef]
- Kingston, D.G.I.; Cassera, M.B. Antimalarial natural products. In Antimalarial Natural Products; Springer: Cham, Switzerland, 2022; Volume 117, pp. 1–106. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Zhou, Y.; Gu, R.; Lu, G.; Zhang, C. Magnoflorine ameliorates collagen-induced arthritis by suppressing the inflammation response via the NF-kappaB/MAPK signaling pathways. J. Inflamm. Res. 2023, 16, 2271–2296. [Google Scholar] [CrossRef]
- Hu, S.Y.; Lin, T.H.; Chen, C.Y.; He, Y.H.; Huang, W.C.; Hsieh, C.Y.; Chen, Y.H.; Chang, W.C. Stephania tetrandra and its active compound coclaurine sensitize NSCLC cells to cisplatin through EFHD2 inhibition. Pharmaceuticals 2024, 17, 1356. [Google Scholar] [CrossRef]
- Bi, F.; Wang, Z.; Guo, Y.; Xia, M.; Zhu, X.; Qiao, W. A combination of magnoflorine and spinosin improves the antidepressant effects on CUMS mouse model. Curr. Drug. Metab. 2024, 25, 71–80. [Google Scholar] [CrossRef]
- Li, L.; Song, W.; Chang, Q.; Sun, Y.; Fang, D.; Qiao, W. The synergistic antidepressant effect: Compatibility of alkaloids with saponins from Ziziphi spinosae Semen. Evid. Based Complement. Alternat. Med. 2022, 2022, 5755980. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.; Ebehairy, L.; Nissapatorn, V.; Rahmatullah, M.; Villegas, J.; Dupa, H.J.; Verzosa, R.C.; Dolma, K.G.; Shabaz, M.; Lanting, S.; et al. Antibacterial phenolic compounds from the flowering plants of Asia and the Pacific: Coming to the light. Pharm. Biol. 2024, 62, 713–766. [Google Scholar] [CrossRef]
- Freitas, M.; Ribeiro, D.; Janela, J.S.; Varela, C.L.; Costa, S.C.; da Silva, E.T.; Fernandes, E.; Roleira, F.M.F. Plant-derived and dietary phenolic cinnamic acid derivatives: Anti-inflammatory properties. Food Chem. 2024, 459, 140080. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lin, Z.; Cortez--Jugo, C.; Qiao, G.G.; Caruso, F. Antimicrobial Phenolic Materials: From Assembly to Function. Angew. Chem. Int. Ed. 2025, 64, e202423654. [Google Scholar] [CrossRef]
- Zhu, C.; Lin, Z.; Jiang, H.; Wei, F.; Wu, Y.; Song, L. Recent advances in the health benefits of phenolic acids in whole grains and the impact of processing techniques on phenolic acids: A comprehensive review. J. Agric. Food Chem. 2024, 72, 24131–24157. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.; Pontifex, M.G.; Vauzour, D. (Poly)phenols and brain health–beyond their antioxidant capacity. FEBS Lett. 2024, 598, 2949–2962. [Google Scholar] [CrossRef]
- Burkard, M.; Piotrowsky, A.; Leischner, C.; Detert, K.; Venturelli, S.; Marongiu, L. The antiviral activity of polyphenols. Mol. Nutr. Food Res. 2025, 69, e70042. [Google Scholar] [CrossRef]
- Fu, W.; Jin, G.; Jimenez-Aleman, G.H.; Wang, X.; Song, J.; Li, S.; Lou, Y.; Li, R. The jasmonic acid-amino acid conjugates JA-Val and JA-Leu are involved in rice resistance to herbivores. Plant Cell Environ. 2022, 45, 262–272. [Google Scholar] [CrossRef]
- Negri, S.; Commisso, M.; Avesani, L.; Guzzo, F. The case of tryptamine and serotonin in plants: A mysterious precursor for an illustrious metabolite. J. Exp. Bot. 2021, 72, 5336–5355. [Google Scholar] [CrossRef]
- Havlova, M.; Dobrev, P.I.; Motyka, V.; Storchova, H.; Libus, J.; Dobra, J.; Malbeck, J.; Gaudinova, A.; Vankova, R. The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant Cell Environ. 2008, 31, 341–353. [Google Scholar] [CrossRef]
- Gangwar, S.; Singh, V.P.; Prasad, S.M.; Maurya, J.N. Modulation of manganese toxicity in Pisum sativum L. seedlings by kinetin. Sci. Hortic. 2010, 126, 467–474. [Google Scholar] [CrossRef]
- De Rybel, B.; Adibi, M.; Breda, A.S.; Wendrich, J.R.; Smit, M.E.; Novak, O.; Yamaguchi, N.; Yoshida, S.; Van Isterdael, G.; Palovaara, J.; et al. Plant development. Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science 2014, 345, 1255215. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Priest, D.M.; Ambrose, S.J.; Vaistij, F.E.; Elias, L.; Higgins, G.S.; Ross, A.R.; Abrams, S.R.; Bowles, D.J. Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana. Plant J. 2006, 46, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Hirai, N.; Matsumoto, C.; Ohigashi, H.; Ohta, D.; Sakata, K.; Mizutani, M. Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant. Physiol. 2004, 134, 1439–1449. [Google Scholar] [CrossRef]
- Tian, D.; Tooker, J.; Peiffer, M.; Chung, S.H.; Felton, G.W. Role of trichomes in defense against herbivores: Comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 2012, 236, 1053–1066. [Google Scholar] [CrossRef]
- Limera, C.; Wang, K.; Xu, L.; Wang, Y.; Zhu, X.; Feng, H.; Sha, Y.; Gong, Y.; Liu, L. Induction of autotetraploidy using colchicine and its identification in radish (Raphanus sativus L.). J. Hortic. Sci. Biotechnol. 2016, 91, 63–70. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lim, J.-M.; Bhoo, S.-H.; Paik, Y.-S.; Hahn, T.-R. Colorimetric determination of amino acids using genipin from Gardenia jasminoides. Anal. Chim. Acta 2003, 480, 267–274. [Google Scholar] [CrossRef]
- Han, D.; Wang, K.; Long, F.; Zhang, W.; Yao, X.; Chen, S. Effects of endophytic fungi on the secondary metabolites of Hordeum bogdanii under alkaline stress. AMB Express 2022, 12, 73. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, H.; Xi, D.; Zhu, Y.; Li, X. Breeding and nutritional quality analysis of a new non-heading Chinese cabbage variety ‘Haiqing 3’. Acta Agric. Shanghai 2023, 39, 8–13. [Google Scholar]
- Jing, S. Comparison of bioactive component contents and antioxidant activities in three varieties of Lonicera caerulea fruits. Non-Wood For. 2019, 37, 95–100. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, S.; Ren, Y.; Li, H.; Zhang, X.; Di, J. Jujube preservation using chitosan film with nano-silicon dioxide. J. Food Eng. 2012, 113, 408–414. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Berrios, J.D.J.; Tang, J.; Derito, C. Bioactivity of antioxidants in extruded products prepared from purple potato and dry pea flours. J. Agric. Food Chem. 2011, 59, 8233–8243. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Sui, J.; Yao, T.; Chen, S.; Yang, M.; He, M.; Ye, T.; Li, X.; Song, T.; Liu, M.; et al. Phenotypic and Metabolic Variations Induced by Autopolyploidization in Chinese Jujube Cultivars. Plants 2025, 14, 3588. https://doi.org/10.3390/plants14233588
Han Y, Sui J, Yao T, Chen S, Yang M, He M, Ye T, Li X, Song T, Liu M, et al. Phenotypic and Metabolic Variations Induced by Autopolyploidization in Chinese Jujube Cultivars. Plants. 2025; 14(23):3588. https://doi.org/10.3390/plants14233588
Chicago/Turabian StyleHan, Yan, Jing Sui, Tong Yao, Shuting Chen, Meng Yang, Miao He, Tingting Ye, Xiaoshan Li, Taoliang Song, Mengjun Liu, and et al. 2025. "Phenotypic and Metabolic Variations Induced by Autopolyploidization in Chinese Jujube Cultivars" Plants 14, no. 23: 3588. https://doi.org/10.3390/plants14233588
APA StyleHan, Y., Sui, J., Yao, T., Chen, S., Yang, M., He, M., Ye, T., Li, X., Song, T., Liu, M., & Liu, P. (2025). Phenotypic and Metabolic Variations Induced by Autopolyploidization in Chinese Jujube Cultivars. Plants, 14(23), 3588. https://doi.org/10.3390/plants14233588






