Responses to Different Magnesium Supply Treatments in the Mature Leaves of Cunninghamia lanceolata Seedlings: Morphological, Physiological, and Structural Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Site
2.2. Different Magnesium Concentration Treatments
2.3. Sample Collection
2.4. Leaf Morphology and Biomass Measurement
2.5. Determination of Mineral Elements in Leaves
2.6. Photosynthetic Physiology Analysis
2.7. Oxidation-Related Indicators and Photosynthetic Enzyme Activities Analysis
2.8. Chloroplast Ultrastructure Observation
2.9. Data Analysis and Structural Equation Model Construction
3. Results
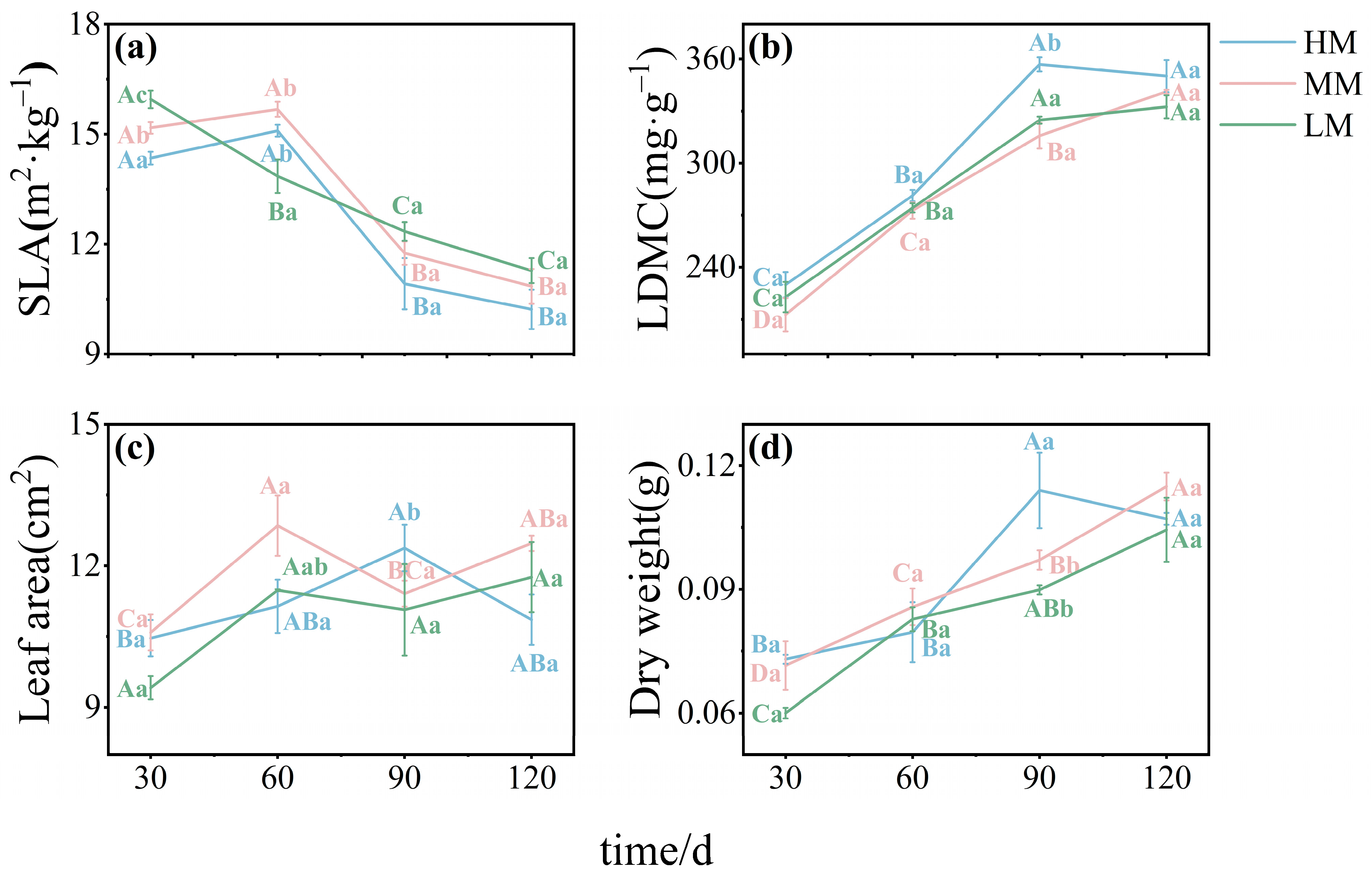
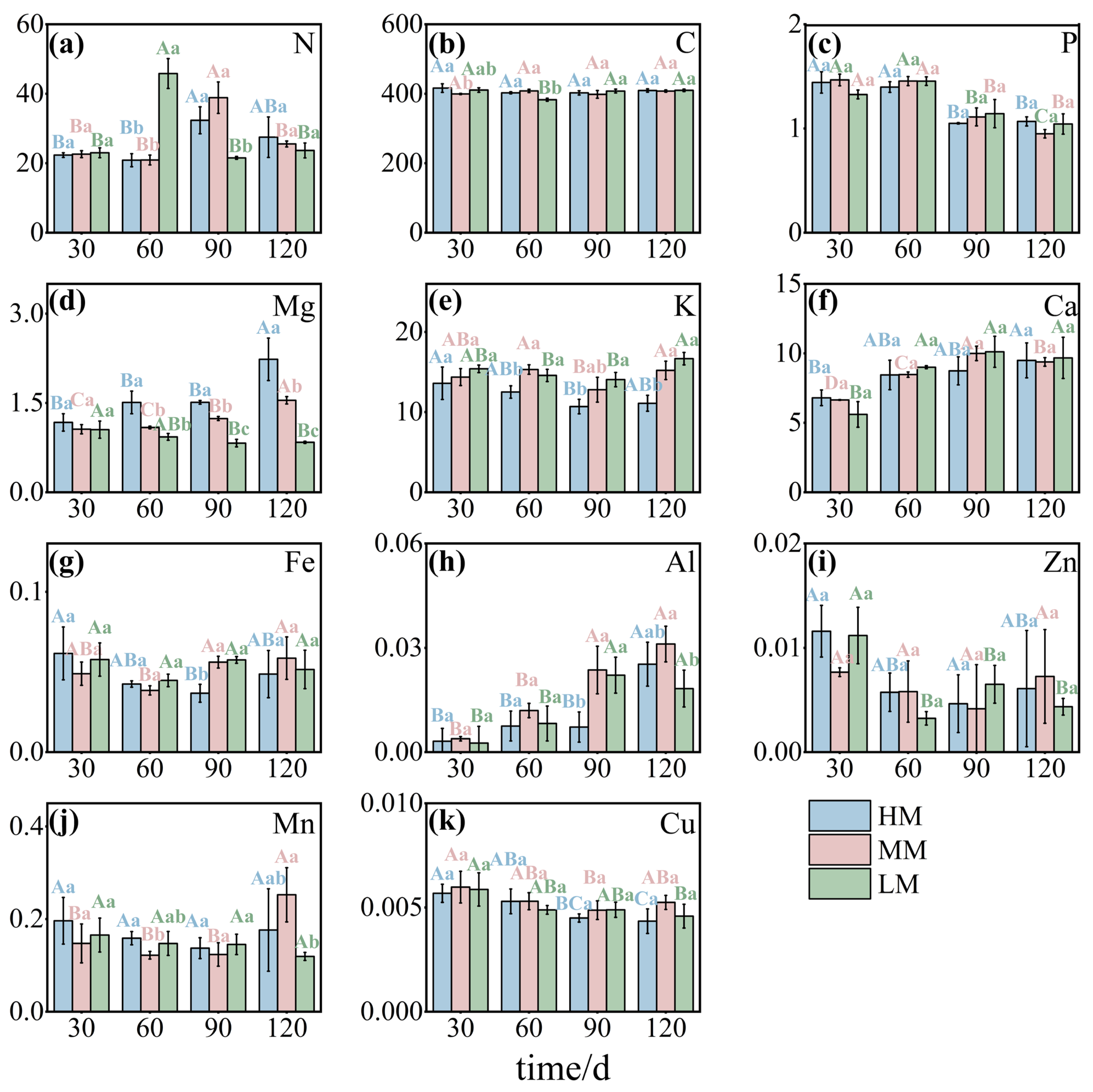
3.1. Effects of Magnesium Treatments on Leaf Morphology and Chemical Traits
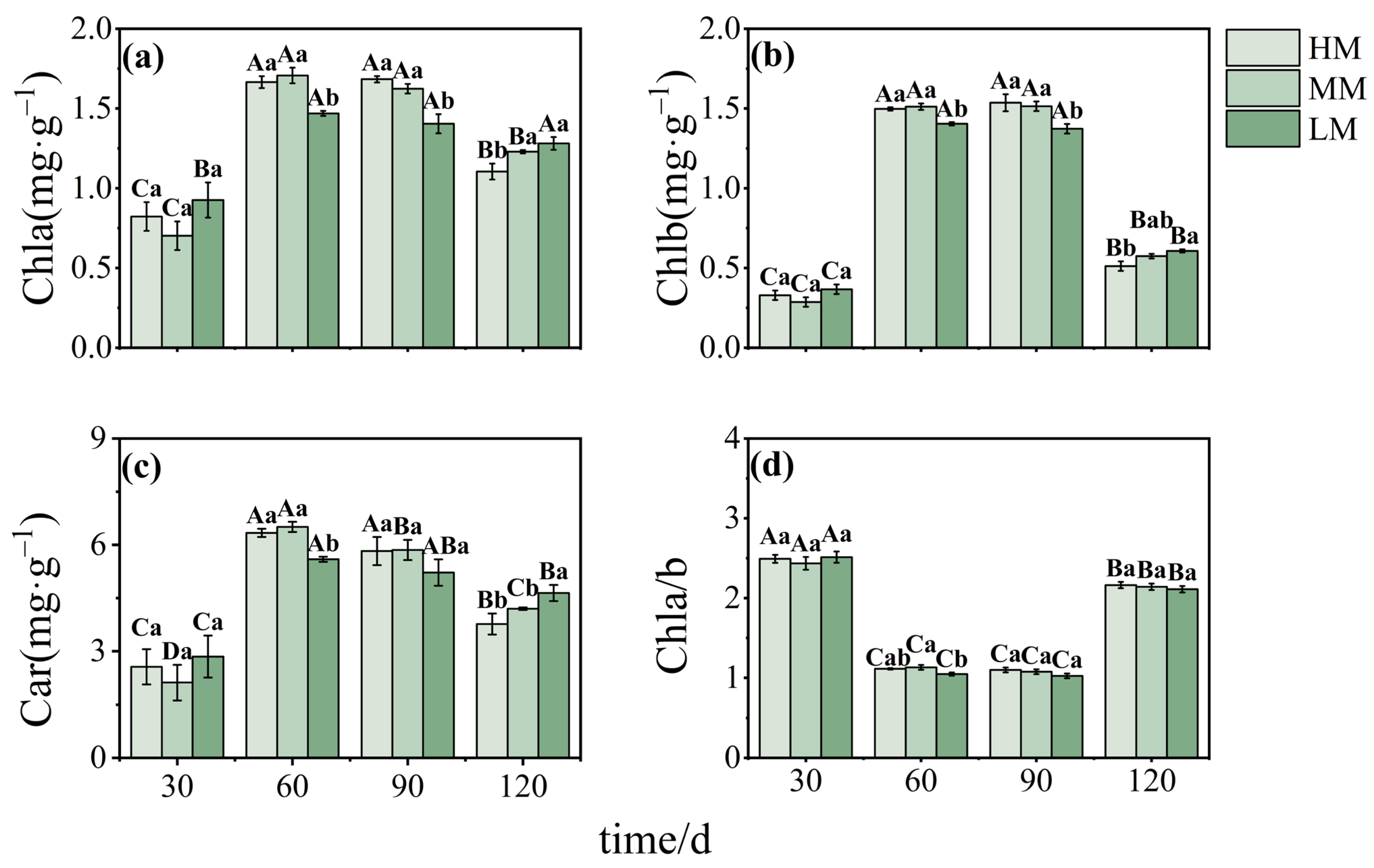
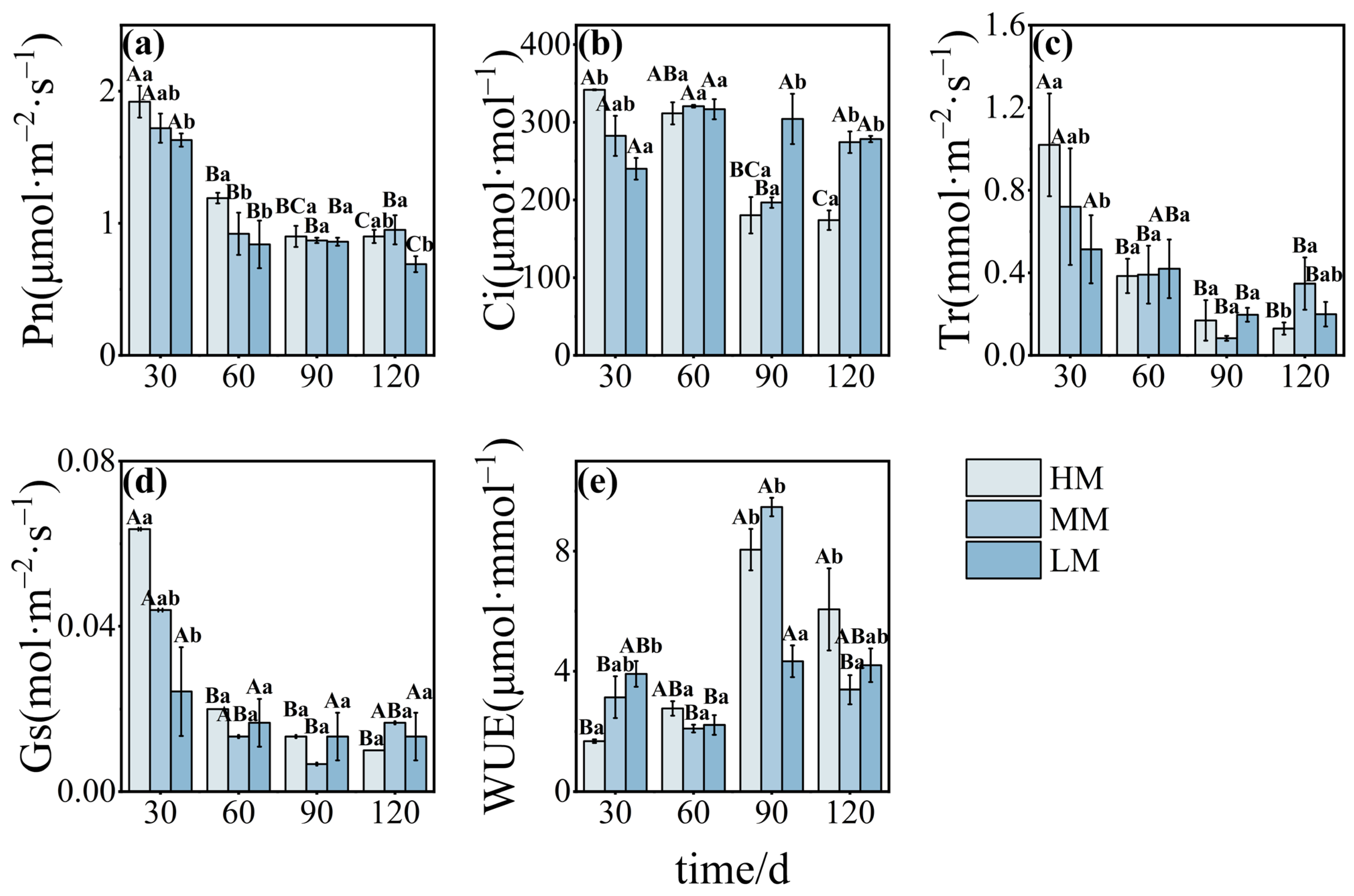
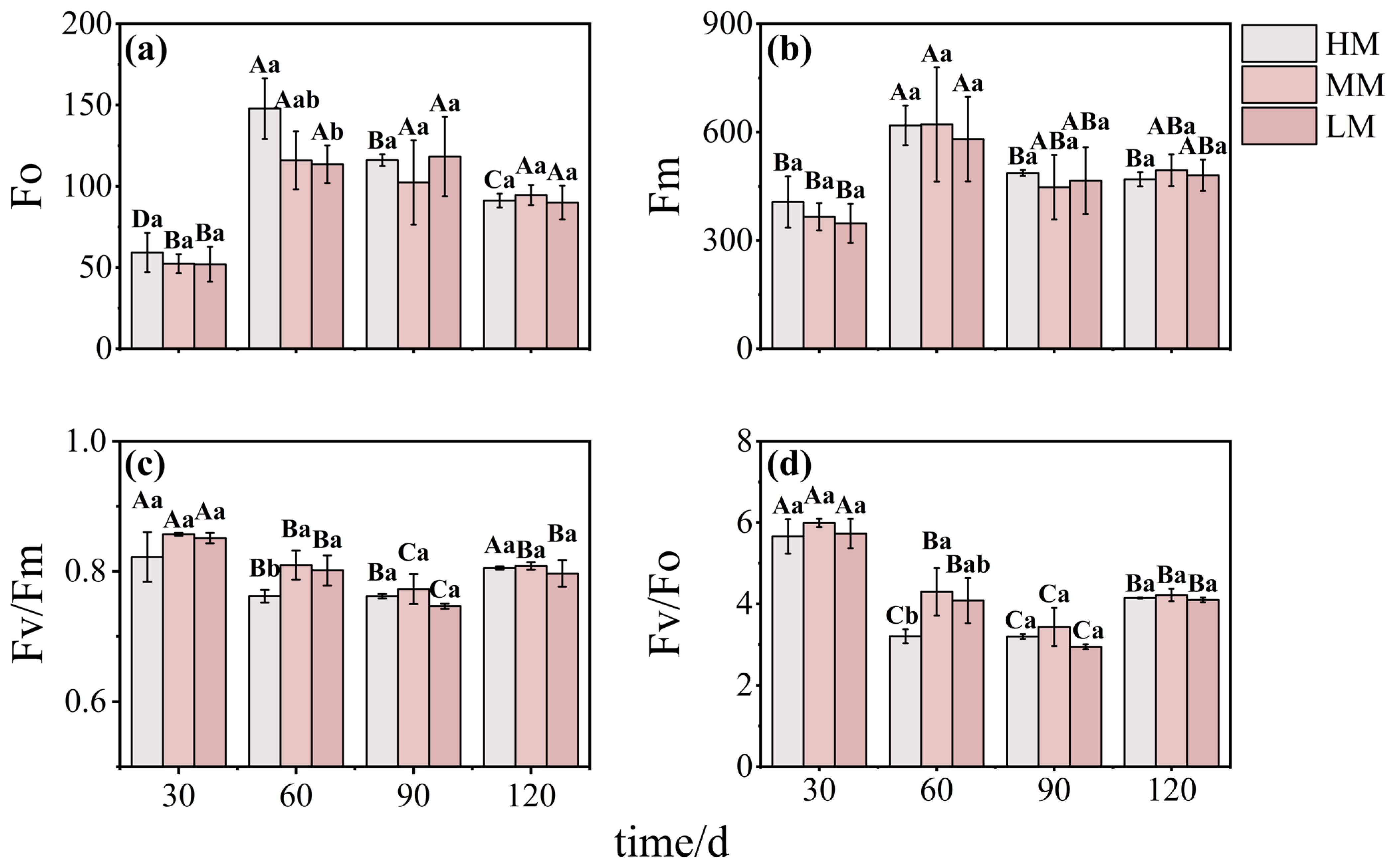
3.2. Magnesium-Mediated Changes in Photosynthetic Performance and Chlorophyll Metabolism
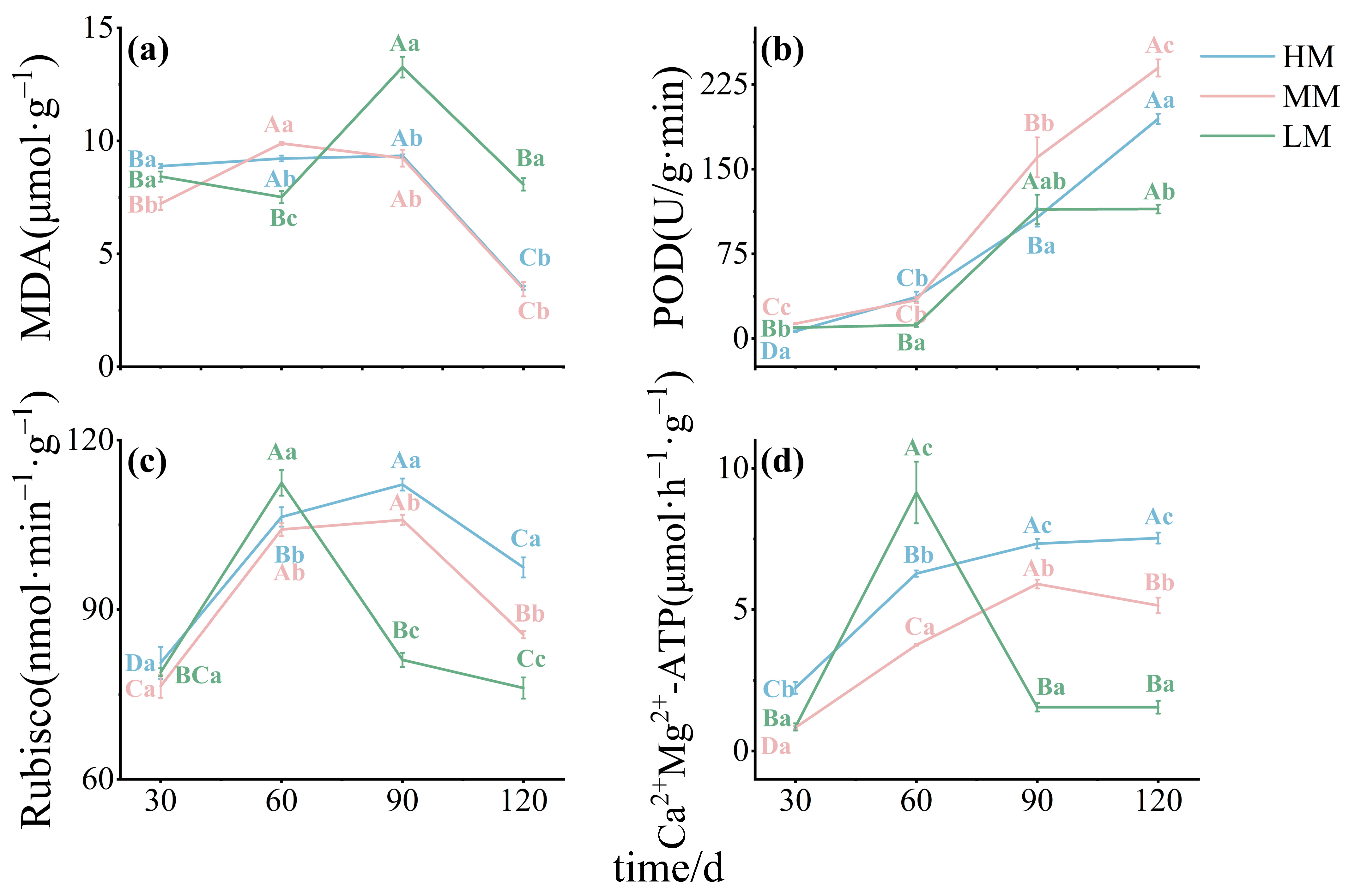
3.3. Effects on Enzyme Activities: Carbon Assimilation and Oxidative Stress
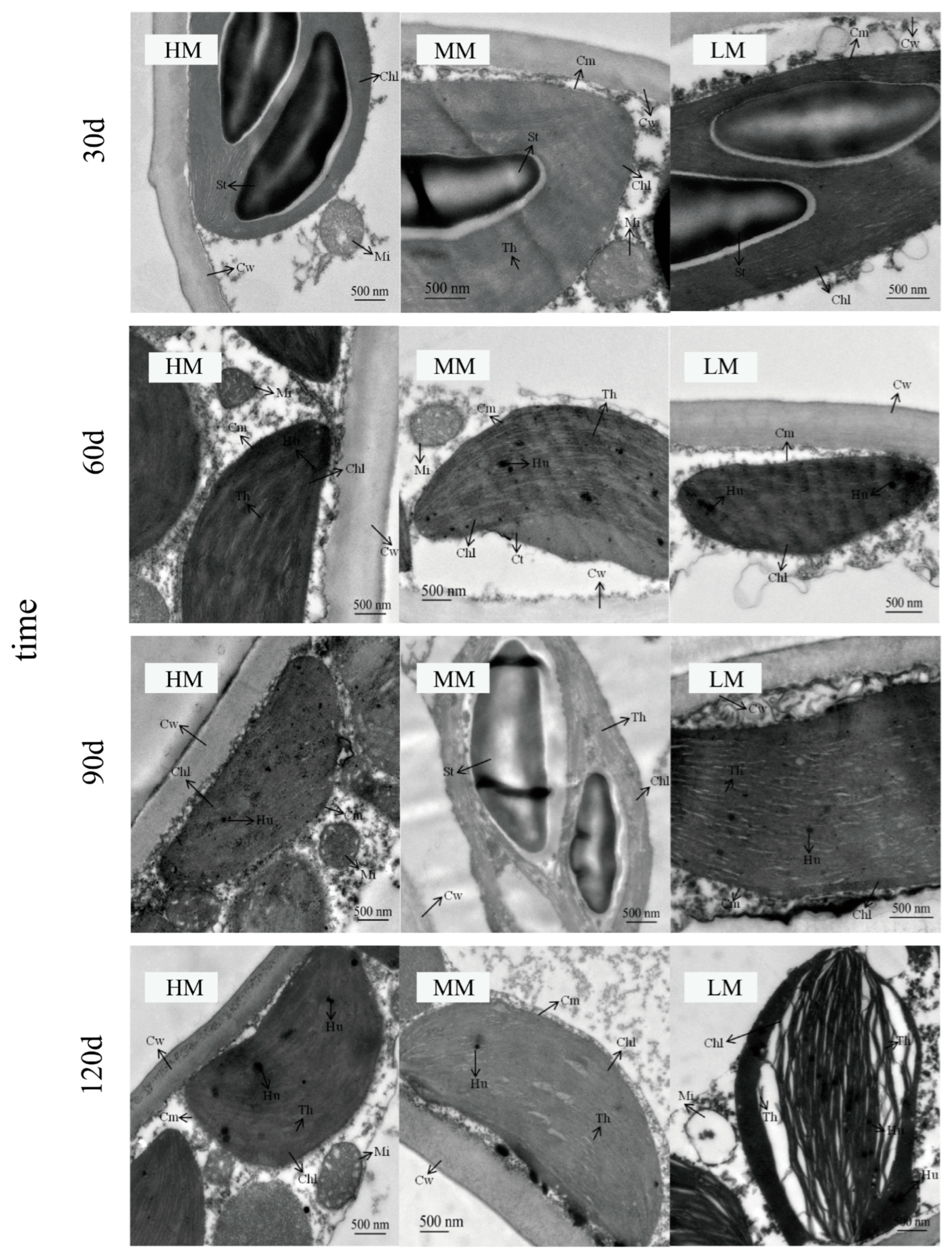
3.4. Magnesium-Induced Changes in Chloroplast Ultrastructure
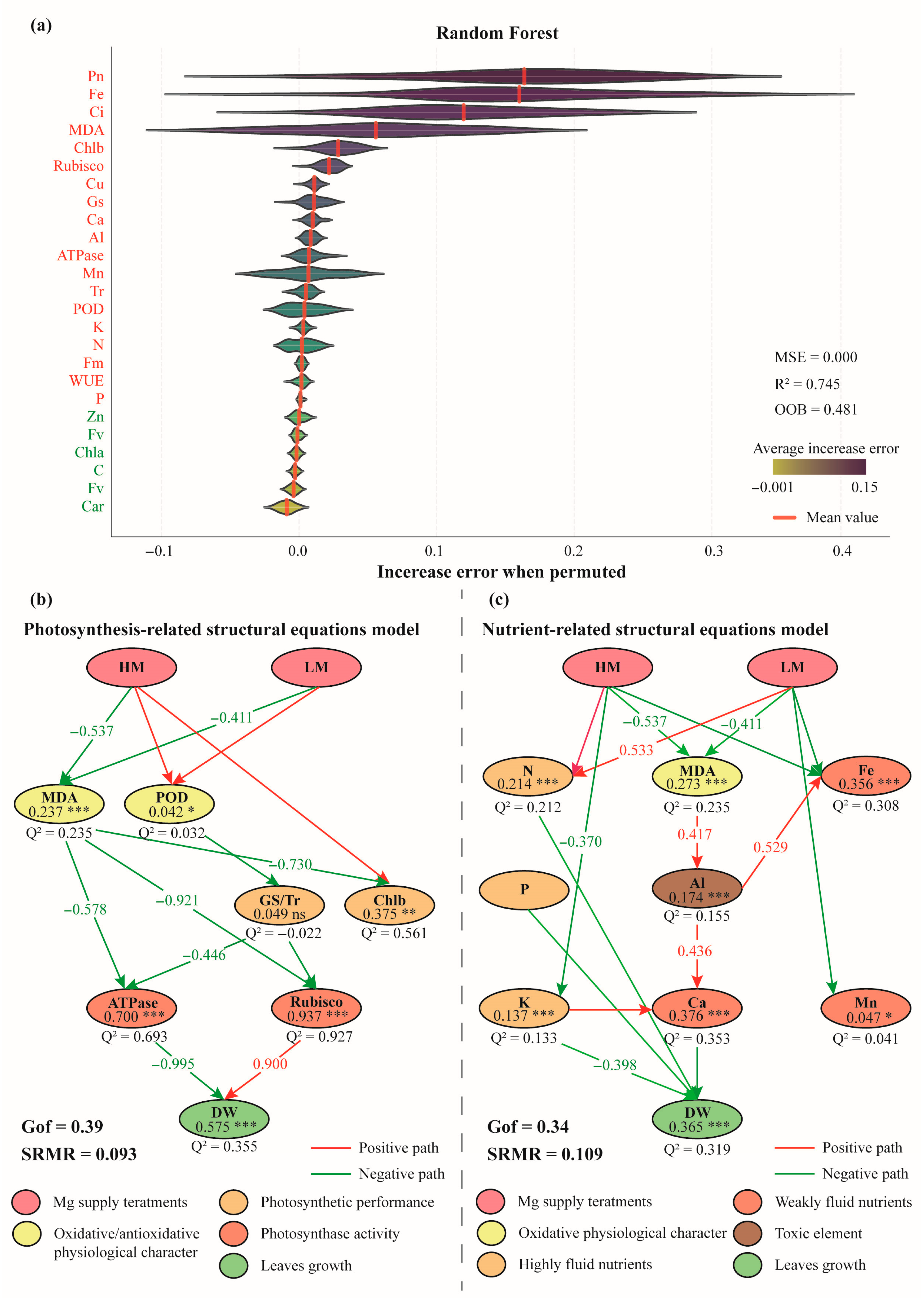
3.5. Key Factors Regulating Mature Leaves Growth Mediates by Different Magnesium Supply
4. Discussion
4.1. Dynamic Changes in Leaf Functional Traits During Development
4.2. Responses of C. lanceolata Leaves to Magnesium Stress
4.3. Growth Characteristics and Regulatory Factors of Mature Leaves in Cunninghamia lanceolata Seedlings Exposed to Magnesium Stress
4.4. Practical Implications for C. lanceolata Plantation Management
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaniv, S.; Gadi, S.; Noam, A. Harnessing Photosynthesis to Produce Electricity Using Cyanobacteria, Green Algae, Seaweeds and Plants. Front. Plant Sci. 2022, 13, 955843. [Google Scholar] [CrossRef]
- Murchie, E.H.; Kefauver, S.; Araus, J.L.; Muller, O.; Rascher, U.; Flood, P.J.; Lawson, T. Measuring the Dynamic Photosynthome. Ann. Bot. 2018, 122, 207–220. [Google Scholar] [CrossRef]
- Legris, M. Light and Temperature Regulation of Leaf Morphogenesis in Arabidopsis. New Phytol. 2023, 240, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, W.; Hu, S.; Liu, M.; Zheng, C.; Sun, X. Effects of Exogenous Melatonin on Photosynthesis and Physiological Characteristics of Chry-Santhemum Seedlings Under High Temperature Stress. J. Appl. Ecol. 2021, 32, 2496–2504. (In Chinese) [Google Scholar] [CrossRef]
- Verryckt, L.T.; Vicca, S.; Langenhove, L.V.; Stahl, C.; Asensio, D.; Urbina, I.; Ogaya, R.; Llusià, J.; Grau, O.; Peguero, G.; et al. Vertical Profiles of Leaf Photosynthesis and Leaf Traits and Soil Nutrients in Two Tropical Rainforests in French Guiana Before and After a 3-Year Nitrogen and Phosphorus Addition Experiment. Earth Syst. Sci. Data 2022, 14, 5–18. [Google Scholar] [CrossRef]
- Zhao, B.; Sui, Y.; Wang, B.; Yuan, F. How Membrane Receptors Mediate Biochemical Responses in Leaf Development Under Environmental Stresses. J. Agric. Food Chem. 2025, 73, 21235–21246. [Google Scholar] [CrossRef]
- Nazir, A.; Zhang, B.; Bilquees, B.; Sadaruddin, C.; Mehtab, R.; Li, J.; Li, Y.; Faisal, H.; Zaid, C.; Tu, P. The Power of Magnesium: Unlocking the Potential for Increased Yield, Quality, and Stress Tolerance of Horticultural Crops. Front. Plant Sci. 2023, 14, 1285512. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, C.; Lou, Y.; Xu, H.; Cheng, Q.; Sun, S.; Xiao, F. High-Density Genetic Map and QTL Analysis in Cunninghamia lanceolate: Insights into Growth and Wood-Color Traits. Forests 2023, 14, 1591. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, Z.; Han, Z.; Zhang, J.; Han, S.; Lin, H. Growth Characteristics of Cunninghamia lanceolata in China. Sci. Rep. 2022, 12, 18179. [Google Scholar] [CrossRef]
- Ren, X.; Jia, J.; Hu, Y.; Han, B.; Peng, P.; Zhang, M.; Liu, M. Cunninghamia lanceolata Cannot Depend Solely on Xylem Embolism Resistance to Withstand Porolonged Seasonal Drought. J. Hydrol. 2024, 645, 132255. [Google Scholar] [CrossRef]
- Zhou, L.; Li, S.; Jia, Y.; Heal, K.V.; He, Z.; Wu, P.; Ma, X. Spatiotemporal Distribution of Canopy Litter and Nutrient Resorption in a Chronosequence of Different Development Stages of Cunninghamia lanceolata in Southeast China. Sci. Total Environ. 2021, 762, 143153. [Google Scholar] [CrossRef]
- Chen, J.; Deng, Z.; Jiang, Z.; Sun, J.; Meng, F.; Zuo, X.-H.; Wu, L.-k.; Cao, G.; Cao, S. Variations of Rhizosphere and Bulk Soil Microbial Community in Successive Planting of Chinese Fir (Cunninghamia lanceolata). Front. Plant Sci. 2022, 13, 954777. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, F.; Wen, Z.; Chen, W.; Zhou, C. Impacts of Successive Chinese Fir Plantations on Soil Carbon and Nitrogen Dynamics: Conclusive Insights from Metagenomic Analysis. J. Environ. Manag. 2025, 376, 124510. [Google Scholar] [CrossRef]
- Farooq, T.H.; Yan, W.; Rashid, M.H.U.; Tigabu, M.; Wu, P. Chinese Fir (Cunninghamia lanceolata) a Green Gold of China with Continues Decline in Its Productivity Over the Successive Rotations: A Review. Appl. Ecol. Environ. Res. 2019, 17, 11055–11067. [Google Scholar] [CrossRef]
- Shen, L.; Ye, S.; Liu, H.; Deng, X.; He, P.; Cheng, F. Linkage Between Leaf-Litter-Soil, Microbial Resource Limitation, and Carbon-Use Efficiency in Successive Chinese Fir (Cunninghamia lanceolata) Plantations. Forests 2023, 14, 357. [Google Scholar] [CrossRef]
- Jing, M.; Zhu, L.; Liu, S.; Cao, Y.; Zhu, Y.; Yan, W. Warming-Induced Drought Leads to Tree Growth Decline in Subtropics: Evidence from Tree Rings in Central China. Front. Plant Sci. 2022, 13, 964400. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, L.; Li, C.; Chen, M.; Zhou, Y.; Meng, M.; Li, Z.; Zhang, J.; Liu, X. Effects of Short-Term Simulated Acid Rain and Nitrogen Deposition on Soil Nutrients and Enzyme Activities in Cunninghamia lanceolata Plantation. Front. Ecol. Evol. 2024, 12, 1365954. [Google Scholar] [CrossRef]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of Magnesium Fertilisers in Agriculture: Plant–soil Continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Qu, S.; Li, H.; Zhang, X.; Gao, J.; Ma, R.; Ma, L.; Ma, J. Effects of Magnesium Imbalance on Root Growth and Nutrient Absorption in Different Genotypes of Vegetable Crops. Plants 2023, 12, 3518. [Google Scholar] [CrossRef] [PubMed]
- Dwaipayan, S.; Soumi, D. Molecular Mechanism of Aluminum Tolerance in Plants: An Overview. In Plant Metal and Metalloid Transporters; Kumar, K., Srivastava, S., Eds.; Springer: Singapore, 2022; pp. 179–205. [Google Scholar]
- Fang, J.; Chen, B.; Wang, F.; Li, W.; Zhang, H.; Fang, J.; Liu, S.; Zheng, Z.; Guo, M.; Niu, S. Nitrogen, Phosphorus, and Potassium Co-Limitation in Terrestrial Ecosystems: A Global Meta-Analysis. Plants People Planet 2024, 6, 1329–1340. [Google Scholar] [CrossRef]
- Yahaya, S.M.; Mahmud, A.A.; Abdullahi, M.; Haruna, A. Recent Advances in the Chemistry of Nitrogen, Phosphorus and Potassium as Fertilizers in Soil: A Review. Pedosphere 2023, 33, 385–406. [Google Scholar] [CrossRef]
- Lu, Z.; Ren, T.; Li, Y.; Cakmak, I.; Lu, J. Nutrient Limitations on Photosynthesis: From Individual to Combinational Stresses. Trends Plant Sci. 2025, 30, 872–885. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, L.; Geng, S.; Ruan, J. Optimization of Nutrient Management Improves Productivity, Quality and Sustainability of Albino Tea Cultivar Baiye-1. Front. Plant Sci. 2024, 15, 1369015. [Google Scholar] [CrossRef]
- Gong, Y.; Alassimone, J.; Varnau, R.; Sharma, N.; S, C.L.; C, B.D. Tuning self-renewal in the Arabidopsis Stomatal Lineage by Hormone and Nutrient Regulation of Asymmetric Cell Division. eLife 2021, 10, e63335. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Li, Z.; Guo, H. New Advances in the Regulation of Leaf Senescence by Classical and Peptide Hormones. Front. Plant Sci. 2022, 13, 923136. [Google Scholar] [CrossRef]
- Gong, X.; Chen, J.; Chen, Y.; He, Y.; Jiang, D. Advancements in Rice Leaf Development Research. Plants 2024, 13, 904. [Google Scholar] [CrossRef]
- Lv, Z.; Zhao, W.; Kong, S.; Li, L.; Lin, S. Overview of Molecular Mechanisms of Plant Leaf Development: A Systematic Review. Front. Plant Sci. 2023, 14, 1293424. [Google Scholar] [CrossRef]
- Wu, Q.; Zheng, D.; Lian, N.; Zhu, X.; Wu, J. Hormonal Regulation and Stimulation Response of Jatropha curcas L. Homolog Overexpression on Tobacco Leaf Growth by Transcriptome Analysis. Int. J. Mol. Sci. 2023, 24, 13183. [Google Scholar] [CrossRef]
- Liu, H.; Han, X.; Ruan, J.; Xu, L.; He, B. Transcriptome Analysis of Ginkgo biloba L. Leaves Across Late Developmental Stages Based on RNA-Seq and Co-Expression Network. Forests 2021, 12, 315. [Google Scholar] [CrossRef]
- Prathibha, V.H.; Rajesh, M.K.; Dinesh, A.; Patil, B.; Daliyamol; Nagaraja, N.R.; Rajkumar; Sabana, A.A.; Gangaraj, K.P.; Thejasri, K.P.; et al. Multi-Gene Phylogeny and Phenotypic Analyses Revealed an Association of Different Colletotrichum Species with Inflorescence Dieback and Leaf Spot of Arecanut in India. Physiol. Mol. Plant Pathol. 2024, 134, 102416. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Z.; Ma, X.; Mulualem, T.; Xing, X.; Jin, S.; Liu, B. Phenotypic Plasticity of Cunninghamia lanceolata (Lamb.) Hook. Seedlings in Response to Varied Light Quality Treatments. Forests 2022, 13, 201. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, B. Effects of Simulated Nitrogen Deposition on the Physiological and Growth Characteristics of Seedlings of Two Typical Subtropical Tree Species. Plants 2025, 14, 2153. [Google Scholar] [CrossRef]
- Hu, R.; Sun, Y.; Wu, B.; Duan, H.; Zheng, H.; Hu, D.; Lin, H.; Tong, Z.; Xu, J.; Li, Y. Somatic Embryogenesis of Immature Cunninghamia lanceolata (Lamb.) Hook Zygotic Embryos. Sci. Rep. 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; He, D.D.; Bai, S.; Zeng, W.Z.; Wang, Z.; Wang, M.; Wu, L.Q.; Chen, Z.C. Physiological and Molecular Advances in Magnesium Nutrition of Plants. Plant Soil 2021, 468, 1–17. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, H.; Tian, H.; Chai, G.; Muhammad, R.; Wang, Q.; Liang, B.; Wu, X. Comprehensive Analysis of the Effects on Photosynthesis and Energy Balance in Tomato Leaves Under Magnesium Deficiency. Plant Physiol. Biochem. 2025, 222, 109671. [Google Scholar] [CrossRef]
- Chaudhry, A.H.; Nayab, S.; Hussain, S.B.; Ali, M.; Zhiyong, P. Current Understandings on Magnesium Deficiency and Future Outlooks for Sustainable Agriculture. Int. J. Mol. Sci. 2021, 22, 1819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Q.; Li, R.; Wei, R.; Geng, X.; Zhang, X.; Wei, H.; Gao, P.; Xu, K.; Dai, Q.; et al. Foliar Magnesium Enhances Rice Salt Tolerance by Improving Photosynthesis and Regulating Ion Homeostasis. Front. Plant Sci. 2025, 16, 1578023. [Google Scholar] [CrossRef]
- Shi, Y.; Jin, X.; Ackah, M.; Amoako, F.K.; Li, J.; Tsigbey, V.E.; Li, H.; Cui, Z.; Sun, L.; Zhao, C.; et al. Comparative Physio-Biochemical and Transcriptome Analyses Reveal Contrasting Responses to Magnesium Imbalances in Leaves of Mulberry (Morus alba L.) Plants. Antioxidants 2024, 13, 516. [Google Scholar] [CrossRef]
- Ye, X.; Chen, X.F.; Deng, C.L.; Yang, L.T.; Lai, N.W.; Guo, J.X.; Chen, L.S. Magnesium-Deficiency Effects on Pigments, Photosynthesis and Photosynthetic Electron Transport of Leaves, and Nutrients of Leaf Blades and Veins in Citrus Sinensis Seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Li, S.; Lu, S.; Wang, J.; Liu, Z.; Yuan, C.; Wang, M.; Guo, J. Divergent Effects of Single and Combined Stress of Drought and Salinity on The Physiological Traits and Soil Properties of Platycladus Orientalis Saplings. Front. Plant Sci. 2024, 15, 1351438. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jin, T.; Li, T.; An, Y.; Dai, X.; Zhao, C.; Qu, T. Euphorbia marginata Alleviate Heavy Metal Ni-Cu Combined Stress by Regulating the Synthesis of Signaling Factors and Flavonoid Organisms. Plants 2025, 14, 2159. [Google Scholar] [CrossRef]
- Thakur, D.; Singh, L.; Chawla, A. Reliability of Leaf Functional Traits after Delayed Measurements. Aust. J. Bot. 2020, 68, 107. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, U.; Poorter, L.; Wright, I.J.; Villar, R. Causes and Consequences of Variation in Leaf Mass Per Area (LMA): A Meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Liu, X.; Li, N.; Ji, T.; Zhou, B.; Wei, M.; Li, J.; Yang, F. Effects of Microbial Agents and Corn Protein Ferment on Physiological Characteristics in Leaves and Yield of Tomato. Ying Yong Sheng Tai Xue Bao 2023, 34, 3039–3044. (In Chinese) [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Kong, L.L.; Huang, Z.Q.; He, Z.M.; Zheng, L.J.; Liu, Z.M.; Wang, M.H. Variations of Water Use Efficiency and Foliar Nutrient Concentrations in Cunninghamia lanceolata Plantations at Different Ages. J. Appl. Ecol. 2017, 28, 1069–1076. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, S.; Wei, C.; Yu, S.; Shi, J.; Zhang, B. Effect of Magnesium Deficiency on Photosynthetic Physiology and Triacylglyceride (TAG) Accumulation of Chlorella Vulgaris. Huan Jing Ke Xue 2014, 35, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K.; Truong, T.B. Evolution of Flexible Non-photochemical Quenching Mechanisms that Regulate Light Harvesting in Oxygenic Photosynthesis. Curr. Opin. Plant Biol. 2013, 16, 307–314. [Google Scholar] [CrossRef]
- Cakmak, I.; Kirkby, E.A. Role of Magnesium in Carbon Partitioning and Alleviating Photooxidative Damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Veneklaas, E.J.; Kuo, J.; Lambers, H. Physiological and Ecological Significance of Biomineralization in Plants. Trends Plant Sci. 2014, 19, 166–174. [Google Scholar] [CrossRef]
- Ye, X.; Gao, Z.; Xu, K.; Li, B.; Ren, T.; Li, X.; Cong, R.; Lu, Z.; Cakmak, I.; Lu, J. Photosynthetic Plasticity Aggravates the Susceptibility of Magnesium-Deficient Leaf to High Light in Rapeseed Plants: The importance of Rubisco and mesophyll conductance. Plant J. 2024, 117, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, Y.; Jeong, B.R. Foliar Silicon Spray before Summer Cutting Propagation Enhances Resistance to Powdery Mildew of Daughter Plants. Int. J. Mol. Sci. 2022, 23, 3803. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Kleczkowski, L.A. Implications of Adenylate Kinase-governed Equilibrium of Adenylates on Contents of Free Magnesium in Plant Cells and Compartments. Biochem. J. 2001, 360 Pt 1, 225–231. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Kleczkowski, L.A. Magnesium and Cell Energetics in Plants under Anoxia. Biochem. J. 2011, 437, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Kleczkowski, L.A. Optimization of CO2 Fixation in Photosynthetic Cells Via Thermodynamic Buffering. Biosystems 2011, 103, 224–229. [Google Scholar] [CrossRef]
- Huang, L.; Lai, U.L.; Yang, S.; Chu, M.; Kuo, C.; Tsai, M.; Sun, C. Delayed Flower Senescence of Petunia Hybrida Plants Transformed with Antisense Broccoli ACC Synthase and ACC Oxidase Genes. Postharvest Biol. Technol. 2007, 46, 47–53. [Google Scholar] [CrossRef]
- Tanaka, A.; Tanaka, R. Chlorophyll Metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef]
- Fatemi, A.; Moaveni, P.; Daneshian, J.; Mozafari, H.; Ghaffari, M. Magnesium Nanoparticles Improve Grain Yield, Oil Percentage, Physiological, and Biochemical Traits of Sunflower (Helianthus annuus L.) under Drought Stress. J. Agric. Sci. Technol. 2022, 24, 665–678. [Google Scholar]
- Reich, P.B. The World-wide ‘Fast-slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Laughlin, D.C. The Intrinsic Dimensionality of Plant Traits and its Relevance to Community Assembly. J. Ecol. 2014, 102, 186–193. [Google Scholar] [CrossRef]
- Setoodeh, A.; Sakinejad, T.; Shokuhfar, A.; Lack, S.; Mojaddam, M. Changes in Assimilate Partitioning and Remobilization and Maize Water Use Efficiency under Influence of Abscisic Acid and Magnesium-Potassium Ratio under Drought Stress Conditions. Commun. Soil Sci. Plant Anal. 2025, 56, 1555–1572. [Google Scholar] [CrossRef]
- Brehelin, C.; Kessler, F. The Plastoglobule: A Bag Full of Lipid Biochemistry Tricks. Photochem. Photobiol. 2008, 84, 1388–1394. [Google Scholar] [CrossRef]
- Xie, R.; Gao, J.; Yang, Z.; Wang, Y.; Tong, L.; Ke, Y.; Li, C.; Zheng, C.; Li, W. Improving High Light Tolerance of Tobacco Plants: Adequate Magnesium Supply Enhances Photosynthetic Performance. Agronomy 2024, 14, 1396. [Google Scholar] [CrossRef]
- Pohland, A.-C.; Bernat, G.; Geimer, S.; Schneider, D. Mg2+ Limitation Leads to A Decrease in Chlorophyll, Resulting in an Unbalanced Photosynthetic Apparatus in The Cyanobacterium Synechocytis sp. PCC6803. Photosynth. Res. 2024, 162, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, L.; Jiang, H.; Li, Y.; Wang, P.; Chen, L. Physiological Impacts of Magnesium-Deficiency in Citrus Seedlings: Photosynthesis, Antioxidant System and Carbohydrates. Trees-Struct. Funct. 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll A Fluorescence as A Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Kovenock, M.; Koven, C.D.; Knox, R.G.; Fisher, R.A.; Swann, A.L.S. Leaf Trait Plasticity Alters Competitive Ability and Functioning of Simulated Tropical Trees in Response to Elevated Carbon Dioxide. Glob. Biogeochem. Cycles 2021, 35, e2020GB006807. [Google Scholar] [CrossRef]
- Yang, L.T.; Zhou, Y.F.; Wang, Y.Y.; Wu, Y.M.; Ye, X.; Guo, J.X.; Chen, L.S. Magnesium Deficiency Induced Global Transcriptome Change in Citrus sinensis Leaves Revealed by RNA-Seq. Int. J. Mol. Sci. 2019, 20, 3129. [Google Scholar] [CrossRef]
- Driever, S.M.; Simkin, A.J.; Alotaibi, S.; Fisk, S.J.; Madgwick, P.J.; Sparks, C.A.; Jones, H.D.; Lawson, T.; Parry, M.A.J.; Raines, C.A. Increased SBPase Activity Improves Photosynthesis and Grain Yield in Wheat Grown in Greenhouse Conditions. Philos. Trans. R. Soc. B-Biol. Sci. 2017, 372, 20160384. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yang, Y.; Liu, T.; Zhang, S.; Huang, W. Responses of Photosystem I Compared with Photosystem II to Combination of Heat Stress and Fluctuating Light in Tobacco leaves. Plant Sci. 2020, 292, 110371. [Google Scholar] [CrossRef]
- Cejudo, F.J.; Sandalio, L.M.; Van Breusegem, F. Understanding Plant Responses to Stress Conditions: Redox-based Strategies. J. Exp. Bot. 2021, 72, 5785–5788. [Google Scholar] [CrossRef]
- Rabhi, M.; Farhat, N.; Krol, M.; Barhoumi, Z.; Ivanov, A.G.; McCarthy, A.; Abdelly, C.; Huner, N.P.A.; Smaoui, A. Effects of Mg Deficiency and Subsequent Recovery on Sulla Carnosa Leaves. Agrochimica 2017, 61, 317–328. [Google Scholar] [CrossRef]
- Parisa, D.; Repnik, U.; Abdalla, M.A.; Muehling, K.H. Leaf Anatomical Adaptation and Chloroplast Ultrastructure Changes Upon Magnesium Foliar Application of Faba Bean (Vicia faba L.) Grown Under Drought Stress. J. Plant Nutr. Soil Sci. 2025, 188, 78–91. [Google Scholar] [CrossRef]
- Minden, V.; Kleyer, M. Internal and External Regulation of Plant Organ Stoichiometry. Plant Biol. 2014, 16, 897–907. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pineros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Khator, K.; Shekhawat, G.S. Nitric oxide Mitigates Salt-induced Oxidative Stress in Brassica Juncea Seedlings by Regulating ROS Metabolism and Antioxidant Defense System. 3 Biotech 2020, 10, 499. [Google Scholar] [CrossRef]
- Patel, M.; Rangani, J.; Kumari, A.; Parida, A.K. Mineral Nutrient Homeostasis, Photosynthetic Performance, and Modulations of Antioxidative Defense Components in Two Contrasting Genotypes of Arachis hypogaea L. (peanut) for Mitigation of Nitrogen and/or Phosphorus Starvation. J. Biotechnol. 2020, 323, 136–158. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of Magnesium Deficiency on Photosynthesis and Carbohydrate Partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium Deficiency in Plants: An Urgent Problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Tang, X.; Wei, C.; Gao, L.; Jia, B.; Lu, X. Similarity in Fine-to-Total Root Mass Ratio Leads to Comparative Plant-Soil Feedbacks between Co-occurring Native and Invasive Plants. J. Plant Ecol. 2021, 14, 33–43. [Google Scholar] [CrossRef]
- Ouni, A.; Abboud, S.; Tlili, D.; Ben Abdelwaheb, S.; Jellali, M.; Bousetta, W.; Mars, M.; Dbara, S. Growth Disturbance, Mineral Nutrient Imbalance, Specific Symptoms and Leaf Characteristics Changes in Two Pomegranate Cultivars Induced by Deficiencies and Excess of Ca, Mg, Zn and Fe. J. Soil Sci. Plant Nutr. 2024, 24, 8291–8305. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Zhang, X.; Gao, J.; Chen, P. Magnesium Deficiency or Excess Hinders Tomato Growth, Potassium and Calcium Uptake. Plant Soil Environ. 2024, 70, 719–730. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.-H.; Zhang, X.-Y.; Zhang, L.-Y.; Zhao, P.-L.; Wen, T.; Zhang, J.-Q.; Xu, W.-L.; Guo, F.; Zhao, H.; et al. Exploring the Effects of Magnesium Deficiency on the Quality Constituents of Hydroponic-Cultivated Tea (Camellia sinensis L.) Leaves. J. Agric. Food Chem. 2021, 69, 14278–14286. [Google Scholar] [CrossRef]
- Kan, B.; Yang, Y.; Du, P.; Li, X.; Lai, W.; Hu, H. Chlorophyll decomposition is accelerated in banana leaves after the long-term magnesium deficiency according to transcriptome analysis. PLoS ONE 2022, 17, e0270610. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Antonopoulou, C.; Sotiropoulos, T.; Chatzissavvidis, C.; Therios, I. Effect of Magnesium on Mineral Nutrition, Chlorophyll, Proline and Carbohydrate Concentrations of Sweet Orange (Citrus sinensis cv. Newhall) Plants. Appl. Sci. 2023, 13, 7995. [Google Scholar] [CrossRef]
- Huang, Z.R.; Zhang, H.; Ye, X.; Lai, N.W.; Yang, L.T.; Guo, J.X.; Chen, L.S. UHPLC-Q-TOF/MS-based metabolomics reveals altered metabolic profiles in magnesium deficient leaves of Citrus sinensis. Sci. Hortic. 2021, 278, 109870. [Google Scholar] [CrossRef]
- Yamaji, N.; Huang, C.F.; Nagao, S.; Yano, M.; Sato, Y.; Nagamura, Y.; Ma, J.F. A Zinc Finger Transcription Factor ART1 Regulates Multiple Genes Implicated in Aluminum Tolerance in Rice. Plant Cell 2009, 21, 3339–3349. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Hoekenga, O.A. The Physiology, Genetics and Molecular Biology of Plant Aluminum Resistance and Toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Lamichhane, S.; Tarpley, L.; Dou, F. Impact of Excess Magnesium Salt Supply on Rice Yield, Physiological Response, and Grain Mineral Content. Sustainability 2023, 15, 15741. [Google Scholar] [CrossRef]
- Guo, J.; Li, X.; Wang, Y.; Hu, W.; Zhang, L.; Luo, Z.; Xu, H.; Chen, L.-S. Magnesium Deficiency Induced Leaf Chlorosis Affects Plant Growth, Mineral Concentration and Fruit Quality in Field Pomelo Trees. J. Agric. Food Res. 2024, 18, 101338. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Jobbagy, E.G.; Jackson, R.B. A Global Meta-analysis of Soil Exchangeable Cations, PH, Carbon, and Nitrogen with Afforestation. Ecol. Appl. 2009, 19, 2228–2241. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Harrison, R. The Effect of Harvest on Forest Soil Carbon: A Meta-Analysis. Forests 2016, 7, 308. [Google Scholar] [CrossRef]
- Vadeboncoeur, M.A. Meta-analysis of Fertilization Experiments Indicates Multiple Limiting Nutrients in Northeastern Deciduous Forests. Can. J. For. Res. 2010, 40, 1766–1780. [Google Scholar] [CrossRef]
- Wang, Y.; Long, Q.; Li, Y.; Kang, F.; Fan, Z.; Xiong, H.; Zhao, H.; Luo, Y.; Guo, R.; He, X.; et al. Mitigating Magnesium Deficiency for Sustainable Citrus Production: A Case Study in Southwest China. Sci. Hortic. 2022, 295, 110832. [Google Scholar] [CrossRef]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium Fertilization Improves Crop Yield in Most Production Systems: A Meta-Analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Risher, J.J.; Sarstedt, M.; Ringle, C.M. When to Use and How to Report the Results of PLS-SEM. Eur. Bus. Rev. 2019, 31, 2–24. [Google Scholar] [CrossRef]
- Lamb, E.; Shirtliffe, S.; May, W. Structural Equation Modeling in the Plant Sciences: An Example Using Yield Components in Oat. Can. J. Plant Sci. 2011, 91, 603–619. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Su, B.; Lu, S.; Han, T.; Wang, F.; Ding, G.; Wu, C.; Cao, G.; Chen, Y. Responses to Different Magnesium Supply Treatments in the Mature Leaves of Cunninghamia lanceolata Seedlings: Morphological, Physiological, and Structural Perspectives. Plants 2025, 14, 3542. https://doi.org/10.3390/plants14223542
Zhang Y, Su B, Lu S, Han T, Wang F, Ding G, Wu C, Cao G, Chen Y. Responses to Different Magnesium Supply Treatments in the Mature Leaves of Cunninghamia lanceolata Seedlings: Morphological, Physiological, and Structural Perspectives. Plants. 2025; 14(22):3542. https://doi.org/10.3390/plants14223542
Chicago/Turabian StyleZhang, Yaling, Bigui Su, Sheng Lu, Tianran Han, Fenglin Wang, Guochang Ding, Chao Wu, Guangqiu Cao, and Yu Chen. 2025. "Responses to Different Magnesium Supply Treatments in the Mature Leaves of Cunninghamia lanceolata Seedlings: Morphological, Physiological, and Structural Perspectives" Plants 14, no. 22: 3542. https://doi.org/10.3390/plants14223542
APA StyleZhang, Y., Su, B., Lu, S., Han, T., Wang, F., Ding, G., Wu, C., Cao, G., & Chen, Y. (2025). Responses to Different Magnesium Supply Treatments in the Mature Leaves of Cunninghamia lanceolata Seedlings: Morphological, Physiological, and Structural Perspectives. Plants, 14(22), 3542. https://doi.org/10.3390/plants14223542






