Pectate Lyase FvePL1 Is Required for Pollen Fertility and Mediates Drought Response in Woodland Strawberry
Abstract
1. Introduction
2. Results
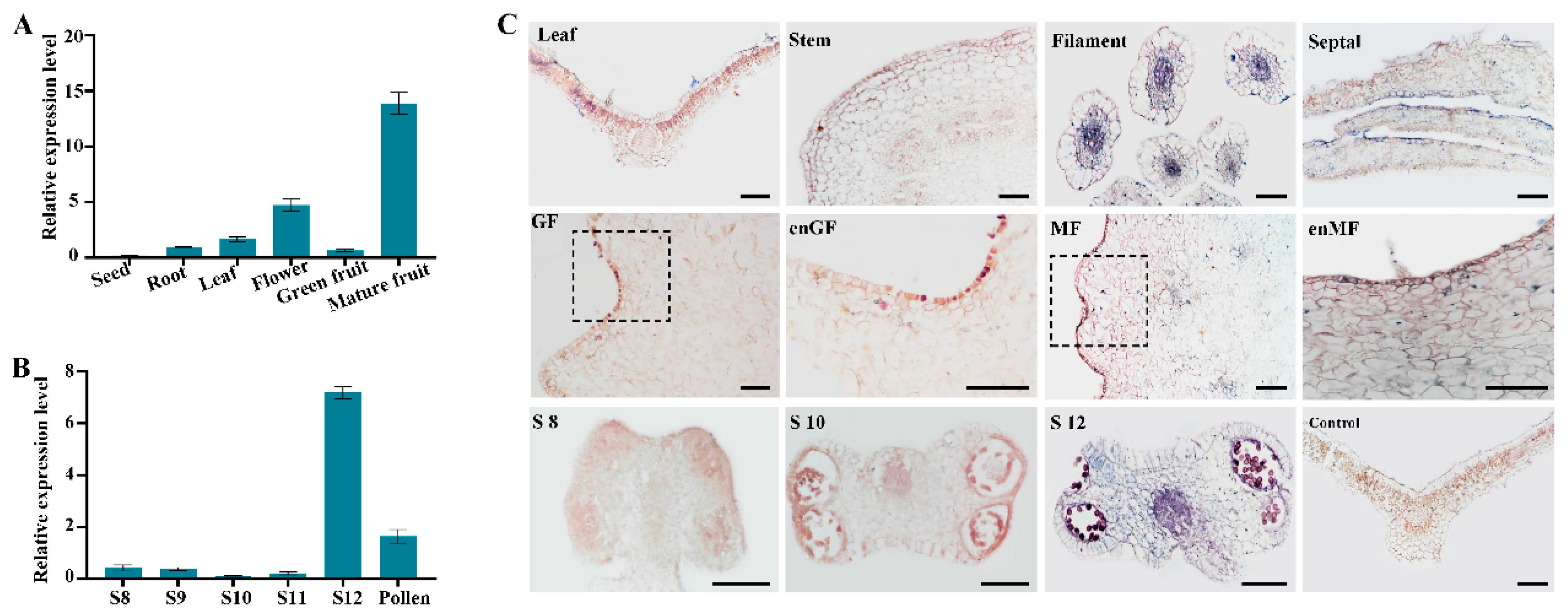
2.1. Molecular Characterization of FvePL1 Which Was Maximumly Expressed During Anther Maturation and Fruit Transformation
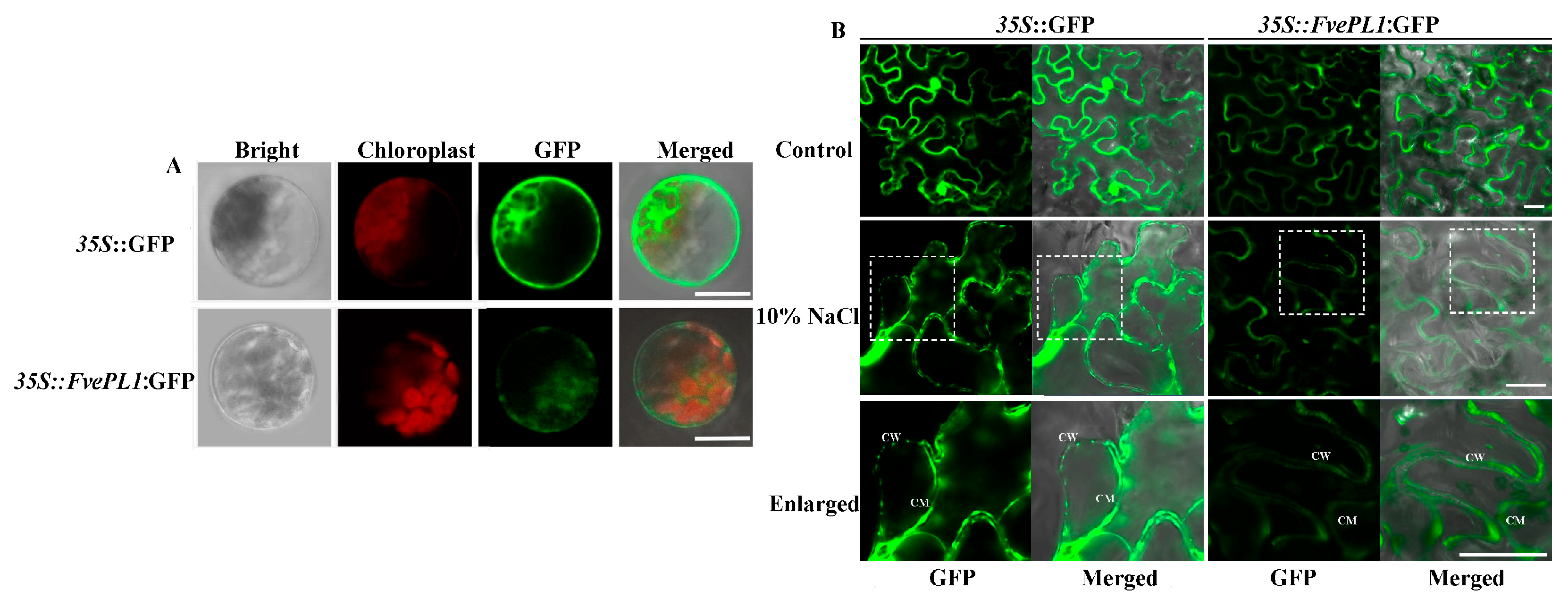
2.2. FvePL1 Was Localized in Cell Wall, Cell Membrane and Cytoplasm
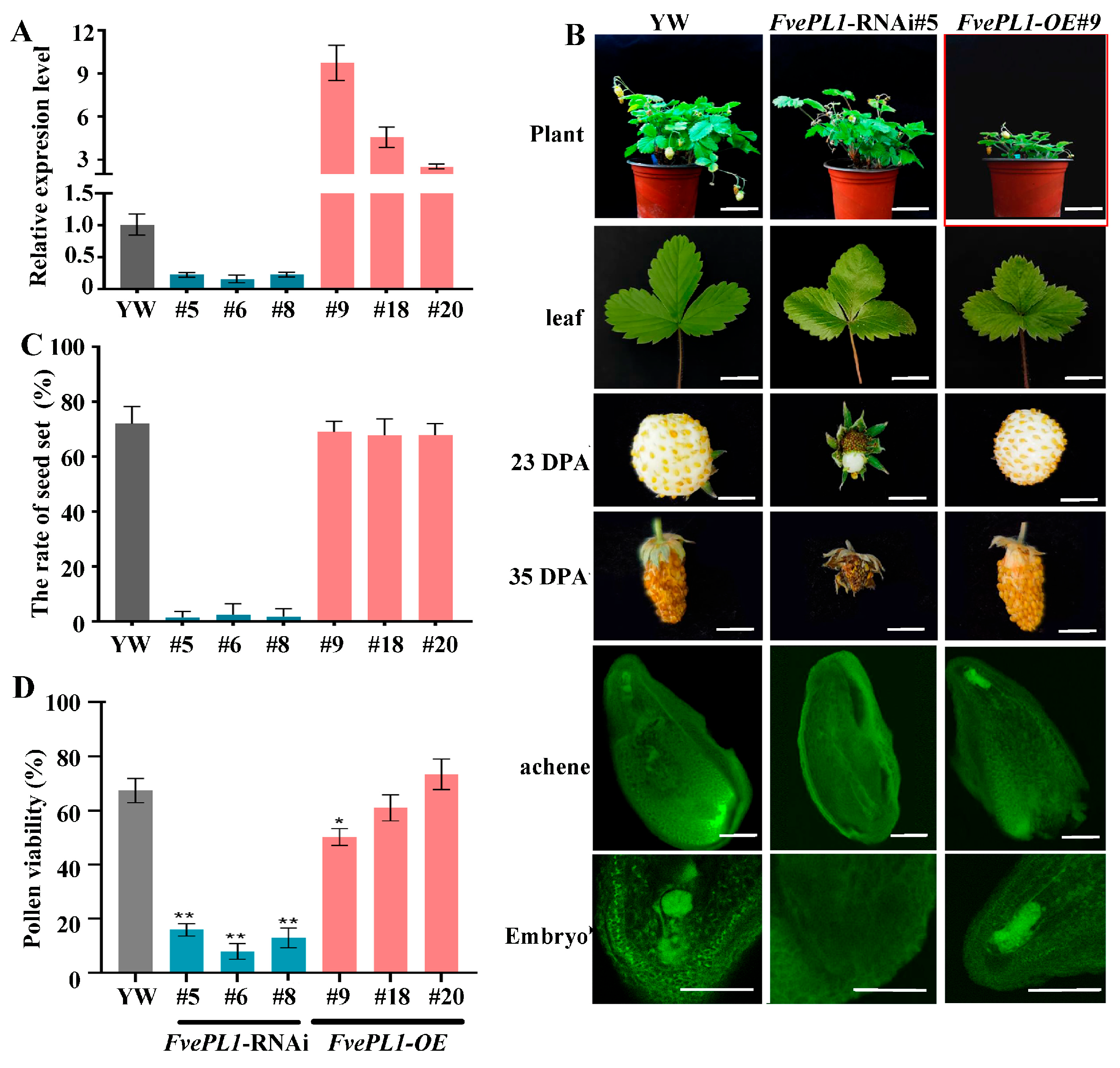
2.3. Reduction in Fvepl1 Resulted in Fruit Defects and Lower Seed Production
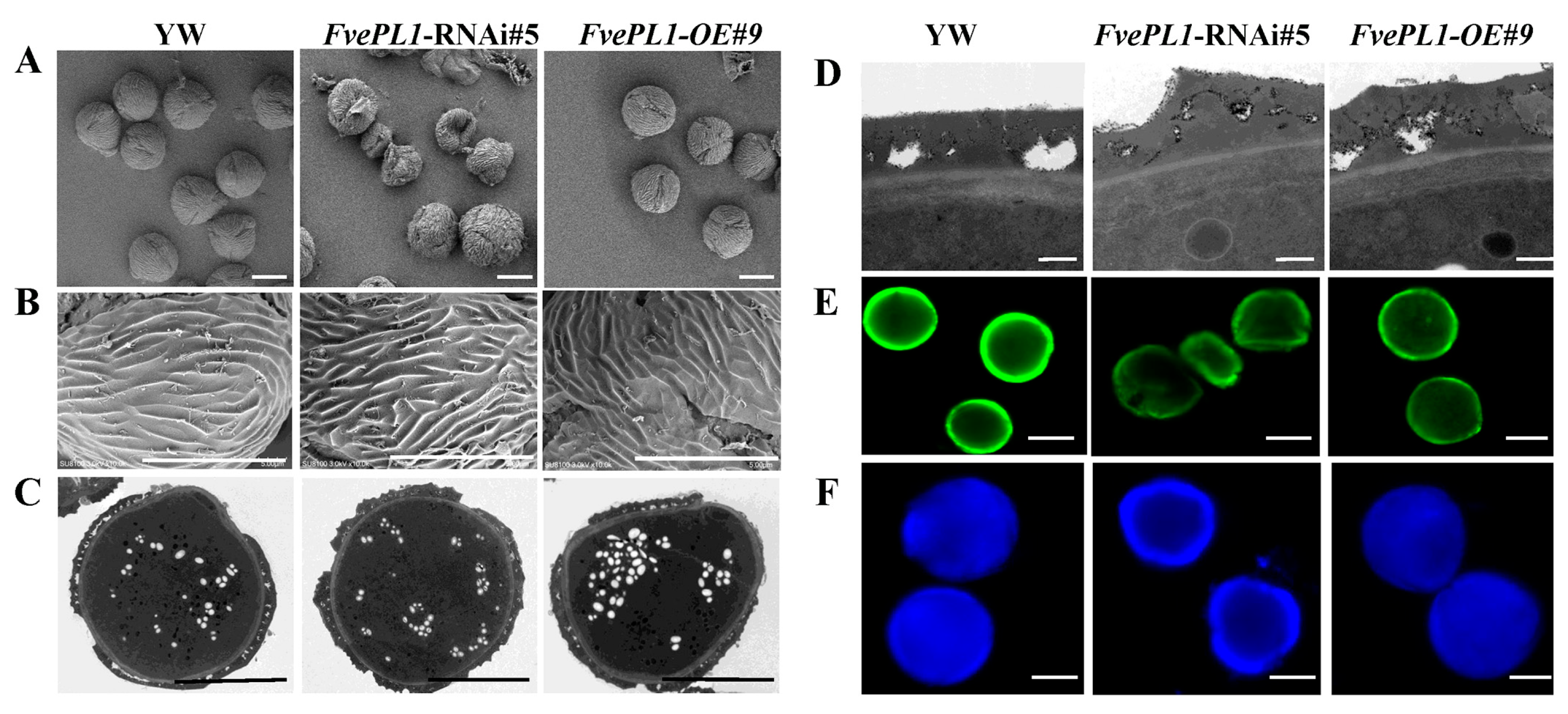
2.4. FvePL1 Is Required for the Development of Exine and Intine of Pollen Grains
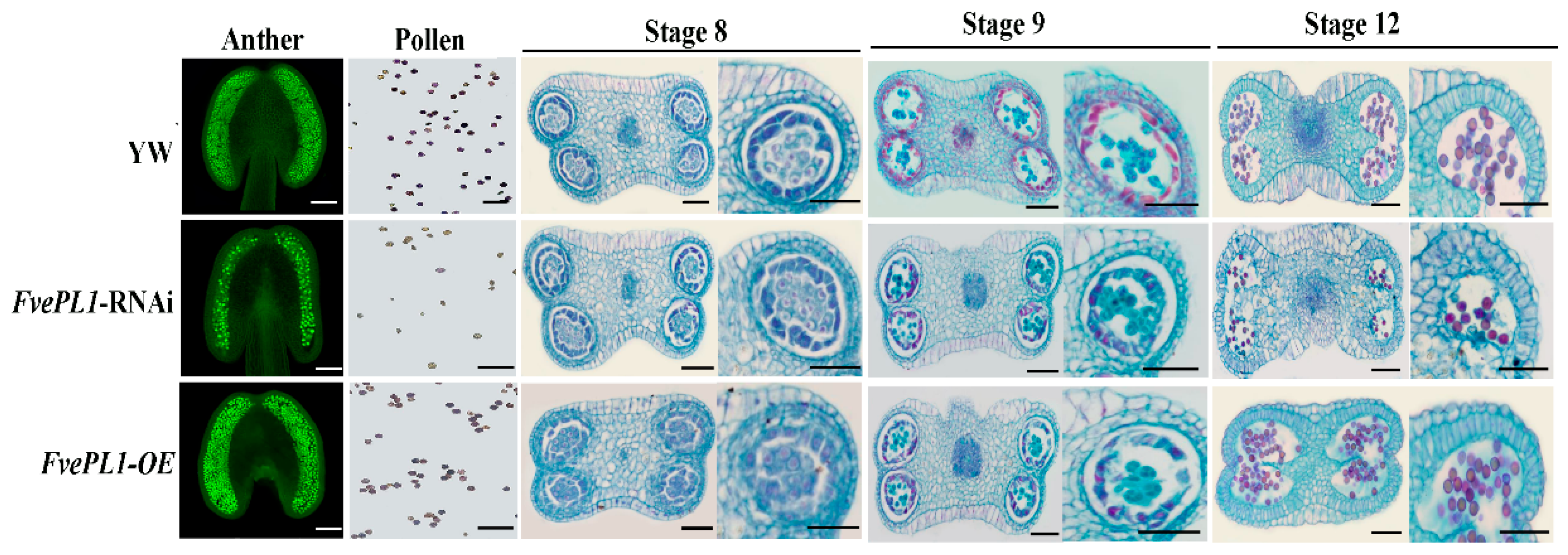
2.5. FvePL1-RNAi Lead to the Decreased Pollen Number Due to Degradation of Tapetum
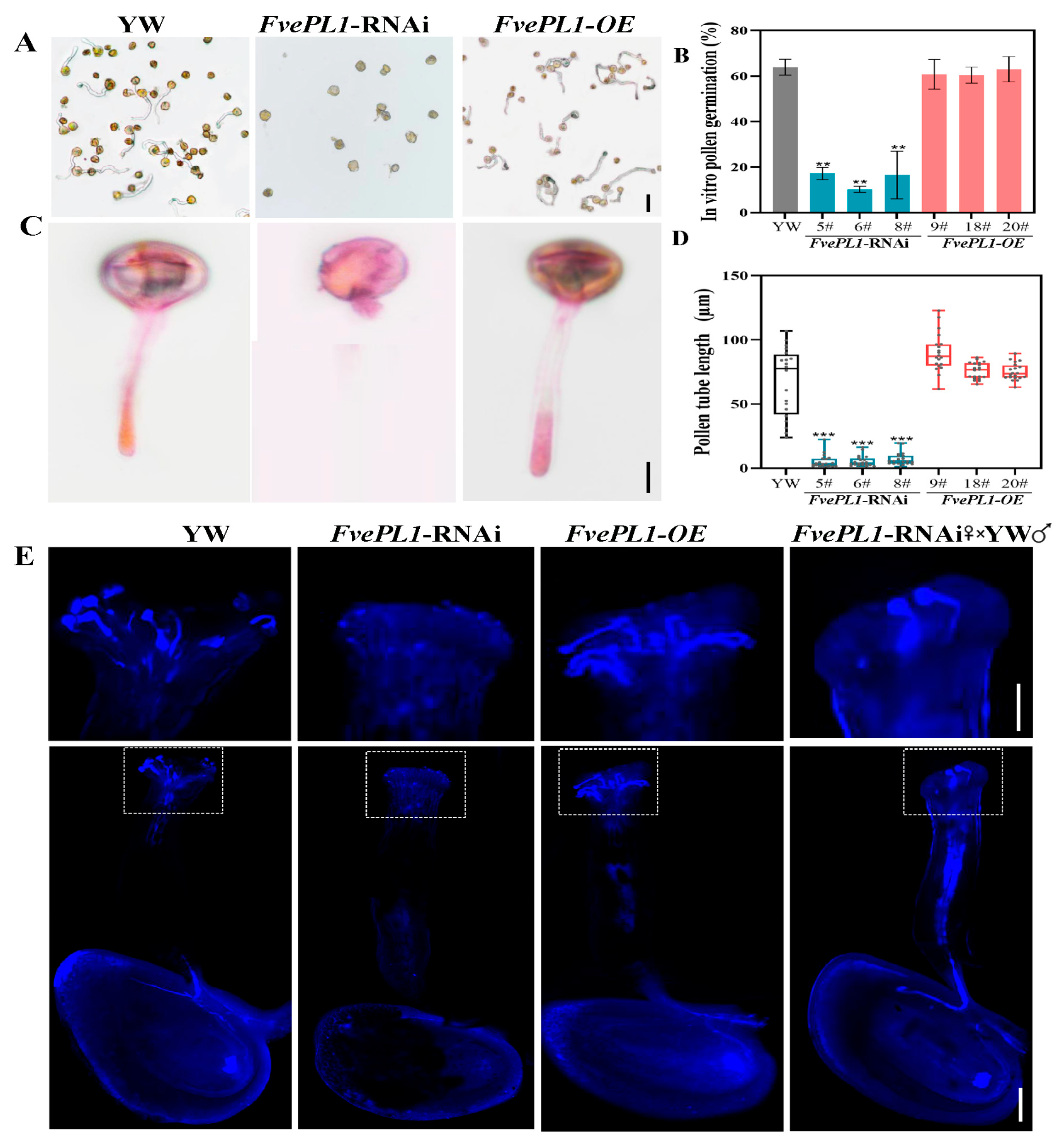
2.6. FvePL1 Is Essential for Pollen Germination
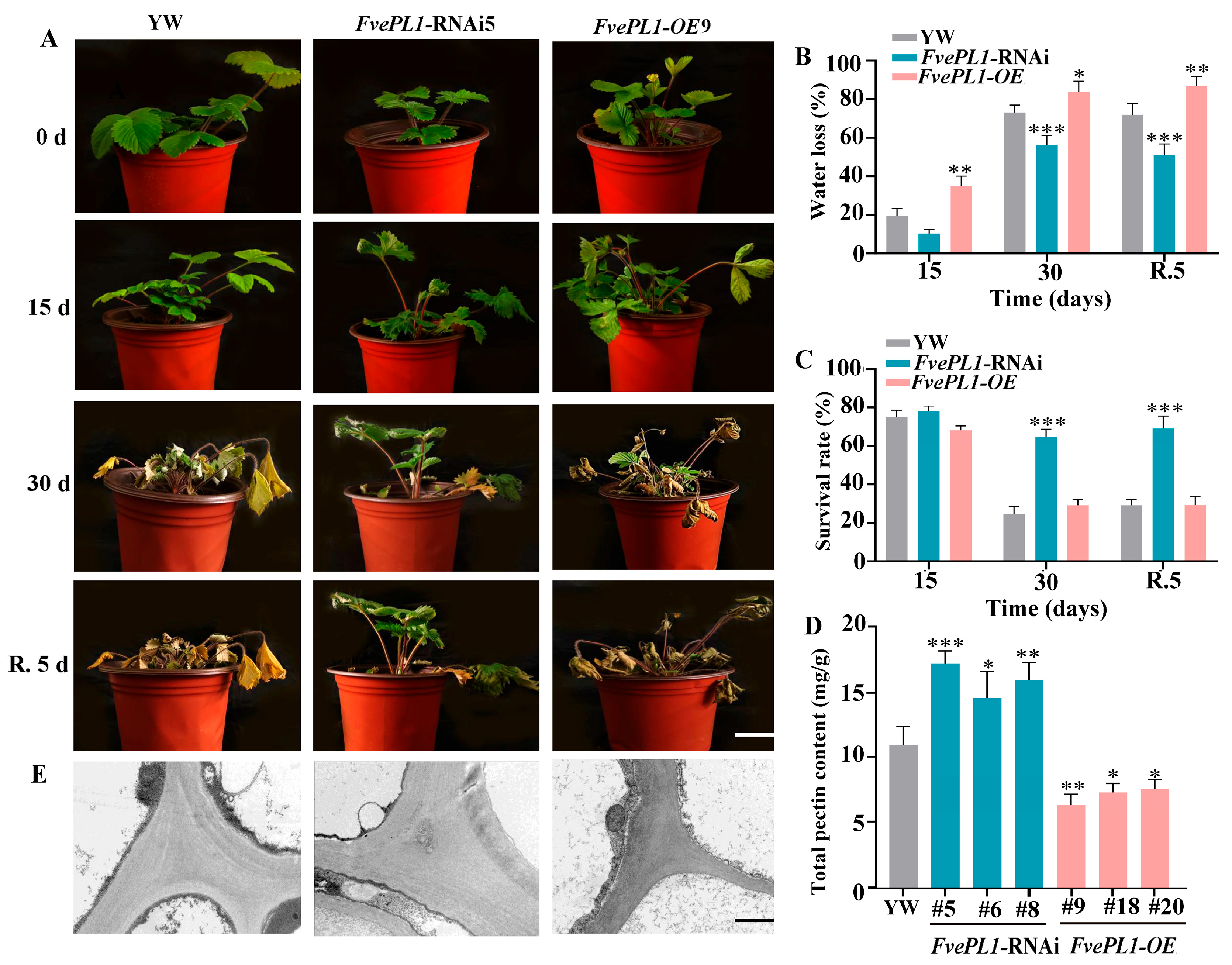
2.7. Downregulation of FvePL1 Improved Drought Tolerance
3. Discussion
3.1. FvePL1 Implicated in Male Gametophyte Development Is Indispensable for Fruit Set
3.2. The Aborted Pollens Caused by the Reduction in Fvepl1 Initiated from the Incomplete and Delayed Degradation of Tapetum and Septum
3.3. Different Regulatory Mechanisms of FvePL1 Influencing Pollen and Fruit Development
3.4. FvePL1 Negatively Mediated Drought Response in Woodland Strawberry
4. Materials and Methods
4.1. Plant and Growth Conditions
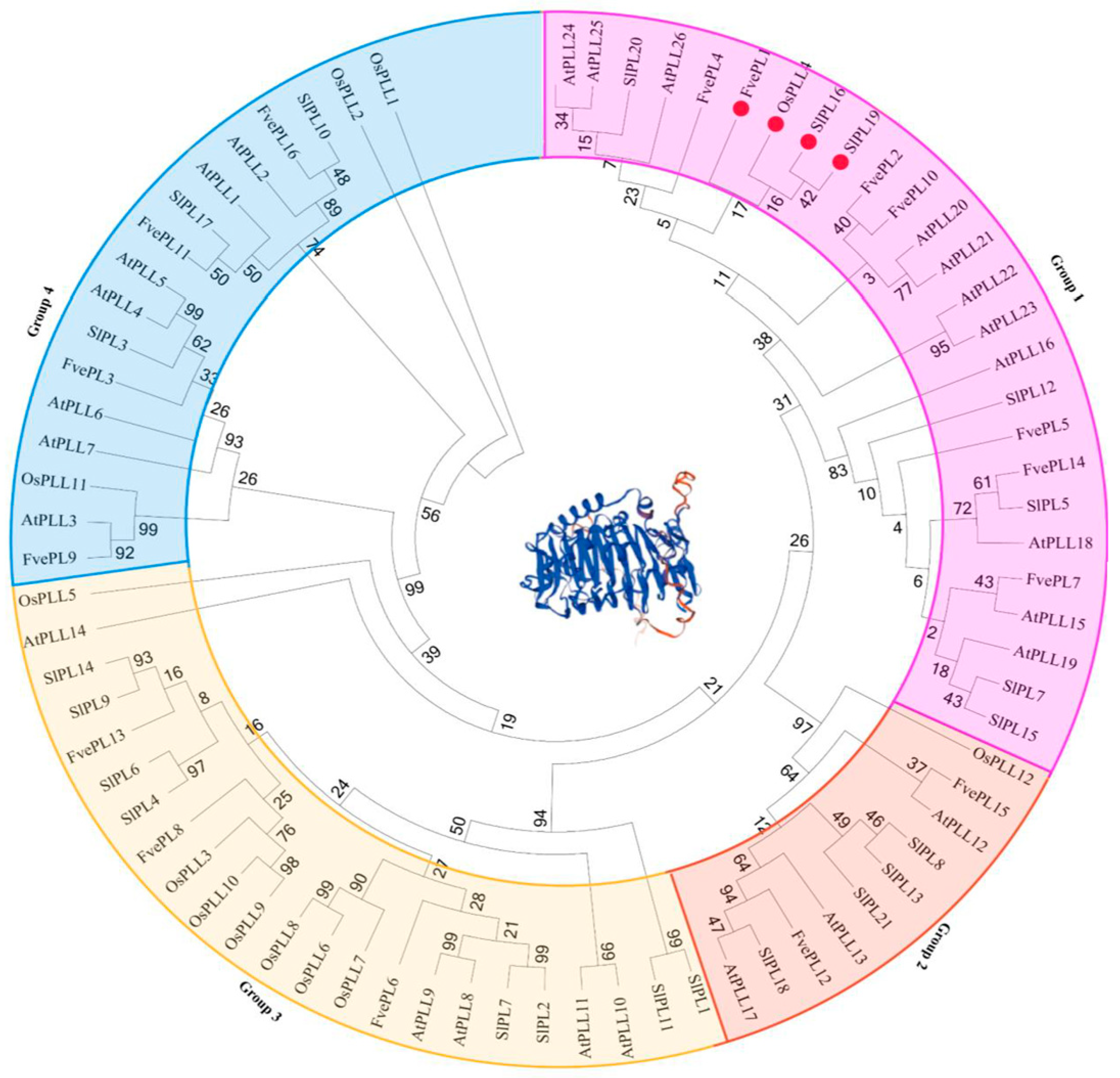
4.2. Phylogenetic Tree Construction
4.3. Subcellular Location
4.4. In Situ Hybridization
4.5. RNAi and Overexpression Constructs and Transgenic Strawberry Generation
4.6. Plasmolysis Assay
4.7. Seed Observation
4.8. RNA Extraction and Quantitative RT-PCR
4.9. Pollen Viability Assay
4.10. Cell Wall Imaging Using SEM and TEM
4.11. Calcofluor White and Auramine O Staining
4.12. Pollen Germination Assays In Vitro and In Vivo
4.13. Drought Stress Treatment, Water Loss and Total Pectin Assay
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PL | pectate lyase |
| YW | Yellow Wonder 5AF7 |
| MTT | 3-(4,5)-dimethylthiahiazo(-z-y1)-2,5-di-phenytetrazoliumromide |
| GFP | green fluorescent protein |
| GDR | Genome database for Rosaceae |
| DPA | day post-anthesis |
Appendix A
| Name | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | Application |
|---|---|---|---|
| FvePL1 | ATGAGGTTAGCTAGCTCGAGG | TTAACATTGACGACCAAACCG | Amplification |
| pM999-FvePL1 | GCTCTAGAATGAGGTTAGCTAGCTCGAGG | GCTCTAGAACATTGACGACCAAACCGGCA | Subcellular location |
| FvePL1-RNAi | GGACTAGTGGTACCACGACGACTGGAATGAGCACG | CGAGCTCGGATCCAGGCTTGCAGTCGTGGATGTG | RNAi line |
| FvePL1-OE | CGGGGTACCATGAGGTTAGCTAGCTCGAGG | CGAGCTCTTAACATTGACGACCAAACCG | Overexpression line |
| q-FvePL1 | GGAAGTGCTGATCCTACCATTA | CTGTGGTCTGAACTCTATGTGT | qRT-PCR |
| Fveactin | CCCAAGTAAGGATGCCCCCATGTTCG | TTGGCAAGGGGAGCAAGACAGTTGGTAG | qRT-PCR |
| FvePL1 | TCTCATGCGCAACAGGGAAT | GTTGCGGCCGAATCCAATAC | ISH |
| Hyg | ACGTTGCAAGACCTGCCTGAAA | TCCAGTCAATGACCGCTGTTATGC | Positive verification |
| Gene ID | Accession Number | Gene ID | Accession Number |
|---|---|---|---|
| OsPLL1 | LOC_Os01g36620 | AtPLL1 | At3g09540 |
| OsPLL2 | LOC_Os01g62000 | AtPLL2 | At3g55140 |
| OsPLL3 | LOC_Os02g12300 | AtPLL3 | At5g09280 |
| OsPLL4 | LOC_Os04g05050 | AtPLL4 | At4g22080 |
| OsPLL5 | LOC_Os05g22800 | AtPLL5 | At4g22090 |
| OsPLL6 | LOC_Os06g05209 | AtPLL6 | At1g11920 |
| OsPLL7 | LOC_Os06g05260 | AtPLL7 | At1g30350 |
| OsPLL8 | LOC_Os06g05272 | AtPLL8 | At1g14420 |
| OsPLL9 | LOC_Os06g38510 | AtPLL9 | At3g02720 |
| OsPLL10 | LOC_Os06g38520 | AtPLL10 | At3g01270 |
| OsPLL11 | LOC_Os08g18970 | AtPLL11 | At5g15110 |
| FvePL1 | FvH4_2g19540 | AtPLL12 | At5g04310 |
| FvePL2 | FvH4_3g00620 | AtPLL13 | At3g54920 |
| FvePL3 | FvH4_3g01680 | AtPLL14 | At5g55720 |
| FvePL4 | FvH4_4g05760 | AtPLL15 | At5g63180 |
| FvePL5 | FvH4_4g25110 | AtPLL16 | At1g67750 |
| FvePL6 | FvH4_4g31900 | AtPLL17 | At3g53190 |
| FvePL7 | FvH4_5g06720 | AtPLL18 | At3g27400 |
| FvePL8 | FvH4_5g15430 | AtPLL19 | At4g24780 |
| FvePL9 | FvH4_5g27090 | AtPLL20 | At3g07010 |
| FvePL10 | FvH4_6g02350 | AtPLL21 | At5g48900 |
| FvePL11 | FvH4_6g14680 | AtPLL22 | At3g24670 |
| FvePL12 | FvH4_6g16670 | AtPLL23 | At4g13210 |
| FvePL13 | FvH4_6g49230 | AtPLL24 | At3g24230 |
| FvePL14 | FvH4_6g52190 | AtPLL25 | At4g13710 |
| FvePL15 | FvH4_7g18400 | AtPLL26 | At1g04680 |
| FvePL16 | FvH4_7g20920 | SlPL1 | Solyc01g010740 |
| SlPL2 | Solyc02g067450 | SlPL12 | Solyc05g014000 |
| SlPL3 | Solyc02g080910 | SlPL13 | Solyc05g055510 |
| SlPL4 | Solyc02g087670 | SlPL14 | Solyc06g071020 |
| SlPL5 | Solyc02g093580 | SlPL15 | Solyc06g071840 |
| SlPL6 | Solyc03g058890 | SlPL16 | Solyc06g083580 |
| SlPL7 | Solyc03g058910 | SlPL17 | Solyc09g005850 |
| SlPL8 | Solyc03g071570 | SlPL18 | Solyc09g008380 |
| SlPL9 | Solyc03g113150 | SlPL19 | Solyc09g061890 |
| SlPL10 | Solyc04g010230 | SlPL20 | Solyc09g091430 |
| SlPL11 | Solyc05g007080 | SlPL21 | Solyc11g008140 |
References
- Kang, C.; Darwish, O.; Geretz, A.; Shahan, R.; Alkharouf, N.; Liu, Z. Genome-scale transcriptomic insights into early-stage fruit development in woodland strawberry Fragaria vesca. Plant Cell 2013, 25, 1960–1978. [Google Scholar] [CrossRef] [PubMed]
- Edlund, A.F.; Swanson, R.; Preuss, D. Pollen and stigma structure and function: The role of diversity in pollination. Plant Cell 2004, 16 (Suppl. S1), S84–S97. [Google Scholar] [CrossRef] [PubMed]
- Hollender, C.A.; Geretz, A.C.; Slovin, J.P.; Liu, Z. Flower and early fruit development in a diploid strawberry, Fragaria vesca. Planta 2012, 235, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Burri, J.T.; Vogler, H.; Laubli, N.F.; Hu, C.; Grossniklaus, U.; Nelson, B.J. Feeling the force: How pollen tubes deal with obstacles. New Phytol. 2018, 220, 187–195. [Google Scholar] [CrossRef]
- Parre, E.; Geitmann, A. Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 2005, 220, 582–592. [Google Scholar] [CrossRef]
- Hou, S.; Shi, J.; Hao, L.; Wang, Z.; Liao, Y.; Gu, H.; Dong, J.; Dresselhaus, T.; Zhong, S.; Qu, L.J. VPS18-regulated vesicle trafficking controls the secretion of pectin and its modifying enzyme during pollen tube growth in Arabidopsis. Plant Cell 2021, 33, 3042–3056. [Google Scholar] [CrossRef]
- Xiang, T.; Yang, R.; Li, L.; Lin, H.; Kai, G. Research progress and application of pectin: A review. J. Food Sci. 2024, 89, 6985–7007. [Google Scholar] [CrossRef]
- Dardelle, F.; Lehner, A.; Ramdani, Y.; Bardor, M.; Lerouge, P.; Driouich, A.; Mollet, J.C. Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiol. 2010, 153, 1563–1576. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Yin, C.; Zhu, J.; Cao, J.; Guo, M.; Chen, G. ABA accelerates postharvest softening in Prunus domestica L. by regulating pectin demethylation and depolymerization via modulation of cell wall metabolism. Food Chem. 2025, 496, 146813. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, L.; Zhang, M.; Wang, H.; Wang, Y.; Zhang, X.; Zhang, K.; Sui, Y.; Qian, J.; Jia, S.; et al. PbrBGAL6 promotes pollen tube growth by influencing apical pectin level in Pyrus bretschneideri. BMC Genom. 2025, 26, 321. [Google Scholar] [CrossRef]
- Rockel, N.; Wolf, S.; Kost, B.; Rausch, T.; Greiner, S. Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J. 2008, 53, 133–143. [Google Scholar] [CrossRef]
- Robil, J.M. The pollen tube’s secret to slick growth? A dab of pectate lyase-like enzyme. Plant Physiol. 2024, 194, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Chebli, Y.; Geitmann, A. Cellular growth in plants requires regulation of cell wall biochemistry. Curr. Opin. Cell Biol. 2017, 44, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Palusa, S.G.; Golovkin, M.; Shin, S.B.; Richardson, D.N.; Reddy, A.S.N. Organ-specific, developmental, hormonal and stress regulation of expression of putative pectate lyase genes in Arabidopsis. New Phytol. 2007, 174, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Chebli, Y.; Geitmann, A. Pectate lyase-like lubricates the male gametophyte’s path toward its mating partner. Plant Physiol. 2023, 194, 9. [Google Scholar] [CrossRef]
- Guo, L.; Luo, X.; Li, M.; Joldersma, D.; Plunkert, M.; Liu, Z. Mechanism of fertilization-induced auxin synthesis in the endosperm for seed and fruit development. Nat. Commun. 2022, 13, 3985. [Google Scholar] [CrossRef]
- Huang, X.; Sun, G.; Wu, Z.; Jiang, Y.; Li, Q.; Yi, Y.; Yan, H. Genome-wide identification and expression analyses of the pectate lyase (PL) gene family in Fragaria vesca. BMC Genom. 2023, 24, 435. [Google Scholar] [CrossRef]
- Santiago-Domenech, N.; Jimenez-Bemudez, S.; Matas, A.J.; Rose, J.K.; Munoz-Blanco, J.; Mercado, J.A.; Quesada, M.A. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. J. Exp. Bot. 2008, 59, 2769–2779. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, L.; Yu, Y.; Lv, M.; Miao, Y.; Cao, J. PECTATE LYASE-LIKE10 is associated with pollen wall development in Brassica campestris. J. Integr. Plant Biol. 2014, 56, 1095–1105. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, L.; Yu, Y.; Liang, Y.; Jiang, J.; Ye, N.; Miao, Y.; Cao, J. PECTATE LYASE-LIKE 9 from Brassica campestris is associated with intine formation. Plant Sci. 2014, 229, 66–75. [Google Scholar] [CrossRef]
- Sun, H.; Hao, P.; Ma, Q.; Zhang, M.; Qin, Y.; Wei, H.; Su, J.; Wang, H.; Gu, L.; Wang, N.; et al. Genome-wide identification and expression analyses of the Pectate lyase (PEL) gene family in cotton (Gossypium hirsutum L.). BMC Genom. 2018, 19, 661. [Google Scholar] [CrossRef]
- Yin, W.Z.; Yang, H.X.; Wang, Y.T.; Feng, P.; Deng, Y.; Zhang, L.S.; He, G.H.; Wang, N. Oryza sativa PECTIN DEFECTIVE TAPETUM1 affects anther development through a pectin-mediated signaling pathway in rice. Plant Physiol. 2022, 189, 1570–1586. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Zhang, D.; Jung, K.H. Molecular basis of pollen germination in cereals. Trends Plant Sci. 2019, 24, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Liu, M.J.; Kita, D.; Jordan, S.S.; Yeh, F.J.; Yvon, R.; Carpenter, H.; Federico, A.N.; Garcia-Valencia, L.E.; Eyles, S.J.; et al. FERONIA controls pectin- and nitric oxide-mediated male-female interaction. Nature 2020, 579, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Zhao, S.Q.; Gu, S.; Cao, X.Y.; Zhang, Y.; Niu, J.F.; Liu, L.; Li, A.R.; Jia, W.S.; Qi, B.X.; et al. FvWRKY48 binds to the pectate lyase FvPLA promoter to control fruit softening in Fragaria vesca. Plant Physiol. 2022, 189, 1037–1049. [Google Scholar] [CrossRef]
- Cheng, M.; Meng, F.; Qi, H.; Mo, F.; Wang, P.; Chen, X.; Wang, A. Escaping drought: The pectin methylesterase inhibitor gene Slpmei27 can significantly change drought resistance in tomato. Plant Physiol. Biochem. 2022, 192, 207–217. [Google Scholar] [CrossRef]
- Zhu, J.; Lee, B.H.; Dellinger, M.; Cui, X.; Zhang, C.; Wu, S.; Nothnagel, E.A.; Zhu, J.K. A cellulose synthase-like protein is required for osmotic stress tolerance in Arabidopsis. Plant J. 2010, 63, 128–140. [Google Scholar] [CrossRef]
- Shahin, L.; Zhang, L.; Mohnen, D.; Urbanowicz, B.R. Insights into pectin O-acetylation in the plant cell wall: Structure, synthesis, and modification. Cell Surf. 2023, 9, 100099. [Google Scholar] [CrossRef]
- Calderone, S.; Mauri, N.; Manga-Robles, A.; Fornale, S.; Garcia-Mir, L.; Centeno, M.L.; Sanchez-Retuerta, C.; Ursache, R.; Acebes, J.L.; Campos, N.; et al. Diverging cell wall strategies for drought adaptation in two maize inbreds with contrasting lodging resistance. Plant Cell Environ. 2024, 47, 1747–1768. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Huang, Y.; Cui, G.; Zhang, X. Exogenous silicon improved the cell wall stability by activating non-structural carbohydrates and structural carbohydrates metabolism in salt and drought stressed Glycyrrhiza uralensis stem. Int. J. Biol. Macromol. 2024, 283, 137817. [Google Scholar] [CrossRef]
- Schumacher, C.; Thümecke, S.; Schilling, F.; Köhl, K.A.-O.; Kopka, J.A.-O.; Sprenger, H.A.-O.; Hincha, D.A.-O.; Walther, D.A.-O.; Seddig, S.; Peters, R.; et al. Genome-wide approach to identify quantitative trait loci for drought tolerance in tetraploid potato (Solanum tuberosum L.). Int. J. Mol. Sci. 2021, 22, 6123. [Google Scholar] [CrossRef]
- Hoedemaekers, K.; Derksen, J.; Hoogstrate, S.W.; Wolters-Arts, M.; Oh, S.A.; Twell, D.; Mariani, C.; Rieu, I. BURSTING POLLEN is required to organize the pollen germination plaque and pollen tube tip in Arabidopsis thaliana. New Phytol. 2015, 206, 255–267. [Google Scholar] [CrossRef]
- Truskina, J.; Bruck, S.; Stintzi, A.; Boeuf, S.; Doll, N.M.; Fujita, S.; Geldner, N.; Schaller, A.; Ingram, G.C. A peptide-mediated, multilateral molecular dialogue for the coordination of pollen wall formation. Proc. Natl. Acad. Sci. USA 2022, 119, e2201446119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Li, N.; Sun, G.; Zhang, L.; Wang, Y.; Jiang, Y.; Yan, H. Pectate Lyase FvePL1 Is Required for Pollen Fertility and Mediates Drought Response in Woodland Strawberry. Plants 2025, 14, 3583. https://doi.org/10.3390/plants14233583
Huang X, Li N, Sun G, Zhang L, Wang Y, Jiang Y, Yan H. Pectate Lyase FvePL1 Is Required for Pollen Fertility and Mediates Drought Response in Woodland Strawberry. Plants. 2025; 14(23):3583. https://doi.org/10.3390/plants14233583
Chicago/Turabian StyleHuang, Xiaolong, Na Li, Guilian Sun, Linfang Zhang, Yuqian Wang, Yu Jiang, and Huiqing Yan. 2025. "Pectate Lyase FvePL1 Is Required for Pollen Fertility and Mediates Drought Response in Woodland Strawberry" Plants 14, no. 23: 3583. https://doi.org/10.3390/plants14233583
APA StyleHuang, X., Li, N., Sun, G., Zhang, L., Wang, Y., Jiang, Y., & Yan, H. (2025). Pectate Lyase FvePL1 Is Required for Pollen Fertility and Mediates Drought Response in Woodland Strawberry. Plants, 14(23), 3583. https://doi.org/10.3390/plants14233583






