Integrating RNA-Seq and Metabolomic Perspectives Reveals the Mechanism of Response to Phosphorus Stress of Potamogeton wrightii
Abstract
1. Introduction
2. Results
2.1. Effects of Different Phosphorus Concentrations on Plant Phenotypes and Physiological Indicators
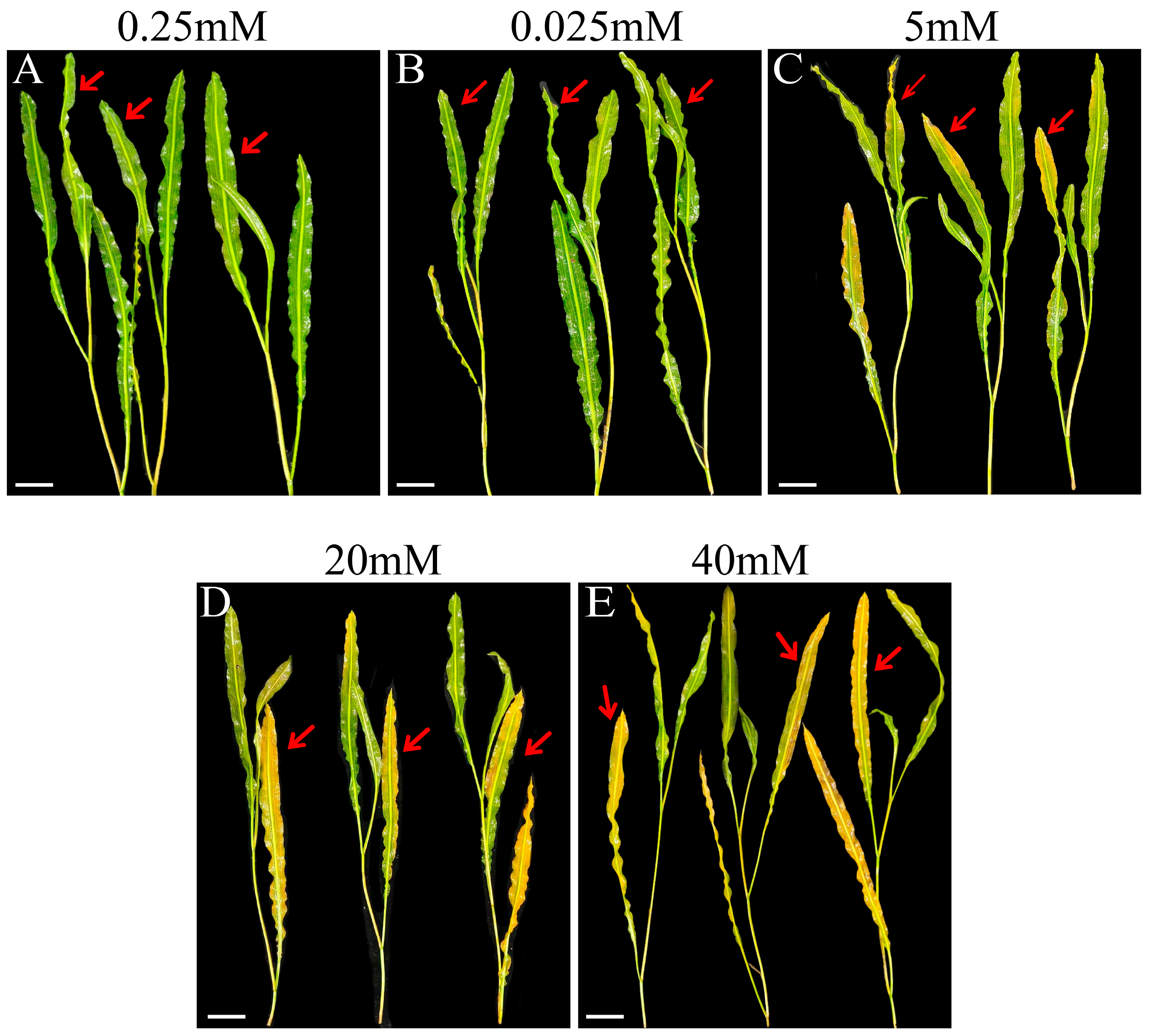
2.1.1. Morphological Differences in Plant Leaves
2.1.2. Determination of Chlorophyll Content, Antioxidant Indices, and Inorganic Phosphorus Content
2.2. RNA-Seq Analysis of P. wrightii Under Different Phosphorus Stress
2.2.1. Quality Assessment of RNA-Seq Data
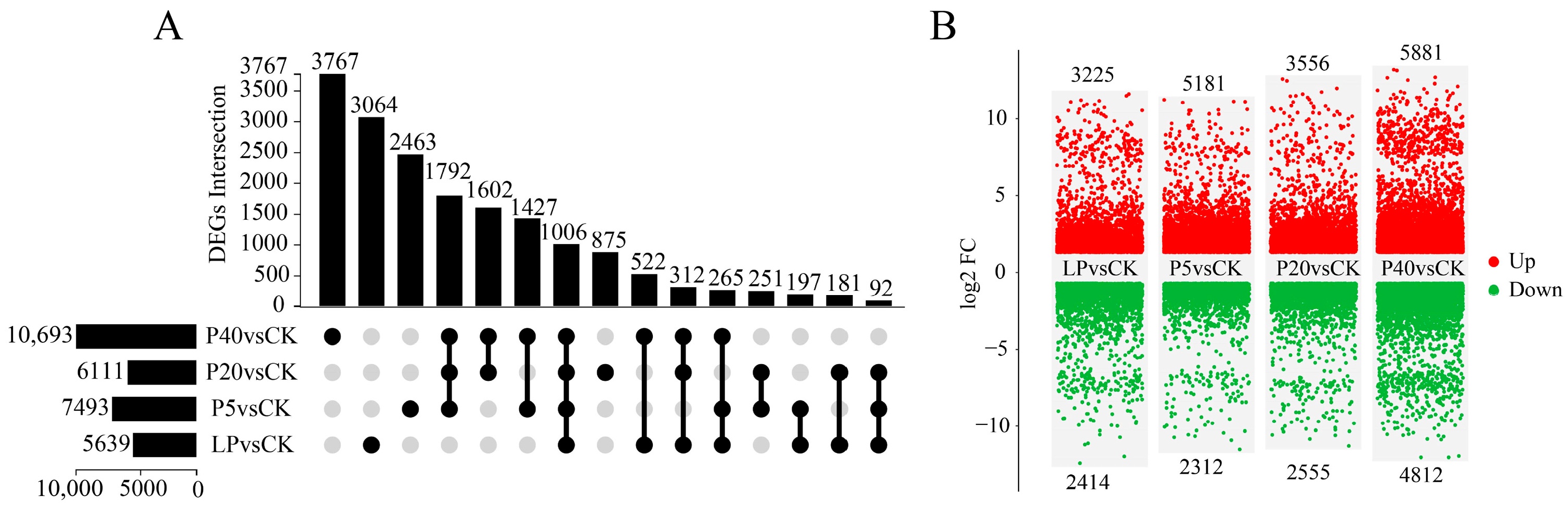
2.2.2. Identification of Differentially Expressed Genes
2.2.3. Expression of DEGs in P. wrightii Under Different Phosphorus Stress Conditions
2.2.4. RT-qPCR Analysis Results
2.3. Metabolome Analysis of P. wrightii Under Different Phosphorus Stress
2.3.1. Qualitative and Quantitative Analysis of Etabolites
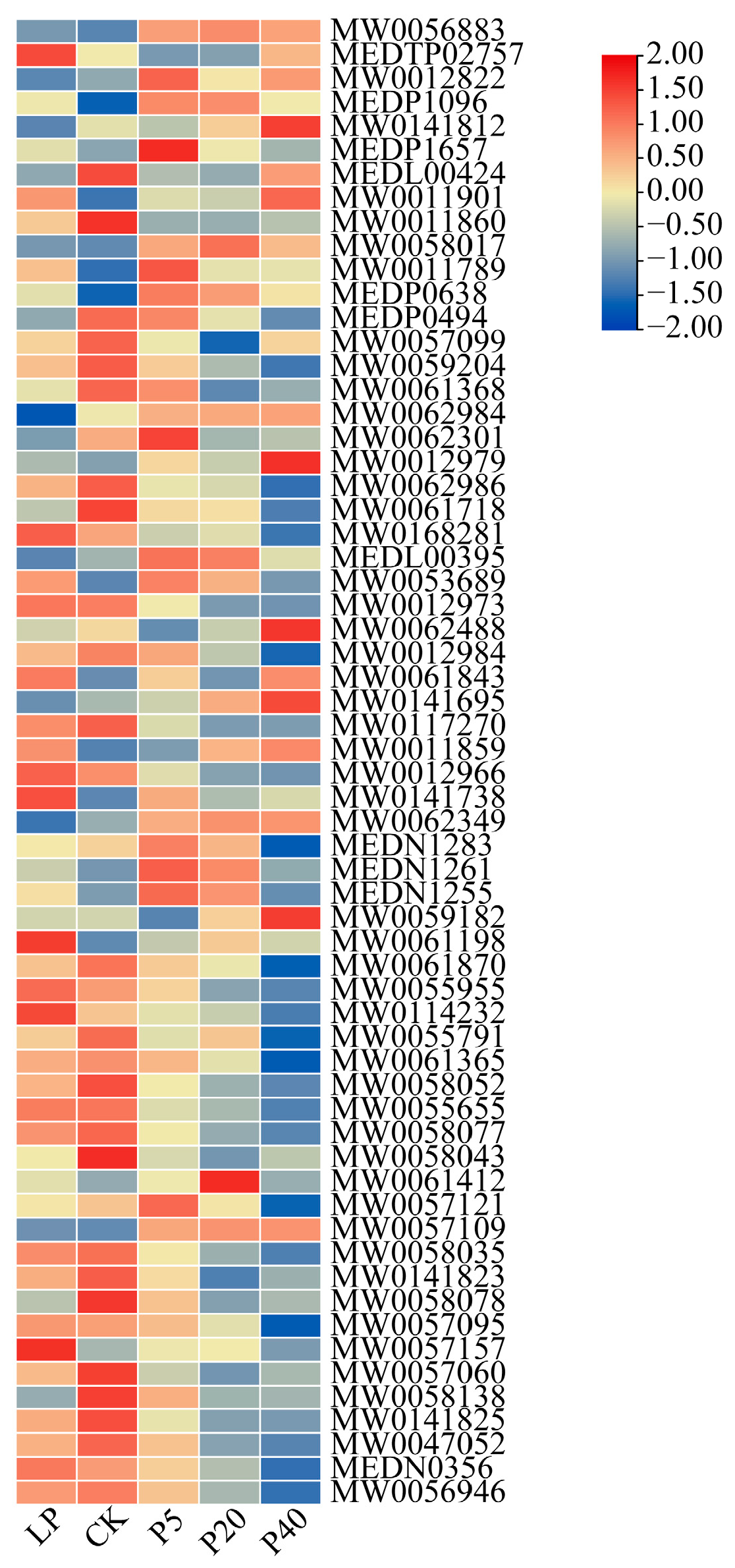
2.3.2. Analysis of Differentially Expressed Metabolites Among Different Treatments
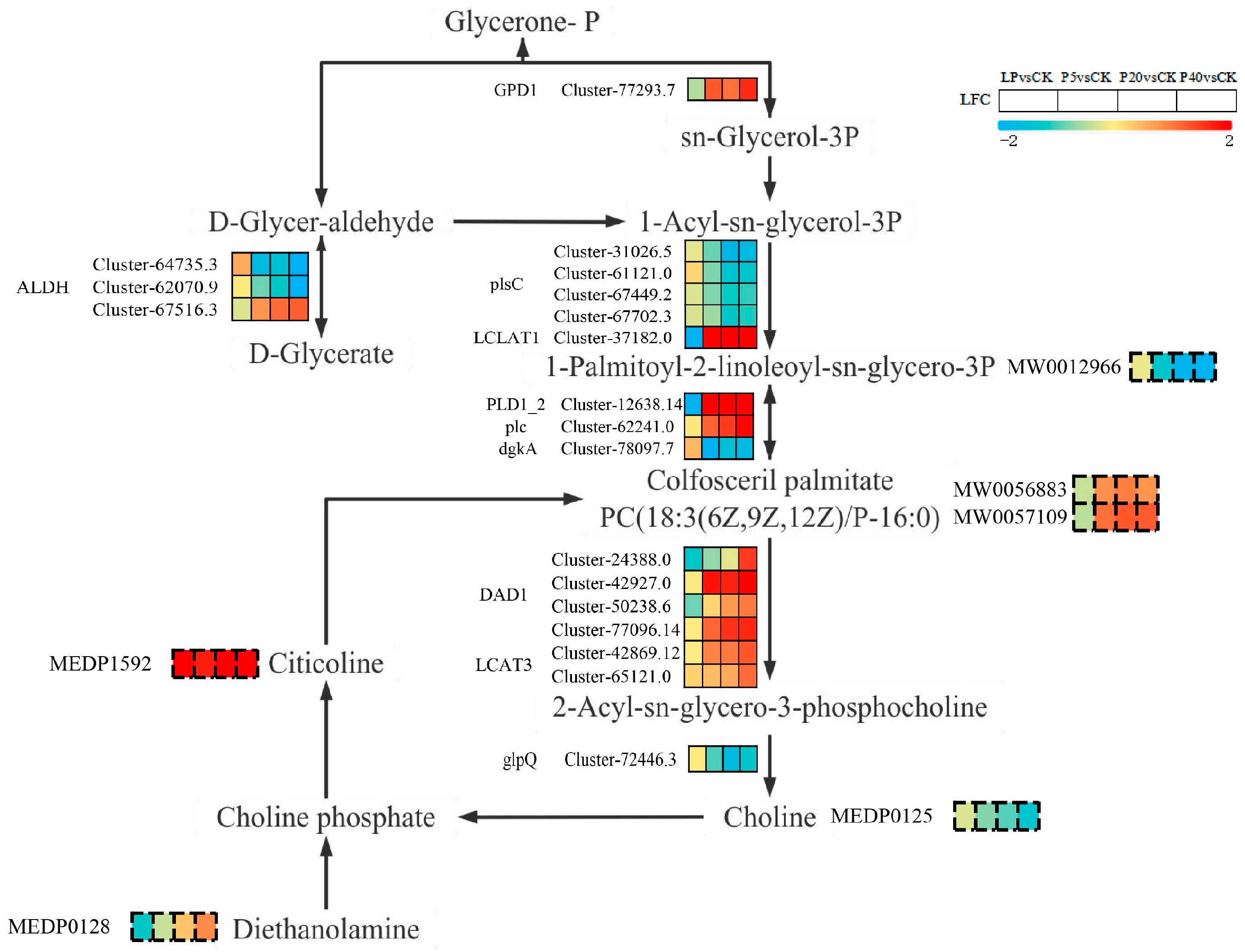
2.4. Combined Transcriptomic and Metabolomic Analysis of the Response Mechanism of P. wrightii to Different Phosphorus Levels
3. Discussion
4. Materials and Methods
4.1. Materials and Sample Preparation
4.2. Determination of Chlorophyll, Antioxidant Indicators, and Phosphorus Content in Plant Leaves
4.3. Leaf RNA-Seq and Data Analysis
4.4. Leaf Metabolome and Data Analysis
4.5. Combined RNA-Seq and Metabolome Analyses
4.6. Analysis of the Expression Characteristics of Key Genes in the Phosphorus Pathway by RT-qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Kuo, H.F.; Chiou, T.J. Intracellular phosphate sensing and regulation of phosphate transport systems in plants. Plant Physiol. 2021, 187, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef]
- Puga, M.I.; Rojas-Triana, M.; De Lorenzo, L.; Leyva, A.; Rubio, V.; Paz-Ares, J. Novel signals in the regulation of Pi starvation responses in plants: Facts and promises. Curr. Opin. Plant Biol. 2017, 39, 40–49. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.B.; Ghahremani, M.; Siebers, M.; Wasaki, J.; Plaxton, W.C. Recent insights into the metabolic adaptations of phosphorus-deprived plants. J. Exp. Bot. 2020, 72, 199–223. [Google Scholar] [CrossRef]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef]
- Li, H.; He, K.; Zhang, Z.; Hu, Y. Molecular mechanism of phosphorous signaling inducing anthocyanin accumulation in Arabidopsis. Plant Physiol. Biochem. 2023, 196, 121–129. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, J.; Zhang, Q.; Li, X.; Li, M.; Yang, Y.; Zhou, J.; Wei, Q.; Zhou, B. RNA-seq and metabolome analyses revealed the response mechanism of apple to different phosphorus stresses. Plant Physiol. Biochem. 2021, 167, 639–650. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Huang, T.; Zhang, X.; Zhang, P.; Xie, H.; Liu, J.; Li, L.; Kong, Z.; Qin, P. RNA-seq and metabolome analyses revealed the response mechanism of quinoa seedlings to different phosphorus stresses. Int. J. Mol. Sci. 2022, 23, 4704. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Wang, K.; Xu, C.; Zou, C.; Xie, C.; Xu, Y.; Li, W.X. Strand-specific RNA-Seq RNA-seq analysis of genotypes with and without low-phosphorus tolerance provides novel insights into phosphorus-use efficiency in maize. BMC Plant Biol. 2016, 16, 222. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.Q.; Wang, G.P.; Zhang, Y.Q.; Hu, X.Y.; Pi, E.X.; Zhu, Y.Y.; Wang, H.Z.; Du, L.Q. Genome-wide identification of phosphate-deficiency-responsive genes in soybean roots by high-throughput sequencing. Plant Soil. 2016, 398, 207–227. [Google Scholar]
- Deng, Q.W.; Luo, X.D.; Chen, Y.L.; Zhou, Y.; Zhang, F.T.; Hu, B.L.; Xie, J.K. RNA-seq analysis of phosphorus stress responsiveness in the seedlings of Dongxiang wild rice (Oryza rufipogon Griff.). Biol. Res. 2018, 51, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, Q.; Pan, J.; Sun, L.; Sun, Y.; Xue, Y.; Song, K. RNA-seq analysis in roots and leaves of wheat seedlings in response to low-phosphorus stress. Sci. Rep. 2019, 9, 19802. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xu, S.; Wang, R.; Sun, Q.; Zuo, J.; Zhuang, X. Transcriptomics Insights into Phosphorus Stress Response of Myriophyllum aquaticum. Int. J. Mol. Sci. 2023, 24, 4874. [Google Scholar] [CrossRef]
- Wu, P.; Shou, H.; Xu, G.; Lian, X. Improvement of phosphorus efficiency in rice on the basis of understanding phosphate signaling and homeostasis. Curr. Opin. Plant Biol. 2013, 16, 205–212. [Google Scholar] [CrossRef]
- Wang, Z.; Ruan, W.; Shi, J.; Zhang, L.; Xiang, D.; Yang, C.; Li, C.; Wu, Z.; Liu, Y.; Yu, Y.; et al. Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc. Natl. Acad. Sci. USA 2014, 111, 14953–14958. [Google Scholar] [CrossRef]
- Wild, R.; Gerasimaite, R.; Jung, J.Y.; Truffault, V.; Pavlovic, I.; Schmidt, A.; Saiardi, A.; Jessen, H.J.; Poirier, Y.; Hothorn, M.; et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 2016, 352, 986–990. [Google Scholar] [CrossRef]
- Puga, M.I.; Mateos, I.; Charukesi, R.; Wang, Z.; Franco-Zorrilla, J.M.; de Lorenzo, L.; Irigoyen, M.L.; Masiero, S.; Bustos, R.; Rodríguez, J.; et al. SPX1 is a phosphate-dependent inhibitor of Phosphate Starvation Response 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14947–14952. [Google Scholar] [CrossRef]
- Lv, Q.; Zhong, Y.; Wang, Y.; Wang, Z.; Zhang, L.; Shi, J.; Wu, Z.; Liu, Y.; Mao, C.; Yi, K.; et al. SPX4 negatively regulates phosphate signaling and homeostasis through its interaction with PHR2 in rice. Plant Cell. 2014, 26, 1586–1597. [Google Scholar] [CrossRef]
- Bustos, R.; Castrillo, G.; Linhares, F.; Puga, M.I.; Rubio, V.; Pérez-Pérez, J.; Solano, R.; Leyva, A.; Paz-Ares, J. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet. 2010, 6, e1001102. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Y.; Liang, Z.; Wu, S.; Guo, H. Internal cycling, not external loading, decides the nutrient limitation in eutrophic lake: A dynamic model with temporal Bayesian hierarchical inference. Water Res. 2017, 116, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gan, X.; Wang, Z.; Jiang, S.; Zheng, X.; Zhao, M.; Zhang, Y.; Fan, C.; Wu, S.; Du, L. Research status on remediation of eutrophic water by submerged macrophytes: A review. Process Saf. Environ. 2023, 169, 671–684. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Babourina, O.; Rengel, Z.; Yang, X.E.; Pu, P.M. Ammonium and nitrate uptake by the floating plant Landoltia punctata. Ann. Bot. 2007, 99, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, S.; Peng, Y.; Li, Y.; Chen, Z.; Xu, H.; Yu, Z.; Zheng, W.; Zheng, T. Effects of marine actinomycete on the removal of a toxicity alga Phaeocystis globose in eutrophication waters. Front. Microbiol. 2015, 6, 474. [Google Scholar] [CrossRef]
- Bakker, E.S.; Van Donk, E.; Declerck, S.A.J.; Helmsing, N.R.; Hidding, B.; Nolet, B.A. Effect of macrophyte community composition and nutrient enrichment on plant biomass and algal blooms. Basic Appl. Ecol. 2010, 11, 432–439. [Google Scholar] [CrossRef]
- Gao, H.; Qian, X.; Wu, H.; Li, H.; Pan, H.; Han, C. Combined effects of submerged macrophytes and aquatic animals on the restoration of a eutrophic water body—A case study of Gonghu Bay, Lake Taihu. Ecol. Eng. 2017, 102, 15–23. [Google Scholar] [CrossRef]
- Chao, C.; Wang, L.; Li, Y.; Yan, Z.; Liu, H.; Yu, D.; Liu, C. Response of sediment and water microbial communities to submerged vegetations restoration in a shallow eutrophic lake. Sci. Total Environ. 2021, 801, 149701. [Google Scholar] [CrossRef]
- Keskinkan, O.; Goksu, M.Z.L.; Yuceer, A.; Basibuyuk, M.; Forster, C.F. Heavy metal adsorption characteristics of a submerged aquatic plant (Myriophyllum spicatum). Process Biochem. 2003, 39, 179–183. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, X.; Han, R.; Xu, X.; Wang, G.; Liu, X.; Bi, F.; Feng, D. Reproduction capacity of Potamogeton crispus fragments and its role in water purification and algae inhibition in eutrophic lakes. Sci. Total Environ. 2017, 580, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Qin, B.; Gao, G.; Cai, X. Submerged macrophyte communities and the controlling factors in large, shallow Lake Taihu (China): Sediment distribution and water depth. J. Great Lakes Res. 2014, 40, 646–655. [Google Scholar] [CrossRef]
- Peng, K.; Luo, C.; Lou, L.; Li, X.; Shen, Z. Bioaccumulation of heavy metals by the aquatic plants Potamogeton pectinatus L. and Potamogeton malaianus Miq. and their potential use for contamination indicators and in wastewater treatment. Sci. Total Environ. 2008, 392, 22–29. [Google Scholar] [CrossRef]
- Han, S.; Xing, Z.; Jiang, H.; Li, W.; Huang, W. Biological adaptive mechanisms displayed by a freshwater plant to live in aquatic and terrestrial environments. Environ. Exp. Bot. 2021, 191, 104623. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, X.; Xia, J.; Liu, G. The effect of temperature, water level and burial depth on seed germination of Myriophyllum spicatum and Potamogeton malaianus. Aquat. Bot. 2010, 92, 28–32. [Google Scholar] [CrossRef]
- Zuo, S.; Wang, H.; Gan, L.D.; Shao, M. Allelopathy appraisal of worm metabolites in the synergistic effect between Limnodrilus hoffmeisteri and Potamogeton malaianus on algal suppression. Ecotoxicol. Environ. Saf. 2019, 182, 109482. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Wei, Q.; Qi, X.; Yin, Q.; Liu, B.; He, K. Response and synergistic effect of microbial community to submerged macrophyte in restoring urban black and smelly water bodies. J. Water Process Eng. 2023, 53, 103906. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lin, W.Y.; Hsiao, Y.M.; Chiou, T.J. Milestones in understanding transport, sensing, and signaling of the plant nutrient phosphorus. Plant Cell. 2024, 36, 1504–1523. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Hu, J.; Wang, H.; Sheng, L.; Dong, X.; Jiang, X. Nitrogen and phosphorus removal in simulated wastewater by two aquatic plants. Environ. Sci. Pollut. Res. 2021, 28, 63237–63249. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, X.; Chen, H.; Lu, C.; Che, F. Promoting effect of submerged plants on phosphorus source and sink in sediments: Response of phosphorus release and microbial community to Vallisneria natans and Potamogeton malaianus growth. Environ. Technol. Innov. 2024, 36, 103899. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, H.; Song, J.; Wu, W.; Li, K.; Zhang, J. Physiological and comparative proteome analyses reveal low-phosphate tolerance and enhanced photosynthesis in a maize mutant owing to reinforced inorganic phosphate recycling. BMC Plant Biol. 2016, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Furlani, A.L.; Bianuccii, E.; Giordanoi, W.; Castroi, S.; Beckeri, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef]
- Lv, S.; Wang, D.; Jiang, P.; Jia, W.; Li, Y. Variation of PHT families adapts salt cress to phosphate limitation under salinity. Plant Cell Environ. 2021, 44, 1549–1564. [Google Scholar] [CrossRef]
- Sun, T.; Li, M.; Shao, Y.; Yu, L.; Ma, F. Comprehensive genomic identification and expression analysis of the phosphate transporter (PHT) gene family in apple. Front. Plant Sci. 2017, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Paszkowski, U.; Kroken, S.; Roux, C.; Briggs, S.P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 13324–13329. [Google Scholar] [CrossRef]
- Mudge, S.R.; Rae, A.L.; Diatloff, E.; Smith, F.W. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J. 2002, 31, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Ai, P.H.; Sun, S.B.; Zhao, J.N.; Fan, X.R.; Xin, W.J.; Guo, Q.; Yu, L.; Shen, Q.R.; Wu, P.; Miller, A.J.; et al. Two rice phosphate transporters, OsPht1; 2 and OsPht1; 6, have different functions and kinetic properties in uptake and translocation. Plant J. 2009, 57, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.; Wang, C.; Arpat, B.A.; Wang, Z.; Poirier, Y.; Tyerman, S.D.; Wu, P.; Shou, H.; Whelan, J. The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol. 2012, 193, 842–851. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, H.; Huang, H.; Duan, K.; Wu, Z.; Wu, P. Regulation of OsSPX1 and OsSPX3 on expression of OsSPX domain genes and Pi-starvation signaling in rice. J. Integr. Plant Biol. 2009, 51, 663–674. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Ying, Y.; Li, S.; Secco, D.; Tyerman, S.; Whelan, J.; Shou, H. Functional characterization of the rice SPX-MFS family reveals a key role of OsSPX-MFS1 in controlling phosphate homeostasis in leaves. New Phytol. 2012, 196, 139–148. [Google Scholar] [CrossRef]
- Liu, H.C.; Rao, L.; Meng, J.H.; Zuo, W.T.; Sun, T.T. Genome-Wide Analysis, Identification, and Transcriptional Profile of the Response to Abiotic Stress of the Purple Acid Phosphatases (PAP) Gene Family in Apple. Int. J. Mol. Sci. 2025, 26, 1011. [Google Scholar] [CrossRef]
- Rubio, V.; Linhares, F.; Solano, R.; Martín, A.C.; Iglesias, J.; Leyva, A.; Paz-Ares, J.A. Conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 2001, 15, 2122–2133. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Z.; Zhu, Y.; Kong, S.; Liu, D. PHOSPHATE RESPONSE 1 family members act distinctly to regulate transcriptional responses to phosphate starvation. Plant Physiol. 2023, 191, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Mühlroth, A.; Winge, P.; El Assimi, A.; Jouhet, J.; Maréchal, E.; Hohmann-Marriott, M.F.; Vadstein, O.; Bones, A.M. Mechanisms of phosphorus acquisition and lipid class remodeling under P limitation in a marine microalga. Plant Physiol. 2017, 175, 1543–1559. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, C.; Fan, J.; Shanklin, J.; Xu, C. Mechanisms and functions of membrane lipid remodeling in plants. Plant J. 2021, 107, 37–53. [Google Scholar] [CrossRef]
- Ding, Z.; Jia, S.; Wang, Y.; Xiao, J.; Zhang, Y. Phosphate stresses affect ionome and metabolome in tea plants. Plant Physiol. Biochem. 2017, 120, 30–39. [Google Scholar] [CrossRef]
- Sato, N. Roles of the acidic lipids sulfoquinovosyl diacylglycerol and phosphatidylglycerol in photosynthesis: Their specificity and evolution. J. Plant Res. 2004, 117, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Xu, C.; Benning, C. Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth. Proc. Natl. Acad. Sci. USA 2002, 99, 5732–5737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, J.; Zhang, Y.; Wang, H.; Wei, S.; Zhang, X.; Zhang, D.; Ma, H.; Ding, Q.; Ma, L. Morphophysiological.; proteomic and metabolomic analyses reveal cadmium tolerance mechanism in common wheat (Triticum aestivum L.). J. Hazard. Mater. 2023, 445, 130499. [Google Scholar] [CrossRef]
- Xiao, S.; Song, W.; Xing, J.; Su, A.; Zhao, Y.; Li, C.; Shi, Z.; Li, Z.; Wang, S.; Zhang, R.; et al. ORF355 confers enhanced salinity stress adaptability to S-type cytoplasmic male sterility maize bymodulating the mitochondrial metabolic homeostasis. J. Integr. Plant Biol. 2023, 65, 656–673. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, L.; Ye, Z.; Xu, L.; Li, Y.; Liu, S.; Song, G.; Zhang, X.B. Engineering of cyanine-based nanoplatform with tunable response toward reactive species for ratiometric NIR-II fluorescent imaging in mice. Sci. Bull. 2023, 68, 2382–2390. [Google Scholar] [CrossRef]
- Liu, X.; Matsumoto, H.; Lv, T.; Zhan, C.; Fang, H.; Pan, Q.; Xu, H.; Fan, X.; Chu, T.; Chen, S.; et al. Phyllosphere microbiome induces host metabolic defence against rice false-smut disease. Nat. Microbiol. 2023, 8, 1419–1433. [Google Scholar] [CrossRef]
- Li, C.; Li, K.; Liu, X.; Ruan, H.; Zheng, M.; Yu, Z.; Gai, J.; Yang, S. Transcription Factor GmWRKY46 Enhanced Phosphate Starvation Tolerance and Root Development in Transgenic Plants. Front. Plant Sci. 2021, 12, 700651. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Regev, A. Full-length RNA-seq assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Dewey, C.N.; Li, B. RSEM: Accurate transcript quantification from rna-seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.-Y.; Dillies, M.-A. SARTools: A DESeq2- and EdgeR-based r pipeline for comprehensive differential analysis of RNA-seq data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef]
- Connell, J.; Bates, H.J.; Geoghegan, I.; Wilson, F.; Harrison, R.J.; Price, R.J. Mutation of the LRG1 Rho-GAP gene is responsible for the hyper branching C-variant phenotype in the quorn mycoprotein fungus Fusarium venenatum A3/5. Fungal Biol. Biotechnol. 2025, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant. 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- French, W.R.; Zimmerman, L.J.; Schilling, B.; Gibson, B.W.; Miller, C.A.; Townsend, R.R.; Sherrod, S.D.; Goodwin, C.R.; McLean, J.A.; Tabb, D.L. Wavelet-based peak detection and a new charge inference procedure for MS/MS implemented in ProteoWizard’s msConvert. J. Proteome Res. 2015, 14, 1299–1307. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Z.; Yu, Q.; Wang, Z.; Diao, L.; Li, D. ToxDAR: A Workflow Software for Analyzing Toxicologically Relevant Proteomic and Transcriptomic Data, from Data Preparation to Toxicological Mechanism Elucidation. Int. J. Mol. Sci. 2024, 25, 9544. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.; Zhou, B.; Tang, M.; Chen, J.; Yang, H.; Xu, X. Integrating RNA-Seq and Metabolomic Perspectives Reveals the Mechanism of Response to Phosphorus Stress of Potamogeton wrightii. Plants 2025, 14, 3556. https://doi.org/10.3390/plants14233556
Pan C, Zhou B, Tang M, Chen J, Yang H, Xu X. Integrating RNA-Seq and Metabolomic Perspectives Reveals the Mechanism of Response to Phosphorus Stress of Potamogeton wrightii. Plants. 2025; 14(23):3556. https://doi.org/10.3390/plants14233556
Chicago/Turabian StylePan, Caiyun, Bing Zhou, Ming Tang, Jingan Chen, Haiquan Yang, and Xiaorong Xu. 2025. "Integrating RNA-Seq and Metabolomic Perspectives Reveals the Mechanism of Response to Phosphorus Stress of Potamogeton wrightii" Plants 14, no. 23: 3556. https://doi.org/10.3390/plants14233556
APA StylePan, C., Zhou, B., Tang, M., Chen, J., Yang, H., & Xu, X. (2025). Integrating RNA-Seq and Metabolomic Perspectives Reveals the Mechanism of Response to Phosphorus Stress of Potamogeton wrightii. Plants, 14(23), 3556. https://doi.org/10.3390/plants14233556







