Pollen Viability and Anomalies in European Hazelnut: Cultivar Traits or Environmental Effect?
Abstract
1. Introduction
2. Results
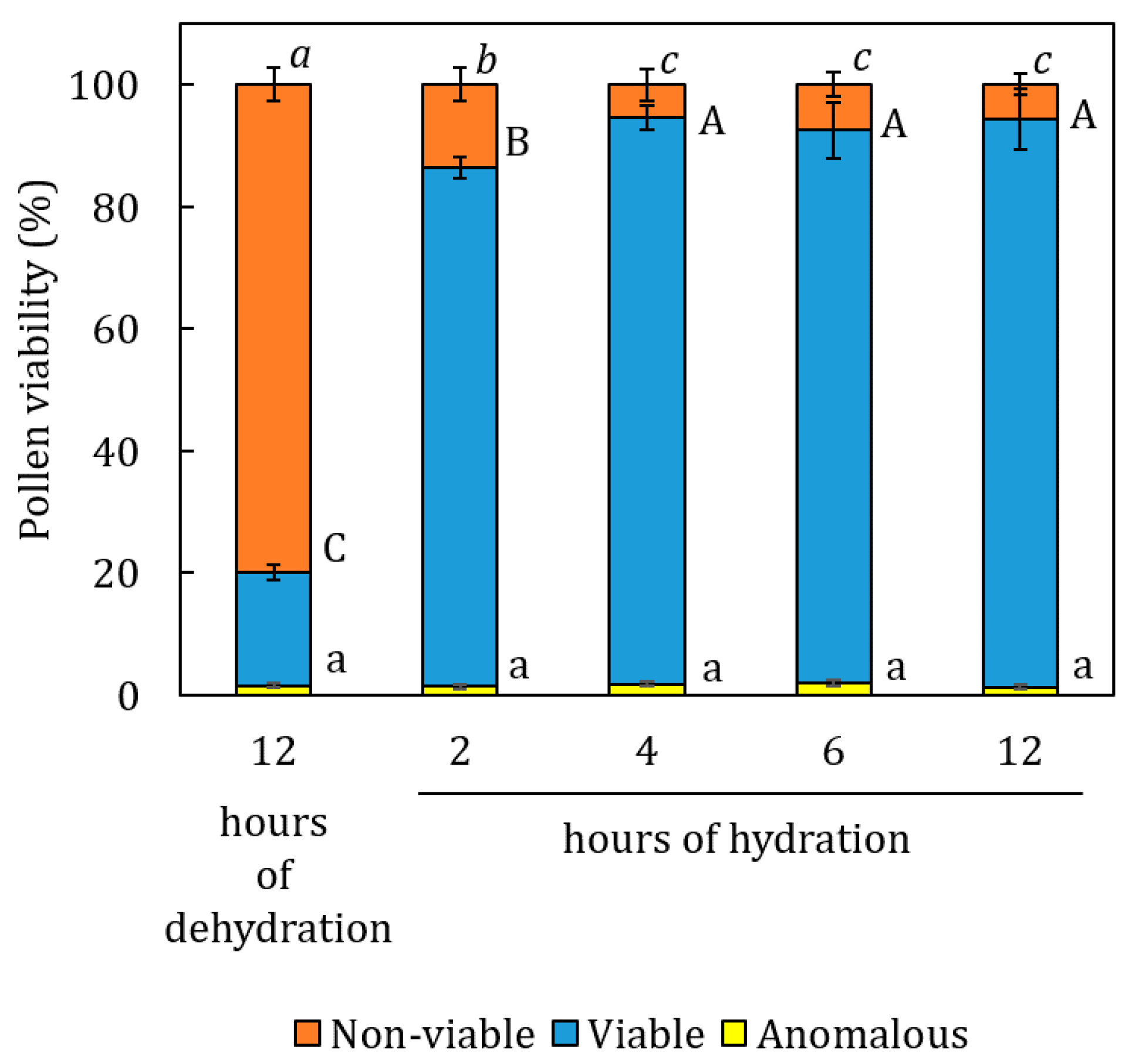
2.1. Pollen Hydration Dynamics
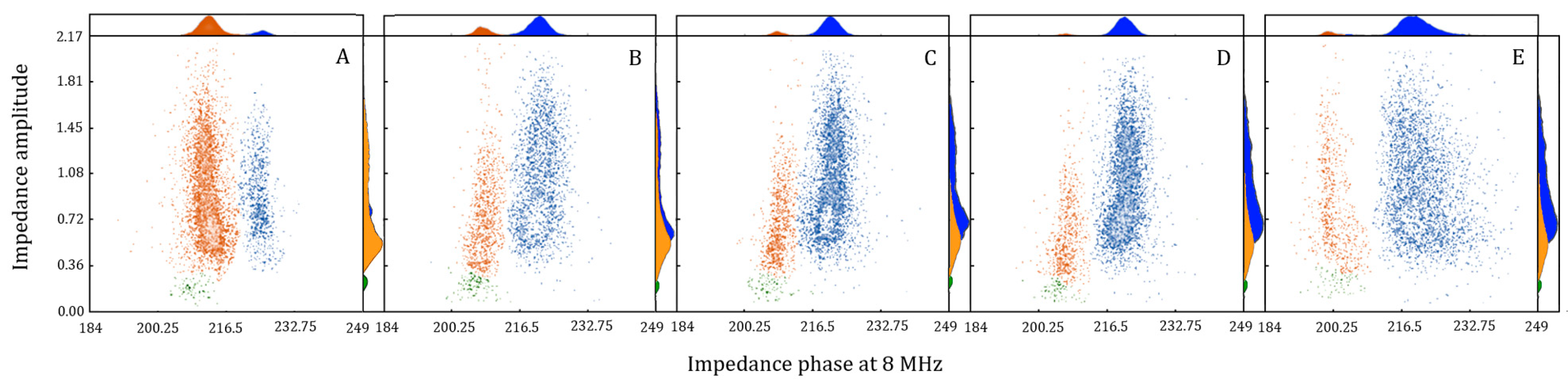
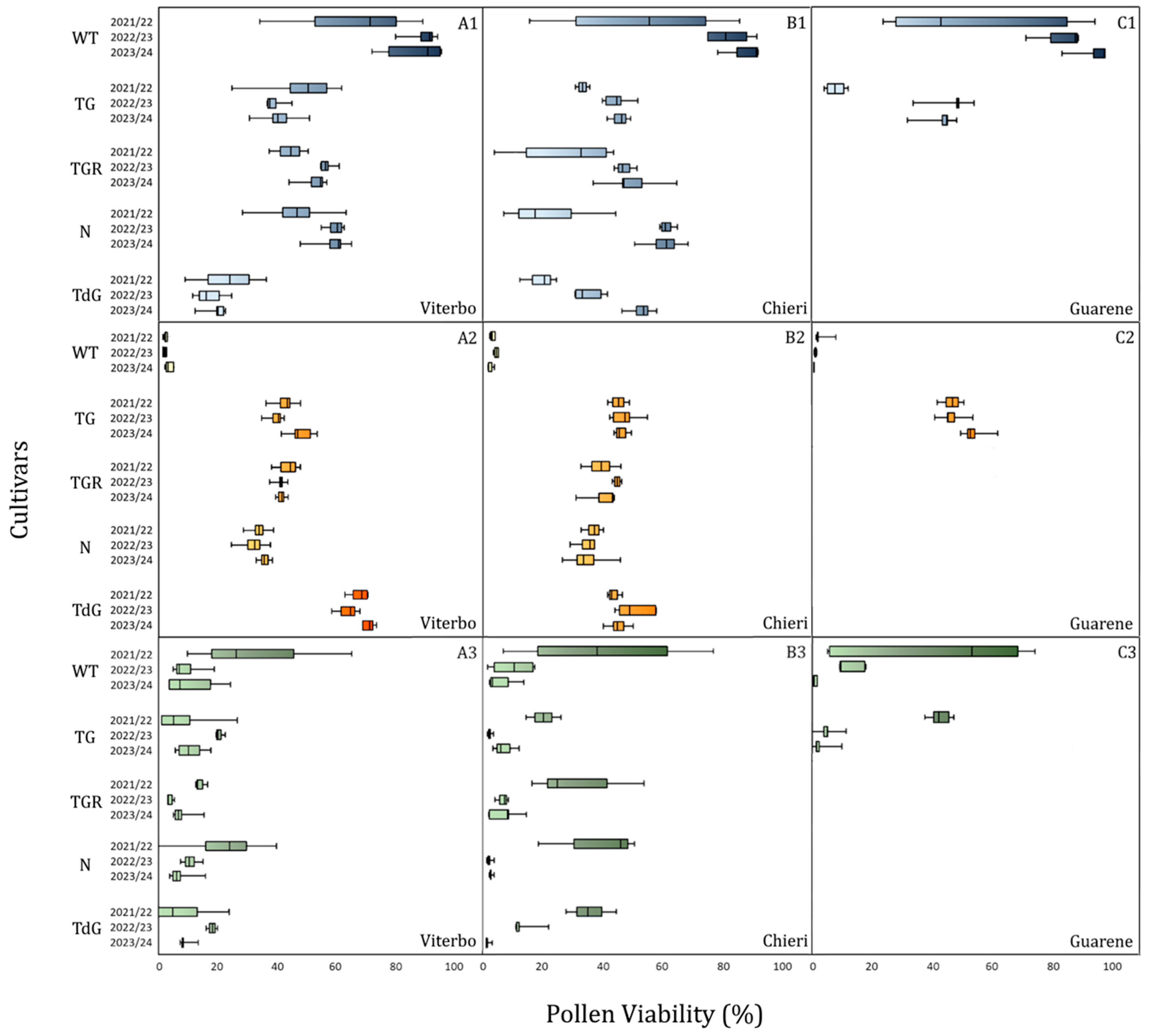
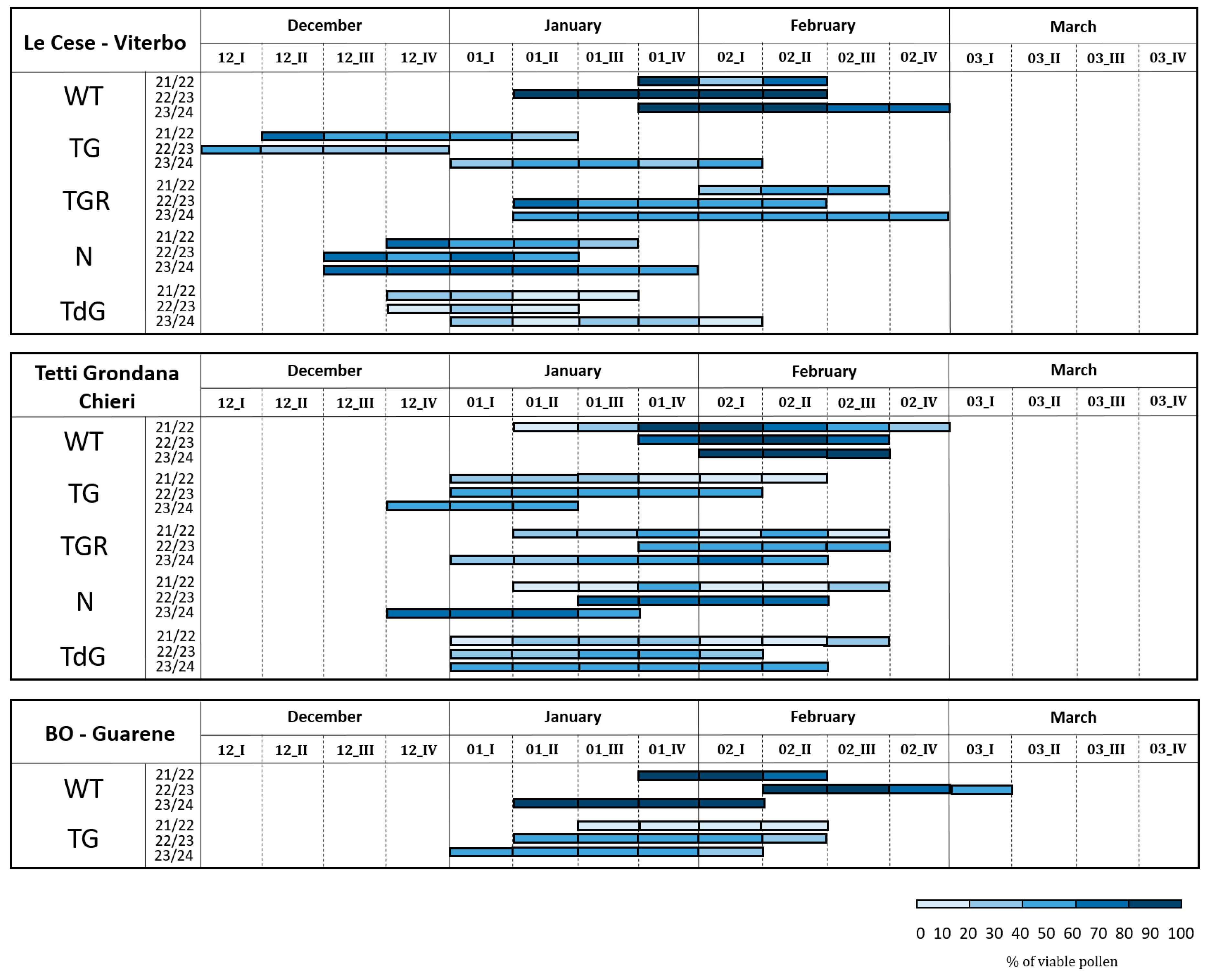
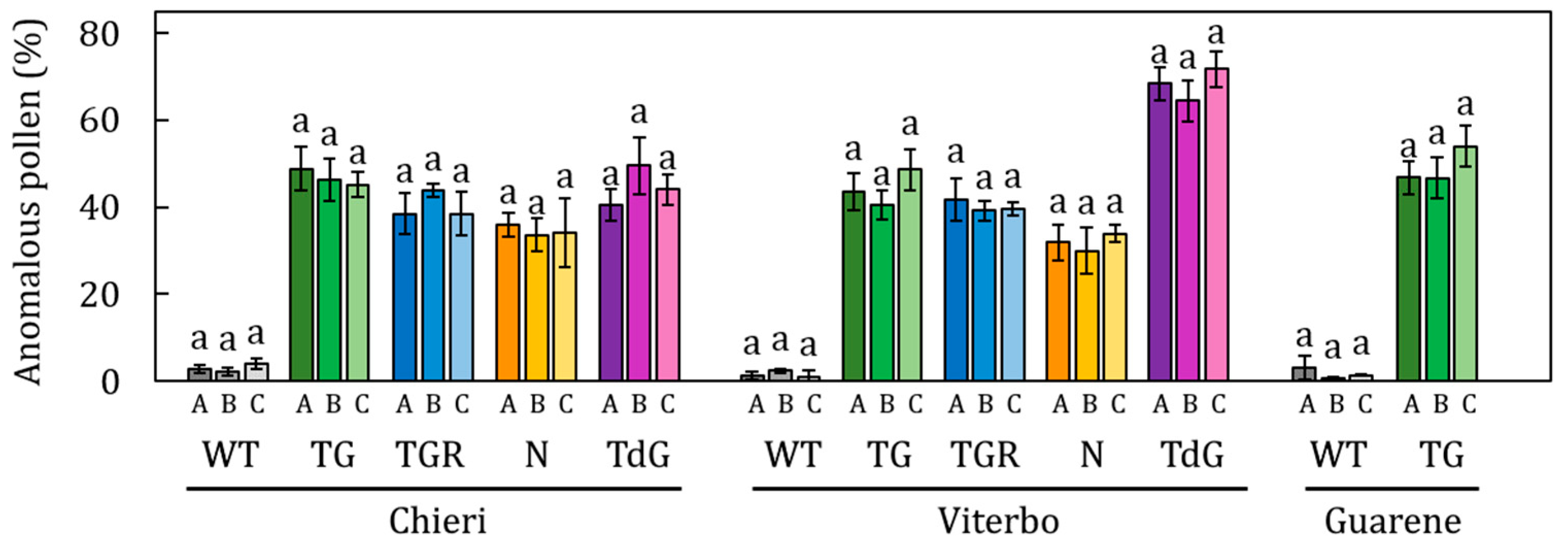
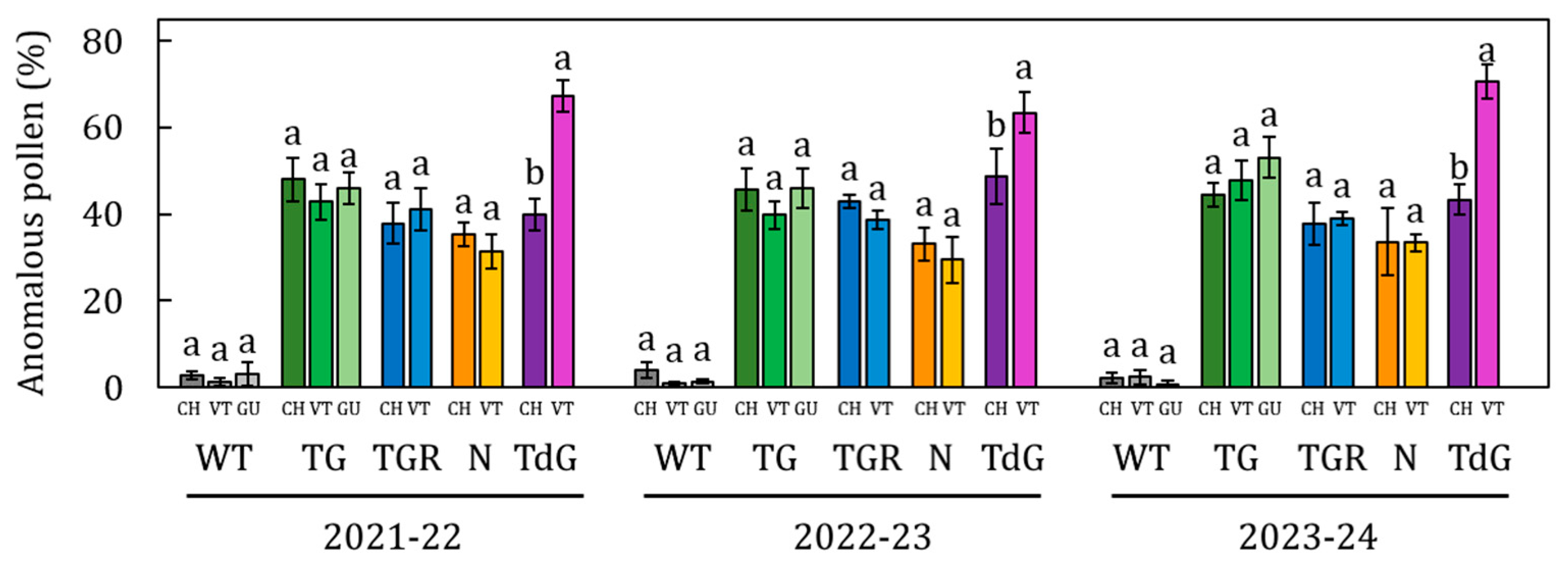
2.2. Pollen Viability
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Study Areas
4.2. Pollen Collection and Conservation
4.3. Study on Pollen Hydration Dynamics
4.4. Pollen Viability Analysis
4.5. Data Analysis Procedure
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/home/en (accessed on 10 March 2025).
- Ellena, M.; Sandoval, P.; Gonzalez, A.; Galdames, R.; Jequier, J.; Contreras, M.; Azocar, G. PRELIMINARY RESULTS OF SUPPLEMENTARY POLLINATION ON HAZELNUT IN SOUTH CHILE. Acta Hortic. 2014, 1052, 121–127. [Google Scholar] [CrossRef]
- Ascari, L.; Siniscalco, C.; Palestini, G.; Lisperguer, M.J.; Suarez Huerta, E.; De Gregorio, T.; Bregaglio, S. Relationships between Yield and Pollen Concentrations in Chilean Hazelnut Orchards. Eur. J. Agron. 2020, 115, 126036. [Google Scholar] [CrossRef]
- Mohr Fuchslocher, J.V.; Navarro Gaete, S.A. Hazelnut Production Areas in Chile, Performance of Cultivars from Oregon State University, and an Equation to Predict Performance. Acta Hortic. 2023, 1379, 21–26. [Google Scholar] [CrossRef]
- WTO World Trade Organization. Available online: https://www.wto.org/search (accessed on 10 March 2025).
- Köksal, A.İ.; Artik, N.; Şimşek, A.; Güneş, N. Nutrient Composition of Hazelnut (Corylus avellana L.) Varieties Cultivated in Turkey. Food Chem. 2006, 99, 509–515. [Google Scholar] [CrossRef]
- Wani, I.A.; Ayoub, A.; Bhat, N.A.; Dar, A.H.; Gull, A. In Antioxidants in Vegetables and Nuts-Properties and Health Benefits; Nayik, G.A., Gull, A., Eds.; Hazelnut. Springer: Singapore, 2020; pp. 559–572. ISBN 978-981-15-7469-6. [Google Scholar]
- Pacchiarelli, A.; Silvestri, C.; Muganu, M.; Cristofori, V. Influence of the Plant Training System on Yield and Nut Traits of European Hazelnut (Corylus avellana L.) Cultivar Nocchione. Agronomy 2025, 15, 345. [Google Scholar] [CrossRef]
- Balta, F.; Yılmaz, M.; Karakaya, O.; Çalışkan, K.; Yarılgaç, T.; Bostan, S.Z.; Balta, M.F.; Uzun, S. Effect of Plant Density on Nut Traits, Nut Yield, Cluster Distribution and Chemical Components in Çakıldak (Corylus avellana L.) Hazelnut Cultivar. Appl. Fruit Sci. 2024, 66, 2295–2305. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A. GENETIC RESOURCES FOR HAZELNUT: STATE OF THE ART AND FUTURE PERSPECTIVES. Acta Hortic. 2009, 845, 33–38. [Google Scholar] [CrossRef]
- Pacchiarelli, A.; Lupo, M.; Ferrucci, A.; Giovanelli, F.; Priori, S.; Pica, A.L.; Silvestri, C.; Cristofori, V. Phenology, Yield and Nut Traits Evaluation of Twelve European Hazelnut Cultivars Grown in Central Italy. Forests 2024, 15, 833. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. AN INTERPRETATION OF THE HYDRODYNAMICS OF POLLEN. Am. J. Bot. 1979, 66, 737–743. [Google Scholar] [CrossRef]
- Nepi, M.; Franchi, G.G.; Padni, E. Pollen Hydration Status at Dispersal: Cytophysiological Features and Strategies. Protoplasma 2001, 216, 171–180. [Google Scholar] [CrossRef]
- Pacini, E.; Guarnieri, M.; Nepi, M. Pollen Carbohydrates and Water Content during Development, Presentation, and Dispersal: A Short Review. Protoplasma 2006, 228, 73–77. [Google Scholar] [CrossRef]
- Zielinski, Q.B. Techniques for Collecting, Handling Germinating, and Storing of Pollen of the Filbert (Corylus Spp.). Euphytica 1968, 17, 121–125. [Google Scholar] [CrossRef]
- Franchi, G.G.; Piotto, B.; Nepi, M.; Baskin, C.C.; Baskin, J.M.; Pacini, E. Pollen and Seed Desiccation Tolerance in Relation to Degree of Developmental Arrest, Dispersal, and Survival. J. Exp. Bot. 2011, 62, 5267–5281. [Google Scholar] [CrossRef]
- Fattahi, R.; Mohammadzedeh, M.; Khadivi-Khub, A. Influence of Different Pollen Sources on Nut and Kernel Characteristics of Hazelnut. Sci. Hortic. 2014, 173, 15–19. [Google Scholar] [CrossRef]
- Ashman, T.-L.; Knight, T.M.; Steets, J.A.; Amarasekare, P.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mazer, S.J.; Mitchell, R.J.; et al. POLLEN LIMITATION OF PLANT REPRODUCTION: ECOLOGICAL AND EVOLUTIONARY CAUSES AND CONSEQUENCES. Ecology 2004, 85, 2408–2421. [Google Scholar] [CrossRef]
- Brewbaker, J.L.; Majumder, S.K. CULTURAL STUDIES OF THE POLLEN POPULATION EFFECT AND THE SELF-INCOMPATIBILITY INHIBITION. Am. J. Bot. 1961, 48, 457–464. [Google Scholar] [CrossRef]
- Hormaza, J.I.; Herrero, M. Dynamics of Pollen Tube Growth under Different Competition Regimes. Sex. Plant Reprod. 1996, 9, 153–160. [Google Scholar] [CrossRef]
- Larrosa, F.H.; Maune, J.F.; Erazzú, L.E.; Camadro, E.L. Meiotic Abnormalities Underlying Pollen Sterility in Wild Potato Hybrids and Spontaneous Populations. Plant Biol. 2012, 14, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Iovane, M.; Aronne, G. High Temperatures during Microsporogenesis Fatally Shorten Pollen Lifespan. Plant Reprod. 2022, 35, 9–17. [Google Scholar] [CrossRef]
- Frenguelli, G.; Ferranti, F.; Tedeschini, E.; Andreutti, R. Volume Changes in the Pollen Grain of Corylus avellana L. (Corylaceae) during Development. Grana 1997, 36, 289–292. [Google Scholar] [CrossRef]
- Novara, C.; Ascari, L.; La Morgia, V.; Reale, L.; Genre, A.; Siniscalco, C. Viability and Germinability in Long Term Storage of Corylus avellana Pollen. Sci. Hortic. 2017, 214, 295–303. [Google Scholar] [CrossRef]
- Ascari, L.; Cristofori, V.; Macrì, F.; Botta, R.; Silvestri, C.; De Gregorio, T.; Huerta, E.S.; Di Berardino, M.; Kaufmann, S.; Siniscalco, C. Hazelnut Pollen Phenotyping Using Label-Free Impedance Flow Cytometry. Front. Plant Sci. 2020, 11, 615922. [Google Scholar] [CrossRef]
- Brandoli, C.; Cristofori, V.; Silvestri, C.; Todeschini, C.; Sgarbi, E. The Development of an Improved Medium for the In Vitro Germination of Corylus avellana L. Pollen. Forests 2024, 15, 1095. [Google Scholar] [CrossRef]
- Brandoli, C.; Dito, G.; Tombesi, S.; Todeschini, C.; Siniscalco, C.; Sgarbi, E. Relationship among Carbohydrates Content, Viability and Germinability in Pollen of European Hazelnut (Corylus avellana L.) Cultivars. Hortic. Environ. Biotechnol. 2025, 66, 741–757. [Google Scholar] [CrossRef]
- Brandoli, C.; Mortada, A.; Todeschini, C.; Siniscalco, C.; Sgarbi, E. The Role of Sucrose in Maintaining Pollen Viability and Germinability in Corylus avellana L.: A Possible Strategy to Cope with Climate Variability. Protoplasma 2024, 262, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Edlund, A.F. Pollen and Stigma Structure and Function: The Role of Diversity in Pollination. Plant Cell Online 2004, 16, S84–S97. [Google Scholar] [CrossRef] [PubMed]
- Salesses, G. Cytological Study of Genus Corylus: A Heterozygotic Translocation in Some Low Male Fertile Varieties of Hazelnut (Corylus avellana). Ann. Amelior. Plantes 1973, 23, 59–66. [Google Scholar]
- Salesses, G.; Bonnet, A. Cytogenetic Studies of Hybrides among Corylus avellana Having Translocations in Heterozygotic States. Cytologia 1988, 53, 407–413. [Google Scholar] [CrossRef]
- Broussard, M.A.; Coates, M.; Martinsen, P. Artificial Pollination Technologies: A Review. Agronomy 2023, 13, 1351. [Google Scholar] [CrossRef]
- Ferrucci, A.; Lupo, M.; Turco, S.; Pavese, V.; Marinoni, D.T.; Botta, R.; Cristofori, V.; Mazzaglia, A.; Silvestri, C. A Roadmap of Tissue Culture and Biotechnology in European Hazelnut (Corylus avellana L.). Plant Physiol. Biochem. 2023, 205, 108167. [Google Scholar] [CrossRef]
- Črepinšek, Z.; Štampar, F.; Kajfež-Bogataj, L.; Solar, A. The Response of Corylus avellana L. Phenology to Rising Temperature in North-Eastern Slovenia. Int. J. Biometeorol. 2012, 56, 681–694. [Google Scholar] [CrossRef]
- Capik, J.M.; Molnar, T.J. Flowering Phenology of Eastern Filbert Blight-Resistant Hazelnut Accessions in New Jersey. HortTechnology 2014, 24, 196–208. [Google Scholar] [CrossRef]
- Shivanna, K.R.; Heslop-Harrison, J. Membrane State and Pollen Viability. Ann. Bot. 1981, 47, 759–770. [Google Scholar] [CrossRef]
- Chicchiriccò, G. Microsporogenesis and Pollen Development in Crocus sativus L. Caryologia 1989, 42, 249–257. Caryologia 1989, 42, 249–257. [Google Scholar] [CrossRef]
- Issarakraisila, M. Effects of Temperature on Pollen Viability in Mango Cv. “Kensington”. Ann. Bot. 1994, 73, 231–240. [Google Scholar] [CrossRef]
- Pacini, E.; Dolferus, R. Pollen Developmental Arrest: Maintaining Pollen Fertility in a World With a Changing Climate. Front. Plant Sci. 2019, 10, 679. [Google Scholar] [CrossRef]
- Jha, P.K.; Materia, S.; Zizzi, G.; Costa-Saura, J.M.; Trabucco, A.; Evans, J.; Bregaglio, S. Climate Change Impacts on Phenology and Yield of Hazelnut in Australia. Agric. Syst. 2021, 186, 102982. [Google Scholar] [CrossRef]
- Lysák, M.A.; Schubert, I. In Plant Genome Diversity Volume 2; Greilhuber, J., Dolezel, J., Wendel, J.F., Eds.; Mechanisms of Chromosome Rearrangements. Springer: Vienna, Austria, 2013; pp. 137–147. ISBN 978-3-7091-1159-8. [Google Scholar]
- Brock, R.D.; Pryor, A.J. An Unstable Minichromosome Generates Variegated Oil Yellow Maize Seedlings. Chromosoma 1996, 104, 575–584. [Google Scholar] [CrossRef]
- Berdnikov, V.A.; Kosterin, O.E.; Bogdanova, V.S. Mortality of Pollen Grains May Result from Errors of Meiosis: Study of Pollen Tetrads in Typha Latifolia L. Heredity 2002, 89, 358–362. Heredity 2002, 89, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Geoportale Regione Piemonte Home—Geoportale Piemonte. Available online: https://geoportale.igr.piemonte.it/cms/ (accessed on 31 March 2025).
- ArpaLazio Piano Della Caratterizzazione Del Lago Di Vico 2012. Available online: https://www.arpalazio.it/documents/20124/40140/PdC_Vico_Regione.pdf(accessed on 30 October 2025).
- Dell’Abate, M.T.; Benedetti, A.; Nardi, P.; Di Bartolomeo, E.; Fabrizio, G. Soil-Plant Relationships in the Cimini-Sabatini Hazelnut District: Plant Nutrition and Soil Fertility Status. Acta Hortic. 2009, 845, 391–398. [Google Scholar] [CrossRef]
- Locardi, E. Tipi Di Ignimbriti Di Magmi Mediterranei: Le Ignimbriti Del Vulcano Di Vico. Atti Soc. Toscana Sci. Nat. 1965, 72, 55–173. [Google Scholar]
- Barbanti, L. Lago Di Vico: Rilevamento Batimetrico e Note Geomorfologiche. Mem. Dellistituto Ital. Idrobiol. 1969, 25, 117–139. [Google Scholar]
- Bertagnini, A.; Sbrana, A. Il Vulcano Di Vico: Stratigrafia Del Complesso Vulcanico e Sequenze Eruttive Delle Formazioni Piroclastiche. Mem. Soc. Geol. Ital. 1986, 35, 699–713. [Google Scholar]
- Global Soil Organic Carbon Map Global Soil Organic Carbon Map (GSOCmap)|FAO SOILS PORTAL|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/global-soil-organic-carbon-map-gsocmap/en/ (accessed on 31 March 2025).
- ArpaLazio Inquadramento Climatologico Regione Lazio 2019. Available online: https://progetti.regione.lazio.it/contrattidifiume/app/uploads/sites/53/C_03_inquadramento-climatologico.pdf (accessed on 30 October 2025).
- Taghavi, T.; Rahemi, A.; Suarez, E. Development of a Uniform Phenology Scale (BBCH) in Hazelnuts. Sci. Hortic. 2022, 296, 110837. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Crowe, J.H.; Crowe, L.M. Effect of Sucrose on Phase Behavior of Membranes in Intact Pollen of Typha Latifolia L., as Measured with Fourier Transform Infrared Spectroscopy. Plant Physiol. 1991, 97, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Speranza, A.; Calzoni, G.L.; Pacini, E. Occurrence of Mono- or Disaccharides and Polysaccharide Reserves in Mature Pollen Grains. Sex. Plant Reprod. 1997, 10, 110–115. [Google Scholar] [CrossRef]
- Pacini, E.; Hesse, M. Cytophysiology of Pollen Presentation and Dispersal. Flora-Morphol. Distrib. Funct. Ecol. Plants 2004, 199, 273–285. [Google Scholar] [CrossRef]
- Brandoli, C.; Sgarbi, E.; Cristofori, V.; Todeschini, C.; Siniscalco, C. Correlation between Carbohydrate Content and Viability in Pollen of Some Italian Hazelnut Cultivars. Acta Hortic. 2025, 1425, 349–356. [Google Scholar] [CrossRef]
- Cheung, K.; Gawad, S.; Renaud, P. Impedance Spectroscopy Flow Cytometry: On-Chip Label-Free Cell Differentiation. Cytometry A 2005, 65A, 124–132. [Google Scholar] [CrossRef]
- Sun, T.; Morgan, H. Single-Cell Microfluidic Impedance Cytometry: A Review. Microfluid. Nanofluidics 2010, 8, 423–443. [Google Scholar] [CrossRef]
- Tai, G.C.C. Analysis of Genotype-Environment Interactions of Potato Yield1. Crop Sci. 1979, 19, 434–438. [Google Scholar] [CrossRef]
| Environment (E) | R2 (E) % | Genotype (G) | R2 (G) % | Phenological Phase (PP) | R2 (PP) % | E × G | E × PP | G × PP | E × G × PP | |
|---|---|---|---|---|---|---|---|---|---|---|
| Season 2021–22 | ||||||||||
| Viable pollen | * | 10.4 | * | 32.7 | * | 7.2 | * | * | * | * |
| Anomalous pollen | * | 1.8 | * | 38.3 | n.s. | n.s. | * | n.s. | n.s. | n.s. |
| Season 2022–23 | ||||||||||
| Viable pollen | * | 25.1 | * | 35.1 | * | 3.4 | * | * | * | * |
| Anomalous pollen | * | 3.7 | * | 51.8 | n.s. | n.s. | * | n.s. | n.s. | n.s. |
| Season 2023–24 | ||||||||||
| Viable pollen | * | 19.9 | * | 44.1 | n.s. | n.s. | * | * | * | * |
| Anomalous pollen | * | 4.1 | * | 36.4 | n.s. | n.s. | * | n.s. | n.s. | n.s. |
| Environment (E) | R2 (E) % | Phenological Phase (PP) | R2 (PP) % | E × PP | |
|---|---|---|---|---|---|
| Season 2021–22 | |||||
| Viable pollen | * | 3.6 | * | 1.7 | * |
| Anomalous pollen | n.s. | n.s. | n.s. | n.s. | n.s. |
| Season 2022–23 | |||||
| Viable pollen | * | 4.7 | * | 16.3 | * |
| Anomalous pollen | * | 6.5 | n.s. | n.s. | n.s. |
| Season 2023–24 | |||||
| Viable pollen | * | 7.7 | * | 38.5 | * |
| Anomalous pollen | * | 0.54 | n.s. | n.s. | n.s. |
| Area | Field Name | Cultivars Analyzed | Coordinates |
|---|---|---|---|
| Chieri, Piedmont | Tetti Grondana | WT, TG, TGR, N, TdG | Lat. 45°02′29″ N, long. 7°50′08″ E, AMSL 327 m |
| Guarene, Piedmont | BO | WT, TG | Lat. 44°44′08″ N, long. 8°02′25″ E, AMSL 167 m |
| Caprarola, Lazio | Le Cese | WT, TG, TGR, N, TdG | Lat. 42°20′00″ N; long. 12°11′00″ E; AMSL 570 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandoli, C.; Demasi, S.; Fochi, V.; Caccialupi, G.; Cristofori, V.; Silvestri, C.; Siniscalco, C.; Todeschini, C.; Sgarbi, E. Pollen Viability and Anomalies in European Hazelnut: Cultivar Traits or Environmental Effect? Plants 2025, 14, 3576. https://doi.org/10.3390/plants14233576
Brandoli C, Demasi S, Fochi V, Caccialupi G, Cristofori V, Silvestri C, Siniscalco C, Todeschini C, Sgarbi E. Pollen Viability and Anomalies in European Hazelnut: Cultivar Traits or Environmental Effect? Plants. 2025; 14(23):3576. https://doi.org/10.3390/plants14233576
Chicago/Turabian StyleBrandoli, Claudio, Sonia Demasi, Valeria Fochi, Giovanni Caccialupi, Valerio Cristofori, Cristian Silvestri, Consolata Siniscalco, Claudio Todeschini, and Elisabetta Sgarbi. 2025. "Pollen Viability and Anomalies in European Hazelnut: Cultivar Traits or Environmental Effect?" Plants 14, no. 23: 3576. https://doi.org/10.3390/plants14233576
APA StyleBrandoli, C., Demasi, S., Fochi, V., Caccialupi, G., Cristofori, V., Silvestri, C., Siniscalco, C., Todeschini, C., & Sgarbi, E. (2025). Pollen Viability and Anomalies in European Hazelnut: Cultivar Traits or Environmental Effect? Plants, 14(23), 3576. https://doi.org/10.3390/plants14233576










