Functional Traits of Herbaceous Plants with Ecological Restoration Potential Under Drought Conditions
Abstract
1. Introduction
2. Results
2.1. Effects of Drought Stress on Herbaceous Plant Growth
2.1.1. Effects of Drought Stress on Seedling Emergence Rate and Plant Height in Leguminous Species
2.1.2. Effects of Drought Stress on Seedling Emergence Rate and Plant Height in Gramineous Species
2.2. Effects of Drought Stress on Functional Traits of Herbaceous Plants
2.2.1. Effects of Drought Stress on Leaf Functional Traits of Herbaceous Plants
2.2.2. Effects of Drought Stress on Root Functional Traits of Herbaceous Plants
2.3. Relative Effects of Drought Stress on Growth Performance and Functional Traits of Herbaceous Plants
2.4. Comprehensive Evaluation of Drought Resistance of Herbaceous Plants
3. Discussion
3.1. Drought Stress Suppresses Seedling Emergence Rate and Growth by Impacting Physiological Processes and Functional Trait
3.2. Root Trait Variation Is a Key Driver of Drought Resistance
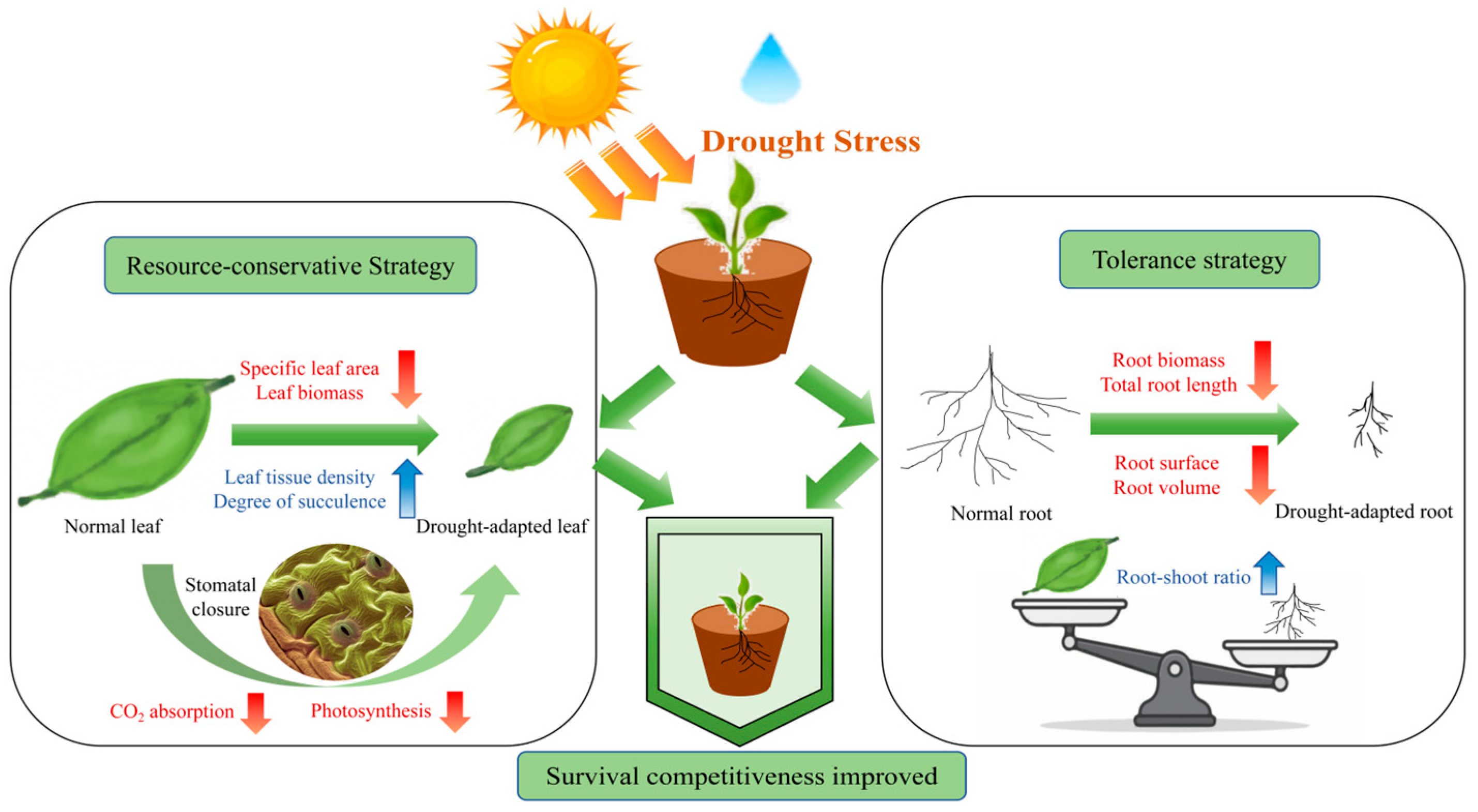
3.3. Plants Adopt Resource-Conservative and Tolerance Strategies to Cope with Drought Stress
4. Materials and Methods
4.1. Test Site and Materials and Methods
4.2. Experimental Design
4.3. Sample Collection and Analysis
4.4. Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Indicator | PC1 | PC2 | PC3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Loading | SE | CI (95%) | Loading | SE | CI (95%) | Loading | SE | CI (95%) | |
| Seedling emergence rate | 0.132 | 0.016 | [0.101, 0.163] | 0.464 | 0.021 | [0.423, 0.505] | −0.241 | 0.026 | [−0.292, −0.190] |
| Plant height | −0.290 | 0.020 | [−0.329, −0.251] | 0.347 | 0.023 | [0.302, 0.392] | 0.183 | 0.027 | [0.130, 0.236] |
| Leaf biomass | 0.394 | 0.018 | [0.359, 0.429] | 0.026 | 0.026 | [−0.025, 0.077] | 0.071 | 0.028 | [0.016, 0.126] |
| Leaf water content | −0.098 | 0.025 | [−0.147, −0.049] | −0.146 | 0.028 | [−0.201, −0.091] | 0.630 | 0.022 | [0.587, 0.673] |
| Degree of succulence | −0.391 | 0.019 | [−0.428, −0.354] | −0.071 | 0.029 | [−0.128, −0.014] | 0.219 | 0.025 | [0.170, 0.268] |
| Specific leaf area | 0.181 | 0.025 | [0.132, 0.230] | 0.259 | 0.024 | [0.212, 0.306] | 0.529 | 0.023 | [0.484, 0.574] |
| Leaf tissue density | −0.353 | 0.019 | [−0.390, −0.316] | 0.053 | 0.028 | [−0.002, 0.108] | −0.374 | 0.024 | [−0.421, −0.327] |
| Root−shoot ratio | −0.215 | 0.027 | [−0.268, −0.162] | 0.353 | 0.022 | [0.310, 0.396] | −0.117 | 0.029 | [−0.174, −0.060] |
| Root biomass | 0.372 | 0.018 | [0.337, 0.407] | 0.138 | 0.025 | [0.089, 0.187] | 0.009 | 0.030 | [−0.050, 0.068] |
| Total root length | −0.222 | 0.024 | [−0.269, −0.175] | 0.425 | 0.021 | [0.384, 0.466] | 0.145 | 0.026 | [0.094, 0.196] |
| Root surface | 0.138 | 0.025 | [0.089, 0.187] | 0.493 | 0.019 | [0.456, 0.530] | 0.035 | 0.031 | [−0.026, 0.096] |
| Root volume | 0.413 | 0.017 | [0.395, 0.446] | 0.023 | 0.027 | [−0.030, 0.076] | −0.059 | 0.029 | [−0.116, −0.002] |
| Indicator | PC1 | PC2 | PC3 | PC4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loading | SE | CI (95%) | Loading | SE | CI (95%) | Loading | SE | CI (95%) | Loading | SE | CI (95%) | |
| Seedling emergence rate | 0.380 | 0.021 | [0.339, 0.421] | 0.069 | 0.031 | [0.008, 0.130] | −0.257 | 0.027 | [−0.310, −0.204] | 0.129 | 0.028 | [0.074, 0.184] |
| Plant height | −0.053 | 0.029 | [−0.110, 0.004] | 0.462 | 0.019 | [0.425, 0.499] | 0.343 | 0.025 | [0.294, 0.392] | −0.161 | 0.027 | [−0.214, −0.108] |
| Leaf biomass | 0.365 | 0.018 | [0.330, 0.400] | −0.236 | 0.025 | [−0.285, −0.187] | 0.003 | 0.029 | [−0.054, 0.060] | −0.168 | 0.026 | [−0.219, −0.117] |
| Leaf water content | −0.228 | 0.024 | [−0.275, −0.181] | −0.270 | 0.022 | [−0.313, −0.227] | 0.537 | 0.020 | [0.498, 0.576] | 0.111 | 0.028 | [0.056, 0.166] |
| Degree of succulence | −0.343 | 0.017 | [−0.376, −0.310] | −0.224 | 0.026 | [−0.275, −0.173] | 0.120 | 0.028 | [0.065, 0.175] | 0.292 | 0.024 | [0.245, 0.339] |
| Specific leaf area | 0.293 | 0.022 | [0.250, 0.336] | −0.074 | 0.030 | [−0.133, −0.015] | 0.539 | 0.019 | [0.502, 0.576] | −0.266 | 0.025 | [−0.315, −0.217] |
| Leaf tissue density | −0.250 | 0.023 | [−0.295, −0.205] | 0.360 | 0.020 | [0.321, 0.399] | −0.332 | 0.023 | [−0.377, −0.287] | −0.087 | 0.029 | [−0.144, −0.030] |
| Root-shoot ratio | 0.080 | 0.032 | [0.017, 0.143] | 0.219 | 0.027 | [0.166, 0.272] | 0.143 | 0.028 | [0.088, 0.198] | 0.838 | 0.015 | [0.809, 0.867] |
| Root biomass | 0.392 | 0.016 | [0.361, 0.423] | −0.126 | 0.028 | [−0.181, −0.071] | −0.005 | 0.030 | [−0.064, 0.054] | 0.190 | 0.025 | [0.141, 0.239] |
| Total root length | 0.047 | 0.031 | [−0.014, 0.108] | 0.488 | 0.018 | [0.453, 0.523] | 0.301 | 0.026 | [0.250, 0.352] | −0.037 | 0.030 | [−0.096, 0.018] |
| Root surface | 0.275 | 0.021 | [0.234, 0.316] | 0.395 | 0.019 | [0.358, 0.432] | 0.034 | 0.029 | [−0.023, 0.091] | 0.005 | 0.031 | [−0.056, 0.066] |
| Root volume | 0.405 | 0.015 | [0.376, 0.434] | −0.103 | 0.027 | [−0.156, −0.050] | 0.013 | 0.029 | [−0.044, 0.070] | 0.112 | 0.026 | [0.061, 0.163] |
| Indicator | PC1 | PC2 | PC3 | PC4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loading | SE | CI (95%) | Loading | SE | CI (95%) | Loading | SE | CI (95%) | Loading | SE | CI (95%) | |
| Seedling emergence rate | 0.340 | 0.023 | [0.295, 0.385] | −0.071 | 0.032 | [−0.134, −0.008] | 0.179 | 0.028 | [0.124, 0.234] | 0.502 | 0.021 | [0.461, 0.543] |
| Plant height | 0.064 | 0.031 | [0.003, 0.125] | 0.467 | 0.018 | [0.432, 0.502] | 0.307 | 0.024 | [0.260, 0.354] | −0.197 | 0.028 | [−0.252, −0.142] |
| Leaf biomass | 0.389 | 0.017 | [0.356, 0.422] | −0.135 | 0.027 | [−0.188, −0.082] | −0.148 | 0.029 | [−0.205, −0.091] | 0.052 | 0.031 | [−0.009, 0.113] |
| Leaf water content | 0.372 | 0.020 | [0.333, 0.411] | −0.041 | 0.030 | [−0.100, 0.018] | 0.089 | 0.027 | [0.036, 0.142] | 0.161 | 0.026 | [0.110, 0.212] |
| Degree of succulence | 0.083 | 0.033 | [0.018, 0.148] | −0.425 | 0.017 | [−0.458, −0.392] | 0.398 | 0.023 | [0.353, 0.443] | −0.141 | 0.029 | [−0.198, −0.084] |
| Specific leaf area | 0.297 | 0.022 | [0.254, 0.340] | 0.099 | 0.029 | [0.042, 0.156] | −0.053 | 0.030 | [−0.112, 0.006] | −0.671 | 0.016 | [−0.702, −0.640] |
| Leaf tissue density | −0.279 | 0.024 | [−0.326, −0.232] | 0.257 | 0.022 | [0.214, 0.300] | 0.386 | 0.025 | [0.337, 0.435] | 0.352 | 0.024 | [0.305, 0.399] |
| Root-shoot ratio | −0.155 | 0.029 | [−0.212, −0.098] | 0.189 | 0.026 | [0.138, 0.240] | −0.700 | 0.019 | [−0.737, −0.663] | 0.187 | 0.027 | [0.134, 0.240] |
| Root biomass | 0.403 | 0.016 | [0.372, 0.434] | 0.142 | 0.025 | [0.093, 0.191] | −0.147 | 0.028 | [−0.202, −0.092] | 0.027 | 0.032 | [−0.036, 0.090] |
| Total root length | 0.101 | 0.030 | [0.042, 0.160] | 0.495 | 0.017 | [0.462, 0.528] | 0.123 | 0.026 | [0.072, 0.174] | −0.066 | 0.030 | [−0.125, −0.007] |
| Root surface | 0.214 | 0.025 | [0.165, 0.263] | 0.440 | 0.019 | [0.403, 0.477] | 0.033 | 0.031 | [−0.028, 0.094] | 0.142 | 0.025 | [0.093, 0.191] |
| Root volume | 0.418 | 0.015 | [0.389, 0.447] | −0.077 | 0.028 | [−0.132, −0.022] | −0.082 | 0.029 | [−0.139, −0.025] | 0.157 | 0.026 | [0.106, 0.208] |
| Species | D Value | SE (D Value) | 95% CI (D Value) | Rank | 95% CI (Rank) |
|---|---|---|---|---|---|
| Elymus dahuricus | 0.671 | 0.018 | [0.625, 0.711] | 1 | [1, 2] |
| Medicago sativa | 0.661 | 0.021 | [0.613, 0.703] | 2 | [1, 4] |
| Agropyron cristatum | 0.522 | 0.020 | [0.470, 0.568] | 3 | [2, 5] |
| Astragalus laxmannii | 0.482 | 0.026 | [0.428, 0.530] | 4 | [3, 6] |
| Agropyron desertorum | 0.168 | 0.028 | [0.110, 0.220] | 5 | [4, 6] |
| Agropyron mongolicum | 0.104 | 0.024 | [0.044, 0.158] | 6 | [5, 6] |
| Species | D Value | SE (D Value ) | 95% CI (D Value ) | Rank | 95% CI (Rank ) |
|---|---|---|---|---|---|
| Agropyron cristatum | 0.830 | 0.016 | [0.859, 0.937] | 1 | [1, 3] |
| Elymus dahuricus | 0.727 | 0.021 | [0.796, 0.882] | 2 | [1, 4] |
| Medicago sativa | 0.577 | 0.024 | [0.554, 0.648] | 3 | [2, 5] |
| Agropyron desertorum | 0.372 | 0.031 | [0.356, 0.454] | 4 | [3, 6] |
| Astragalus laxmannii | 0.355 | 0.026 | [0.321, 0.423] | 5 | [3, 6] |
| Agropyron mongolicum | 0.210 | 0.027 | [0.258, 0.364] | 6 | [5, 6] |
| Species | D Value | SE (D Value ) | 95% CI (D Value ) | Rank | 95% CI (Rank ) |
|---|---|---|---|---|---|
| Agropyron cristatum | 0.901 | 0.020 | [0.859, 0.937] | 1 | [1, 3] |
| Elymus dahuricus | 0.842 | 0.017 | [0.796, 0.882] | 2 | [1, 4] |
| Medicago sativa | 0.604 | 0.021 | [0.554, 0.648] | 3 | [2, 5] |
| Agropyron mongolicum | 0.408 | 0.025 | [0.356, 0.454] | 4 | [3, 6] |
| Agropyron desertorum | 0.375 | 0.026 | [0.321, 0.423] | 5 | [3, 6] |
| Astragalus laxmannii | 0.314 | 0.030 | [0.258, 0.364] | 6 | [5, 6] |
References
- Gui, D.; Liu, Q.; Martínez-Valderrama, J.; Abd-Elmabod, S.K.; Zeeshan, A.; Xu, Z.; Lei, J. Desertification Baseline: A Bottleneck for Addressing Desertification. Earth Sci. Rev. 2024, 257, 110984. [Google Scholar] [CrossRef]
- Islam, W.; Zeng, F.; Alotaibi, M.O.; Khan, K.A. Unlocking the potential of soil microbes for sustainable desertification management. Earth Sci. Rev. 2024, 252, 104738. [Google Scholar] [CrossRef]
- Gao, Y.; Tariq, A.; Zeng, F.; Sardans, J.; Zhang, Z.; Islam, W.; Xu, M.; Penuelas, J. “Fertile islands” beneath three desert vegetation on soil phosphorus fractions, enzymatic activities, and microbial biomass in the desert-oasis transition zone. Catena 2022, 212, 106090. [Google Scholar] [CrossRef]
- Xiu, X.; Wu, B.; Chen, Q.; Li, Y.; Pang, Y.; Jia, X.; Zhu, J.; Lu, Q. Spatio-temporal dynamics of desertification in China from 1970 to 2019: A meta-analysis. J. Arid Land 2025, 17, 1189–1214. [Google Scholar] [CrossRef]
- Yu, X.; Lei, J.; Gao, X. An over review of desertification in Xinjiang, Northwest China. J. Arid Land 2022, 14, 1181–1195. [Google Scholar] [CrossRef]
- Wang, X.; Geng, X.; Liu, B.; Cai, D.; Li, D.; Xiao, F.; Zhu, B.; Hua, T.; Lu, R.; Liu, F. Desert ecosystems in China: Past, present, and future. Earth Sci. Rev. 2022, 234, 104206. [Google Scholar] [CrossRef]
- Xie, Y.; Dang, X.; Zhou, Y.; Hou, Z.; Li, X.; Jiang, H.; Zhou, D.; Wang, J.; Hai, C.; Zhou, R. Using sediment grain size characteristics to assess effectiveness of mechanical sand barriers in reducing erosion. Sci. Rep. 2020, 10, 14009. [Google Scholar] [CrossRef]
- Liu, X.; Tahir, M.; Li, C.; Chen, C.; Xin, Y.; Zhang, G.; Cheng, M.; Yan, Y. Mixture of alfalfa, orchardgrass, and tall fescue produces greater biomass yield in southwest China. Agronomy 2022, 12, 2425. [Google Scholar] [CrossRef]
- Yang, S.; Qu, Z. Cost analysis of sand barriers in desertified regions based on the land grid division model. J. Arid Land 2022, 14, 978–992. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, M.; Liu, Y.; Lopez-Vicente, M. Litter cover promotes biocrust decomposition and surface soil functions in sandy ecosystem. Geoderma 2020, 374, 114429. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, D.; Tang, Y.; Liu, L. Land surface roughness impacted by typical vegetation restoration projects on aeolian sandy lands in the Yarlung Zangbo River valley, southern Tibetan plateau. Int. Soil Water Conserv. Res. 2022, 10, 109–118. [Google Scholar] [CrossRef]
- Wang, L.; Lu, P.; Feng, S.; Hamel, C.; Sun, D.; Siddique, K.H.M.; Gan, G. Strategies to improve soil health by optimizing the plant–soil–microbe–anthropogenic activity nexus. Agric. Ecosyst. Environ. 2024, 359, 108750. [Google Scholar] [CrossRef]
- Phogole, B.; Sethusa, M.T.; Yessoufou, K. Policy and Land Degradation Are Neglected in the Desertification, Land Degradation, and Drought Research Landscape in South Africa: Evidence from a Systematic Review and Bibliometric Analysis. Land Degrad. Dev. 2025, 36, 4017–4030. [Google Scholar] [CrossRef]
- Xu, C.; Ke, Y.; Zhou, W.; Luo, W.; Ma, W.; Song, L.; Smith, M.D.; Hoover, D.L.; Wilcox, K.R.; Fu, W.; et al. Resistance and resilience of a semi-arid grassland to multi-year extreme drought. Ecol. Indic. 2021, 131, 108139. [Google Scholar] [CrossRef]
- Cheng, Z.; Qian, Z.; Huang, B.; Feng, G.; Sun, G. Increased drought impacts on vegetation productivity in drylands under climate change. Geophys. Res. Lett. 2025, 52, e2025GL115616. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Bufford, J.L.; Hulme, P.E. Increased adaptive phenotypic plasticity in the introduced range in alien weeds under drought and flooding. Biol. Invasions 2021, 23, 2675–2688. [Google Scholar] [CrossRef]
- Barros, V.; Oliveira, M.T.; Santos, M.G. Low foliar construction cost and strong investment in root biomass in Calotropis procera, an invasive species under drought and recovery. Flora 2021, 280, 151848. [Google Scholar] [CrossRef]
- Bakhtiari, E.S.; Mousavi, A.; Yadegari, M.; Haghighati, B.; Martínez-García, P.J. Physiological and Biochemical Responses of Almond (Prunus dulcis) Cultivars to Drought Stress in Semi-Arid Conditions in Iran. Plants 2025, 14, 734. [Google Scholar] [CrossRef]
- Ivanova, L.A.; Yudina, P.K.; Ronzhina, D.A.; Ivanov, L.A.; Hölzel, N. Quantitative mesophyll parameters rather than whole-leaf traits predict response of C3 steppe plants to aridity. New Phytol. 2018, 217, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Bai, W.; Zhang, Y.; Zhang, W. Multi-dimensional patterns of variation in root traits among coexisting herbaceous species in temperate steppes. J. Ecol. 2018, 106, 2320–2331. [Google Scholar] [CrossRef]
- Yan, S.; Weng, B.; Jing, L.; Bi, W.; Yan, D. Adaptive pathway of summer maize under drought stress: Transformation of root morphology and water absorption law. Front. Earth Sci. 2022, 10, 1020553. [Google Scholar] [CrossRef]
- Kozub, P.C.; Cavagnaro, J.B.; Cavagnaro, P.F. Exploiting genetic and physiological variation of the native forage grass Trichloris crinita for revegetation in arid and semi-arid regions: An integrative review. Grass Forage Sci. 2018, 73, 257–271. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, J.; Wu, X.; Zhang, Y.; Jia, H.; Shi, Y. Full-length transcriptome of in Medicago sativa L. roots in response to drought stress. Front. Genet. 2023, 13, 1086356. [Google Scholar] [CrossRef]
- Gong, W.; Ma, L.; Gao, Q.; Wei, B.; Zhang, J.; Liu, X.; Gong, P.; Wang, Z.; Zhao, G. Construction of a high-density genetic linkage map and identification of flowering-related QTL in erect milkvetch (Astragalus adsurgens). Crop J. 2022, 10, 1141–1150. [Google Scholar] [CrossRef]
- Huang, J.; Dai, J.; Scarpa, F.; Wang, Y.; Ji, J.; Mao, Z. Random root distribution affects the mechanical properties of the soil-root composite and root reinforcement. Catena 2025, 255, 108896. [Google Scholar] [CrossRef]
- Mellish, A.; Coulman, B.; Ferdinandez, Y. Genetic relationships among selected crested wheatgrass cultivars and species determined on the basis of AFLP markers. Crop Sci. 2002, 42, 1662–1668. [Google Scholar] [CrossRef]
- Yan, X.; Wu, X.; Sun, F.; Nie, H.; Du, X.; Li, X.; Fang, Y.; Zhai, Y.; Zhao, Y.; Fan, B.; et al. Cloning and functional study of AmGDSL1 in Agropyron mongolicum. Int. J. Mol. Sci. 2024, 25, 9467. [Google Scholar] [CrossRef] [PubMed]
- Robins, G.J.; Jensen, B.K.; Waldron, L.B.; Bushman, B.S. Irrigation amount and ploidy affect the turfgrass potential of crested wheatgrass (Agropyron cristatum). Grassl. Sci. 2020, 66, 48–53. [Google Scholar] [CrossRef]
- de Souza, G.A.R.; Baroni, D.F.; Bernado, W.d.P.; Santos, A.R.; Barcellos, L.C.d.S.; Barcelos, L.F.T.; Correia, L.Z.; de Almeida, C.M.; Verdin Filho, A.C.; Rodrigues, W.P.; et al. Leaf to Root Morphological and Anatomical Indicators of Drought Resistance in Coffea Canephora After Two Stress Cycles. Agriculture 2025, 15, 574. [Google Scholar] [CrossRef]
- Cao, K.; Mu, Y.; Lu, S.; Zhao, Y. Regulatory Mechanisms of Exogenous Gibberellin on Seed Germination and Transcriptomic Responses in Lomatogonium rotatum. Genes 2025, 16, 878. [Google Scholar] [CrossRef]
- Chabili, A.; Hakkoum, Z.; Minaoui, F.; Douma, M.; Meddich, A.; Loudiki, M. Germination screen of eco-extracts from soil cyanobacteria and microalgae for their biostimulant effects on wheat seeds emergence and vigor. Algal Res. 2025, 89, 104087. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Secchi, M.A.; Fernandez, J.A.; Stamm, M.J.; Durrett, T.; Prasad, P.V.; Messina, C.D.; Ciampitti, I.A. Effects of heat and drought on canola (Brassica napus L.) yield, oil, and protein: A meta-analysis. Field Crop Res. 2023, 293, 108848. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, H.; Chen, Y.; Zhang, L.; Kudusi, K.; Song, J. Effects of drought and salt stress on seed germination of ephemeral plants in desert of northwest China. Front. Ecol. Evol. 2022, 10, 1026095. [Google Scholar] [CrossRef]
- Gao, S.; Fan, Y.; Yu, M.; Zhang, J.; Wang, J. Effects of drought stress on seed germination and seedling growth of alfalfa with different seed coat colors. Legume Res. 2023, 46, 1339–1344. [Google Scholar] [CrossRef]
- Jupa, R.; Rosell, J.A.; Pittermann, J. Bark structure is coordinated with xylem hydraulic properties in branches of five Cupressaceae species. Plant Cell Environ. 2024, 47, 1439–1451. [Google Scholar] [CrossRef]
- Pan, Q.; Xue, C.; Meng, L.; Gao, Y.; Yu, M.; Geng, L.; Guan, P.; Qu, B. Flexible resource allocation-efficient water use strategies facilitate invasion of invasive Vine Sicyos angulatus L. Biology 2024, 13, 392. [Google Scholar] [CrossRef]
- Luo, Y.; Li, G.; Yan, G.; Liu, H.; Turner, N.C. Morphological Features and Biomass Partitioning of Lucerne Plants (Medicago sativa L.) Subjected to Water Stress. Agronomy 2020, 10, 322. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Zhao, D.; Wei, M.; Wang, X.; Aqeel, M.; Ran, J.; Deng, J. Morpho-physiological adaptations to drought stress in nitrogen-fixing and non-nitrogen-fixing plants. Front. Ecol. Evol 2024, 12, 1407882. [Google Scholar] [CrossRef]
- Philadelphi, S.M.; Malisch, C.S.; Eriksen, J.; Högy, P. Grasses, legumes and forbs respond differently to compound drought-heatwave events during establishment. Plant Soil 2025. prepublish. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, M.; Huang, X.; Liu, E. Evaluation of drought resistance and index screening of foxtail millet cultivars. J. Water Clim. Change 2023, 14, 2384–2396. [Google Scholar] [CrossRef]
- Sun, F.; Chen, Q.; Chen, Q.; Jiang, M.; Gao, W.; Qu, Y. Screening of key drought tolerance indices for cotton at the flowering and boll setting stage using the dimension reduction method. Front. Plant Sci. 2021, 12, 619926. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Y.; Zhao, Z.; Liu, W.; Jiang, C.; Li, J.; Zhang, Z.; Zhang, H.; Zhang, Y.; Wang, X.; et al. RRS1 shapes robust root system to enhance drought resistance in rice. New Phytol. 2023, 238, 1146–1162. [Google Scholar] [CrossRef]
- Perlikowski, D.; Augustyniak, A.; Skirycz, A.; Pawłowicz, I.; Masajada, K.; Michaelis, A.; Kosmala, A. Efficient Root Metabolism Improves Drought Resistance of Festuca arundinacea. Plant Cell Physiol. 2020, 61, 492–504. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, X.; Liang, J.; Zhang, S.; Wang, G.; Liu, Y. Physiological mechanisms and drought resistance assessment of four dominant species on the Loess Plateau under drought stress. Physiol. Plant. 2025, 177, e70261. [Google Scholar] [CrossRef]
- Bayat, H.; Nemati, H.; Tehranifar, A.; Gazanchian, A. Screening Different Crested Wheatgrass (Agropyron cristatum (L.) Gaertner.) Accessions for Drought Stress Tolerance. Arch. Agron. Soil Sci. 2016, 62, 769–780. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, X.; Liu, W.; Liang, G.; Zhang, Y. Germplasm resources and drought resistance evaluation of Siberian wildrye (Elymus sibiricus L.) in the Tibetan Plateau. Braz. J. Bot. 2023, 46, 743–756. [Google Scholar] [CrossRef]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root plasticity under abiotic stress. Plant Physiol. 2021, 187, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Fry, E.L.; Evans, A.L.; Sturrock, C.J.; Bullock, J.M.; Bardgett, R.D. Root architecture governs plasticity in response to drought. Plant Soil 2018, 433, 189–200. [Google Scholar] [CrossRef]
- Ke, X.; Xiao, H.; Peng, Y.; Xia, X.; Wang, X. Nitrogen deficiency modulates carbon allocation to promote nodule nitrogen fixation capacity in soybean. Exploration 2023, 4, 20230104. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, L.; Liu, H.; He, W.; Shi, L.; Xu, C.; Xiang, C. Coarsened Soil Reduces Drought Resistance of Fibrous-Rooted Species on Degraded Steppe. Ecol. Indic. 2022, 145, 109644. [Google Scholar] [CrossRef]
- Yang, Y.; McCormack, L.M.; Li, F.; Bao, W.; Wu, N.; Hu, H.; Wu, Y.; Wang, Z.; Wei, D.; Yang, T.; et al. Variation in root hair traits in 75 xerophytic species: Constraints of phylogeny, trait trade-offs and environment. Funct. Ecol. 2025, 39, 1594–1605. [Google Scholar] [CrossRef]
- Eller, C.B.; Lima, A.L.; Oliveira, R.S. Cloud Forest Trees with Higher Foliar Water Uptake Capacity and Anisohydric Behavior Are More Vulnerable to Drought and Climate Change. New Phytol. 2016, 211, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Liu, H.; Guan, W.; Li, J.; Li, J. Drought affects the coordination of belowground and aboveground resource-related traits in Solidago canadensis in China. Ecol. Evol. 2019, 9, 9948–9960. [Google Scholar] [CrossRef]
- Zhang, H.; Lan, Y.; Jiang, C.; Cui, Y.; He, Y.; Deng, J.; Lin, M.; Ye, S. Leaf traits explain the growth variation and nitrogen response of Eucalyptus urophylla × Eucalyptus grandis and Dalbergia odorifera in mixed culture. Plants 2024, 13, 988. [Google Scholar] [CrossRef]
- Sun, S.; Liu, X.; Zhao, X.; Medina-Roldánd, E.; He, Y.; Lv, P.; Hu, H. Annual Herbaceous Plants Exhibit Altered Morphological Traits in Response to Altered Precipitation and Drought Patterns in Semiarid Sandy Grassland, Northern China. Front. Plant Sci. 2022, 13, 756950. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, H.; Li, Q.; Liu, X.; Zhang, Z.; Bian, S. Study on dry matter accumulation and leaf response to light and CO2 of maize under irrigation quota. Cereal Res. Commun. 2020, 48, 173–178. [Google Scholar] [CrossRef]
- Khan, R.; Ma, X.H.; Hussain, Q.; Chen, K.L.; Farooq, S.; Asim, M.; Ren, X.C.; Shah, S.H.; Shi, Y. Transcriptome and anatomical studies reveal alterations in leaf thickness under long-term drought stress in tobacco. J. Plant Physiol. 2023, 281, 153920. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The Physiology of Plant Responses to Drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.; He, Z.; Ma, D.; Sun, W.; Xu, X.; Tian, Q. Effects of Drought Stress on Leaf Functional Traits and Biomass Characteristics of Atriplex canescens. Plants 2024, 13, 2006. [Google Scholar] [CrossRef]
- Qian, H.; Tong, J.; Li, S.; Xie, J.; Wang, Z.; Li, Y.; Lu, S. Plant trait networks reveal the ecological strategies of Arabidopsis thaliana along ontogeny. Ecosphere 2025, 16, e70180. [Google Scholar] [CrossRef]
- Dao, J.; Xing, Y.; Chen, C.; Chen, M.; Wang, Z.; Chen, Y. Changes in shoot and root adaptations of fibrous-root and taproot crops in response to different drought types: A meta-analysis. Agric. Water Manag. 2025, 309, 109320. [Google Scholar] [CrossRef]
- Gusain, S.; Kumari, K.; Joshi, R. Physiological, hormonal and molecular dynamics of root system architectural response to drought stress signaling in crops. Rhizosphere 2024, 31, 100922. [Google Scholar] [CrossRef]
- Jiang, S.; Tang, Y.; Fan, R.; Bai, S.; Wang, X.; Huang, Y.; Li, W.; Ji, W. Response of Carex breviculmis to phosphorus deficiency and drought stress. Front. Plant Sci. 2023, 14, 1203924. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.S.; Silva, M.N.; Carvalho, S.M.P.; Santos, C.S.; Vasconcelos, M.W. Low water supply differentially affects the growth, yield and mineral profile of kabuli and desi chickpeas (Cicer arietinum). Ann. Appl. Biol. 2024, 184, 37–49. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, B.; Ciais, P.; Luo, Y.; Peng, C.; Tian, Y.; Wu, X. Higher sensitivity of deep soil root productivity to precipitation changes. Glob. Ecol. Biogeogr. 2025, 34, e70121. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, C.; Wang, L.; Han, X.; Zhu, Y.; Liu, J.; Yang, X. Functional trait responses of Sophora alopecuroides L. seedlings to diverse environmental stresses in the desert steppe of Ningxia China. Plants 2023, 13, 69. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, K.; Nan, L. Differences of morphological and physiological responses of sainfoin varieties/lines under simulated drought stresses. Grassl. Sci. 2024, 70, 133–150. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Bertero, D.; Hoang, D.T.; Long, N.V. Variation in Quinoa roots growth responses to drought stresses. J. Agron. Crop Sci. 2022, 208, 830–840. [Google Scholar] [CrossRef]
- GB/T 32136-2015; Grade of Agricultural Drought. China Meteorological Administration: Beijing, China, 2015.
- Hestrin, R.; Kan, M.; Lafler, M.; Wollard, J.; Kimbrel, J.A.; Ray, P.; Blazewicz, S.J.; Stuart, R.; Craven, K.; Firestone, M.; et al. Plant-Associated Fungi Support Bacterial Resilience Following Water Limitation. ISME J. 2022, 16, 2752–2762. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, Q.; Ge, W.; Liu, Q.; Kong, D.; Yin, H. Coordination of Leaf and Root Economic Space in Alpine Coniferous Forests on the Tibetan Plateau. Plant Soil 2023, 496, 555–568. [Google Scholar] [CrossRef]
- Yan, G.; Luo, X.; Liang, C.; Han, S.; Han, S.; Liu, G.; Yin, L.; Wang, X.; Zhang, Z.; Xu, L.; et al. Nitrogen Deposition Enhances Soil Organic Carbon Sequestration Through Plant-Soil-Microbe Synergies. J. Ecol. 2025, 113, 2889–2904. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Y.; Li, Y.; Yu, H.; Shao, J.; Zhao, Z.; Qiao, Y.; Liu, C.; Liu, S.; Gao, C. Optimizing irrigation strategies for sustainable crop productivity and reduced groundwater consumption in a winter wheat-maize rotation system. J. Environ. Manag. 2023, 348, 119469. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Blackwood, C.B. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1311–1313. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Liu, L.; Li, H.; Wang, X.; Si, A.; Yu, Y. Screening and Comprehensive Evaluation of Drought Resistance in Cotton Germplasm Resources at the Germination Stage. Plants 2025, 14, 2191. [Google Scholar] [CrossRef]
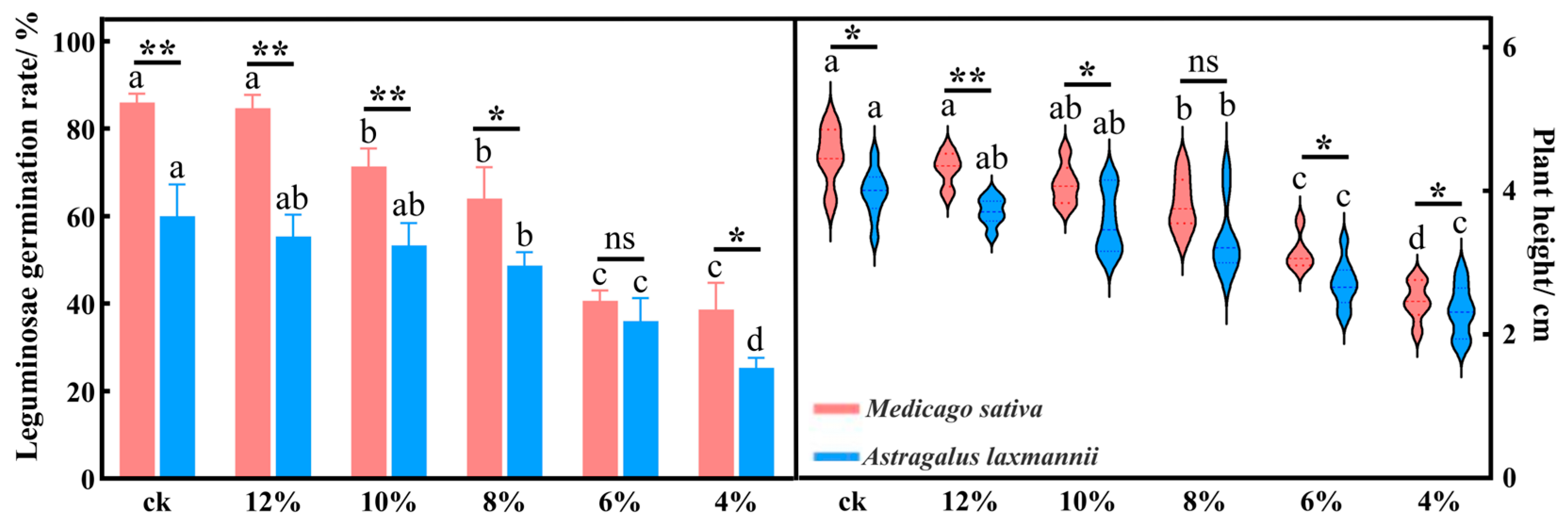
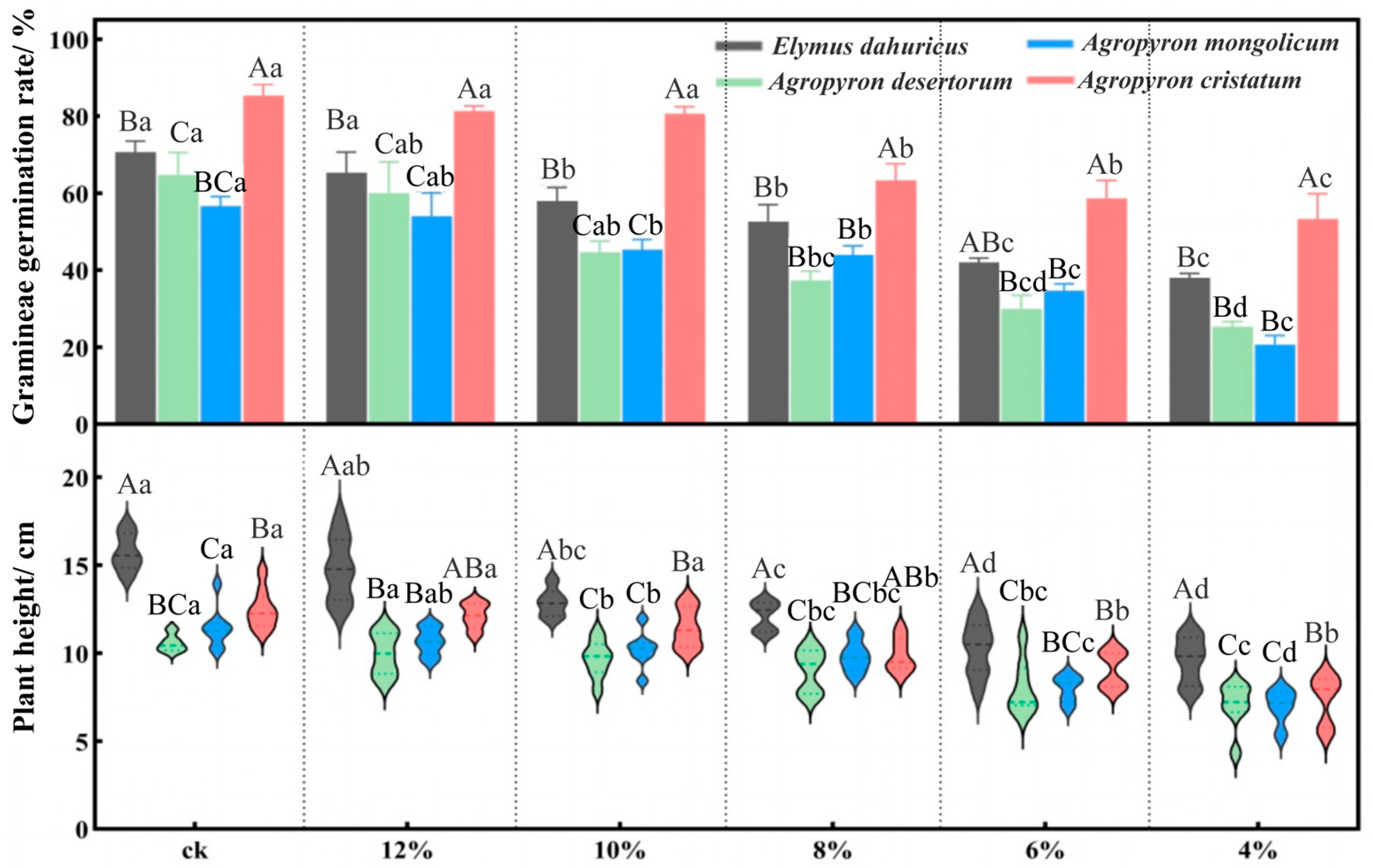

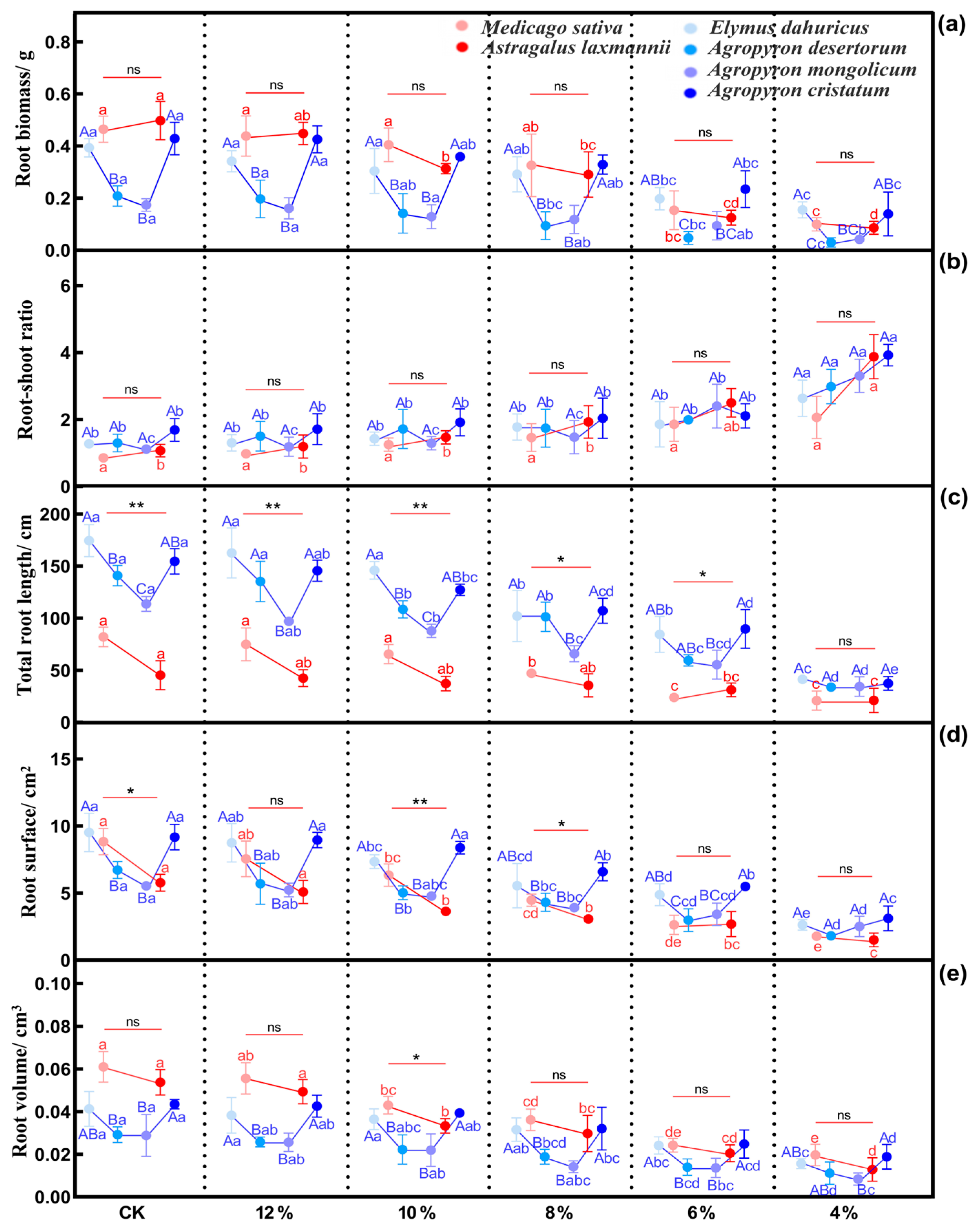
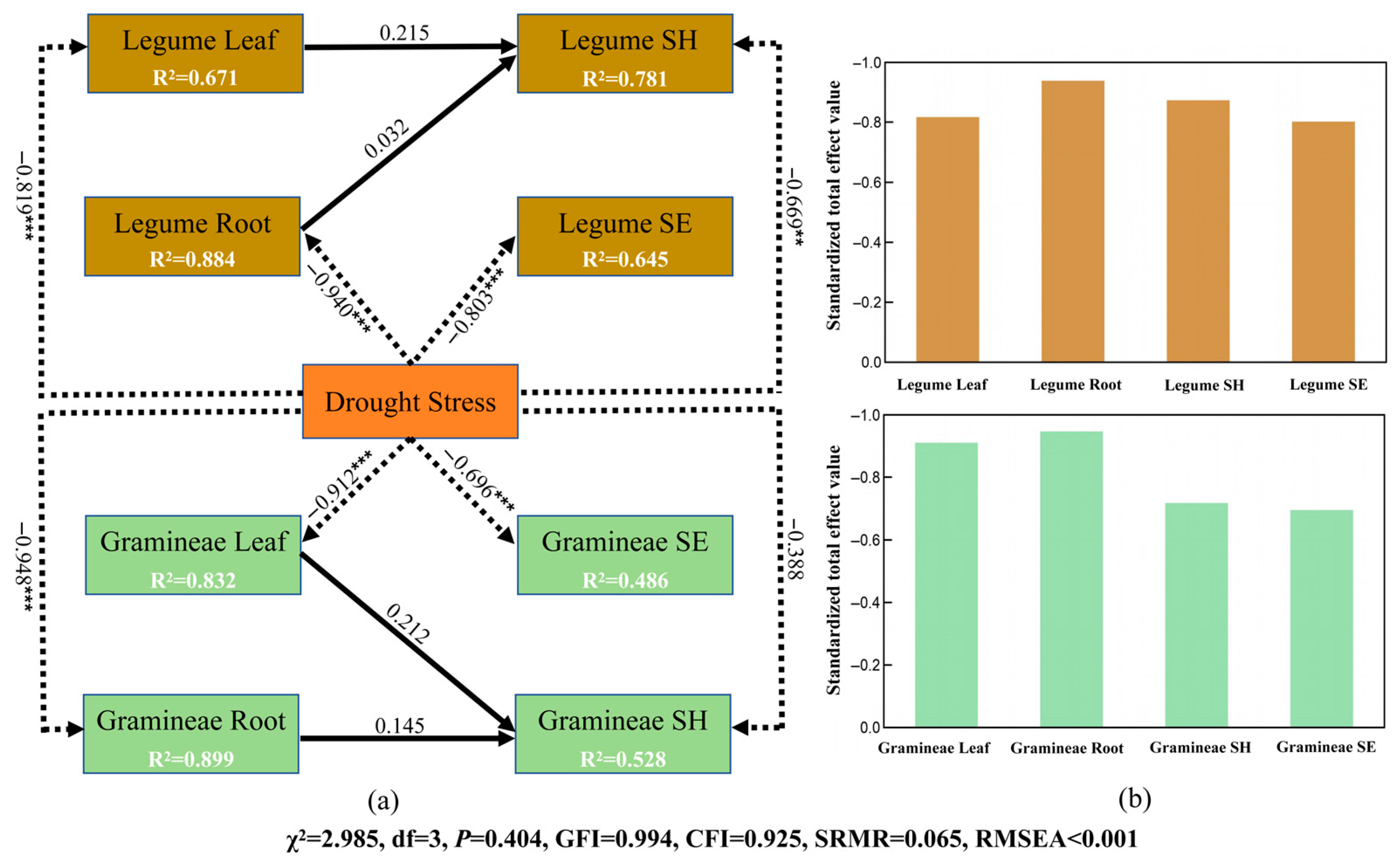

| Indicator | Mild Drought | Moderate Drought | Severe Drought | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | PC3 | PC4 | |
| Seedling emergence rate | 0.132 | 0.464 | −0.241 | 0.380 | 0.069 | −0.257 | 0.129 | 0.340 | −0.071 | 0.179 | 0.502 |
| Plant height | −0.290 | 0.347 | 0.183 | −0.053 | 0.462 | 0.343 | −0.161 | 0.064 | 0.467 | 0.307 | −0.197 |
| Leaf biomass | 0.394 | 0.026 | 0.071 | 0.365 | −0.236 | 0.003 | −0.168 | 0.389 | −0.135 | −0.148 | 0.052 |
| Leaf water content | −0.098 | −0.146 | 0.630 | −0.228 | −0.270 | 0.537 | 0.111 | 0.372 | −0.041 | 0.089 | 0.161 |
| Degree of succulence | −0.391 | −0.071 | 0.219 | −0.343 | −0.224 | 0.120 | 0.292 | 0.083 | −0.425 | 0.398 | −0.141 |
| Specific leaf area | 0.181 | 0.259 | 0.529 | 0.293 | −0.074 | 0.539 | −0.266 | 0.297 | 0.099 | −0.053 | −0.671 |
| Leaf tissue density | −0.353 | 0.053 | −0.374 | −0.250 | 0.360 | −0.332 | −0.087 | −0.279 | 0.257 | 0.386 | 0.352 |
| Root-shoot ratio | −0.215 | 0.353 | −0.117 | 0.080 | 0.219 | 0.143 | 0.838 | −0.155 | 0.189 | −0.700 | 0.187 |
| Root biomass | 0.372 | 0.138 | 0.009 | 0.392 | −0.126 | −0.005 | 0.190 | 0.403 | 0.142 | −0.147 | 0.027 |
| Total root length | −0.222 | 0.425 | 0.145 | 0.047 | 0.488 | 0.301 | −0.037 | 0.101 | 0.495 | 0.123 | −0.066 |
| Root surface | 0.138 | 0.493 | 0.035 | 0.275 | 0.395 | 0.034 | 0.005 | 0.214 | 0.440 | 0.033 | 0.142 |
| Root volume | 0.413 | 0.023 | −0.059 | 0.405 | −0.103 | 0.013 | 0.112 | 0.418 | −0.077 | −0.082 | 0.157 |
| Eigenvalue | 5.739 | 3.483 | 1.885 | 5.775 | 3.564 | 1.405 | 1.067 | 5.352 | 3.703 | 1.36 | 1.084 |
| Contribution rate/% | 47.825 | 29.026 | 15.707 | 48.123 | 29.703 | 11.705 | 8.893 | 44.599 | 30.859 | 11.33 | 9.034 |
| Cumulative contribution rate/% | 47.825 | 76.851 | 92.558 | 48.123 | 77.826 | 89.531 | 98.423 | 44.599 | 75.458 | 86.788 | 95.822 |
| Drought Degree | Species | Comprehensive Indicator Value | Membership Function Value | D Value | Rank | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cl1 | Cl2 | Cl3 | Cl4 | μ1 | μ2 | μ3 | μ4 | ||||
| Mild drought | Medicago sativa | 3.443 | −0.344 | −0.869 | - | 1 | 0.364 | 0.181 | - | 0.661 | 2 |
| Astragalus laxmannii | 1.852 | −2.050 | 0.660 | - | 0.742 | 0 | 0.579 | - | 0.482 | 4 | |
| Elymus dahuricus | 0.105 | 1.895 | 2.279 | - | 0.459 | 0.841 | 1 | - | 0.671 | 1 | |
| Agropyron desertorum | −2.728 | −0.727 | 0.230 | - | 0 | 0.282 | 0.467 | - | 0.168 | 5 | |
| Agropyron mongolicum | −2.429 | −1.415 | −0.736 | - | 0.048 | 0.135 | 0.216 | - | 0.104 | 6 | |
| Agropyron cristatum | −0.242 | 2.641 | −1.564 | - | 0.403 | 1 | 0 | - | 0.522 | 3 | |
| Moderate drought | Medicago sativa | 2.712 | −1.431 | −1.226 | −0.973 | 1 | 0.291 | 0 | 0.004 | 0.577 | 3 |
| Astragalus laxmannii | −0.633 | −2.961 | 0.484 | 1.205 | 0.416 | 0 | 0.514 | 1 | 0.355 | 5 | |
| Elymus dahuricus | 1.492 | 0.743 | 2.097 | −0.717 | 0.787 | 0.704 | 1 | 0.121 | 0.727 | 2 | |
| Agropyron desertorum | −2.473 | 1.042 | −0.033 | 0.295 | 0.095 | 0.761 | 0.359 | 0.584 | 0.372 | 4 | |
| Agropyron mongolicum | −3.017 | 0.309 | −0.600 | −0.981 | 0 | 0.622 | 0.188 | 0 | 0.210 | 6 | |
| Agropyron cristatum | 1.919 | 2.298 | −0.722 | 1.172 | 0.862 | 1 | 0.152 | 0.985 | 0.830 | 1 | |
| Severe drought | Medicago sativa | 1.761 | −3.044 | 0.983 | 0.633 | 0.893 | 0 | 1 | 0.748 | 0.604 | 3 |
| Astragalus laxmannii | −0.737 | −1.492 | −2.052 | −0.603 | 0.399 | 0.301 | 0 | 0.336 | 0.314 | 6 | |
| Elymus dahuricus | 2.301 | 1.334 | 0.601 | −1.613 | 1 | 0.849 | 0.874 | 0 | 0.842 | 2 | |
| Agropyron desertorum | −2.530 | 0.246 | 0.567 | −0.133 | 0.044 | 0.638 | 0.863 | 0.493 | 0.375 | 5 | |
| Agropyron mongolicum | −2.753 | 0.843 | 0.635 | 0.326 | 0 | 0.754 | 0.886 | 0.646 | 0.408 | 4 | |
| Agropyron cristatum | 1.958 | 2.113 | −0.734 | 1.389 | 0.932 | 1 | 0.434 | 1 | 0.901 | 1 | |
| Indicator | Water Content % | pH | OM g·kg−1 | TN g·kg−1 | TP g·kg−1 | TK g·kg−1 | AN mg·kg−1 | AP mg·kg−1 | AK mg·kg−1 |
|---|---|---|---|---|---|---|---|---|---|
| Value | 0.33 | 8.2 | 2.14 | 1.09 | 0.31 | 1.87 | 12.33 | 0.52 | 125.7 |
| Indicator | Unit | Measurement Method | |
|---|---|---|---|
| Growth | seedling emergence rate | % | Emergence Number/Seed Number × 100% |
| plant height | cm | Measurement with a ruler (precion:1mm). | |
| Leaf | leaf biomass | g | Dry in a 75 °C oven for 48 h and weigh |
| leaf water content | % | (Leaf Fresh Weight-Leaf Dry Weight)/Leaf Fresh Weight × 100% | |
| degree of succulence | g·g−1 | Leaf Fresh Weight/Leaf Dry Weight | |
| specific leaf area | cm2·g−1 | Leaf Surface Area/Leaf Dry Weight | |
| leaf tissue density | g·cm−3 | Leaf Biomass/Leaf Volume | |
| Root system | root biomass | g | Dry in a 75 °C oven for 48 h and weigh |
| root-shoot ratio | / | Underground Biomass/Aboveground Biomass | |
| total root length, root surface, root volume | cm, cm2, cm3 | Root Scanner: Epson Expression 11000XL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, T.; Li, Y.; Wu, Y.; Yang, Q.; Wang, S.; Huang, Z.; Li, Q.; Zhou, X.; Zheng, T.; Pei, X.; et al. Functional Traits of Herbaceous Plants with Ecological Restoration Potential Under Drought Conditions. Plants 2025, 14, 3552. https://doi.org/10.3390/plants14233552
Zou T, Li Y, Wu Y, Yang Q, Wang S, Huang Z, Li Q, Zhou X, Zheng T, Pei X, et al. Functional Traits of Herbaceous Plants with Ecological Restoration Potential Under Drought Conditions. Plants. 2025; 14(23):3552. https://doi.org/10.3390/plants14233552
Chicago/Turabian StyleZou, Tong, Yujie Li, Yanling Wu, Qingwen Yang, Shuangcheng Wang, Zhenfu Huang, Qiang Li, Xiaohui Zhou, Tianliang Zheng, Xiangjun Pei, and et al. 2025. "Functional Traits of Herbaceous Plants with Ecological Restoration Potential Under Drought Conditions" Plants 14, no. 23: 3552. https://doi.org/10.3390/plants14233552
APA StyleZou, T., Li, Y., Wu, Y., Yang, Q., Wang, S., Huang, Z., Li, Q., Zhou, X., Zheng, T., Pei, X., & Li, J. (2025). Functional Traits of Herbaceous Plants with Ecological Restoration Potential Under Drought Conditions. Plants, 14(23), 3552. https://doi.org/10.3390/plants14233552






