Metabolomics Reveals the Mechanism of Browning Inhibition by Transient Light Quality in Tea Plant Tissue Culture
Abstract
1. Introduction
2. Results
2.1. Leaf Morphology Identification of LJ43 Which Exposed to Irradiations of 3 Different Light
2.2. Amino Acid (AA), Polyphenols (PP) and Phenol-Ammonia Ratio (PP/AA) Contents Identification of LJ43 Exposed Under 3 Different Lights
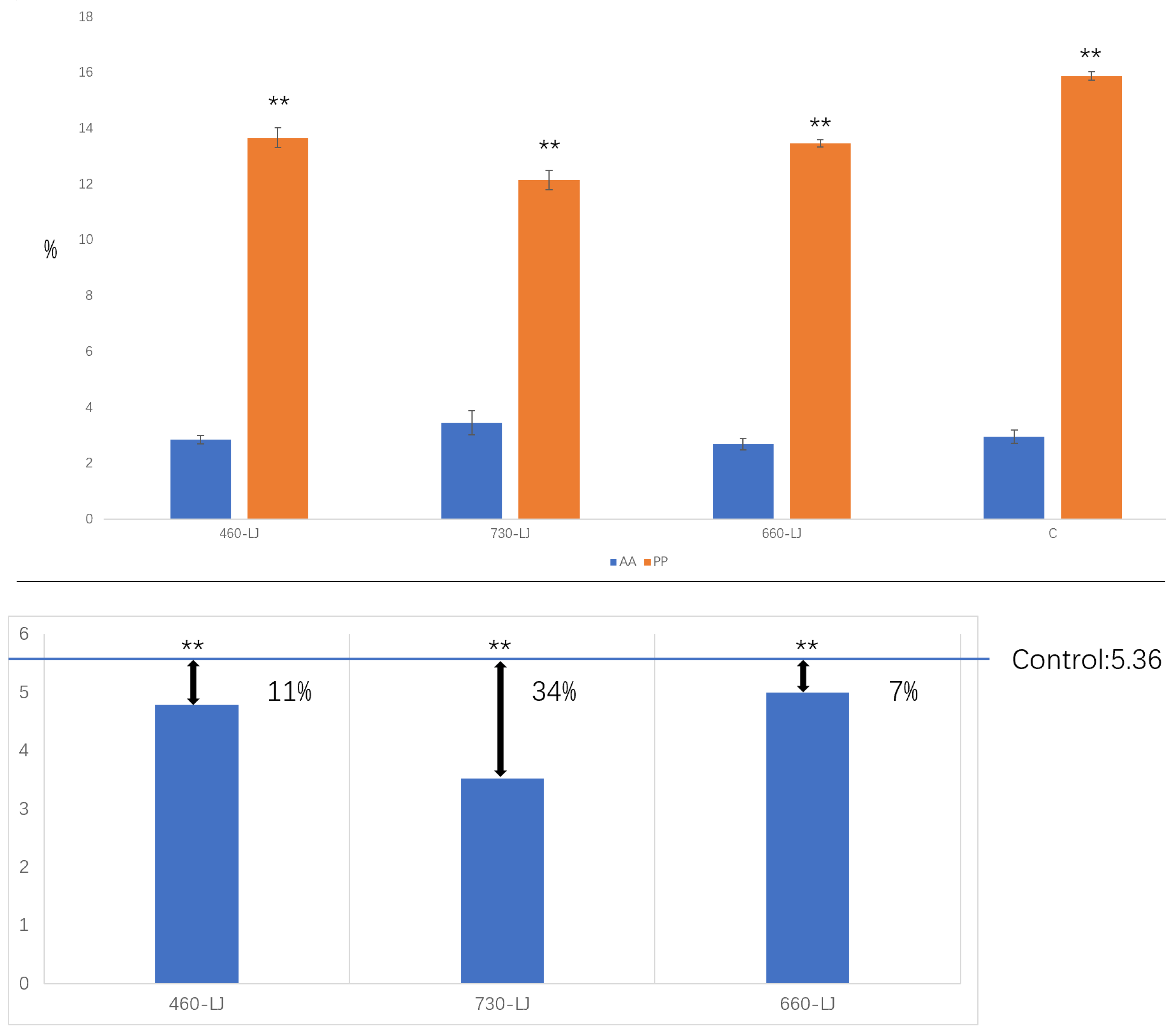
2.3. Tissue Culture and Browning Rate Statistics
| Browning Rate | |
|---|---|
| Control | 11.3% a |
| 660 nm group | 8.0% b |
| 730 nm group | 2.0% c |
| 460 nm group | 7.0% b |
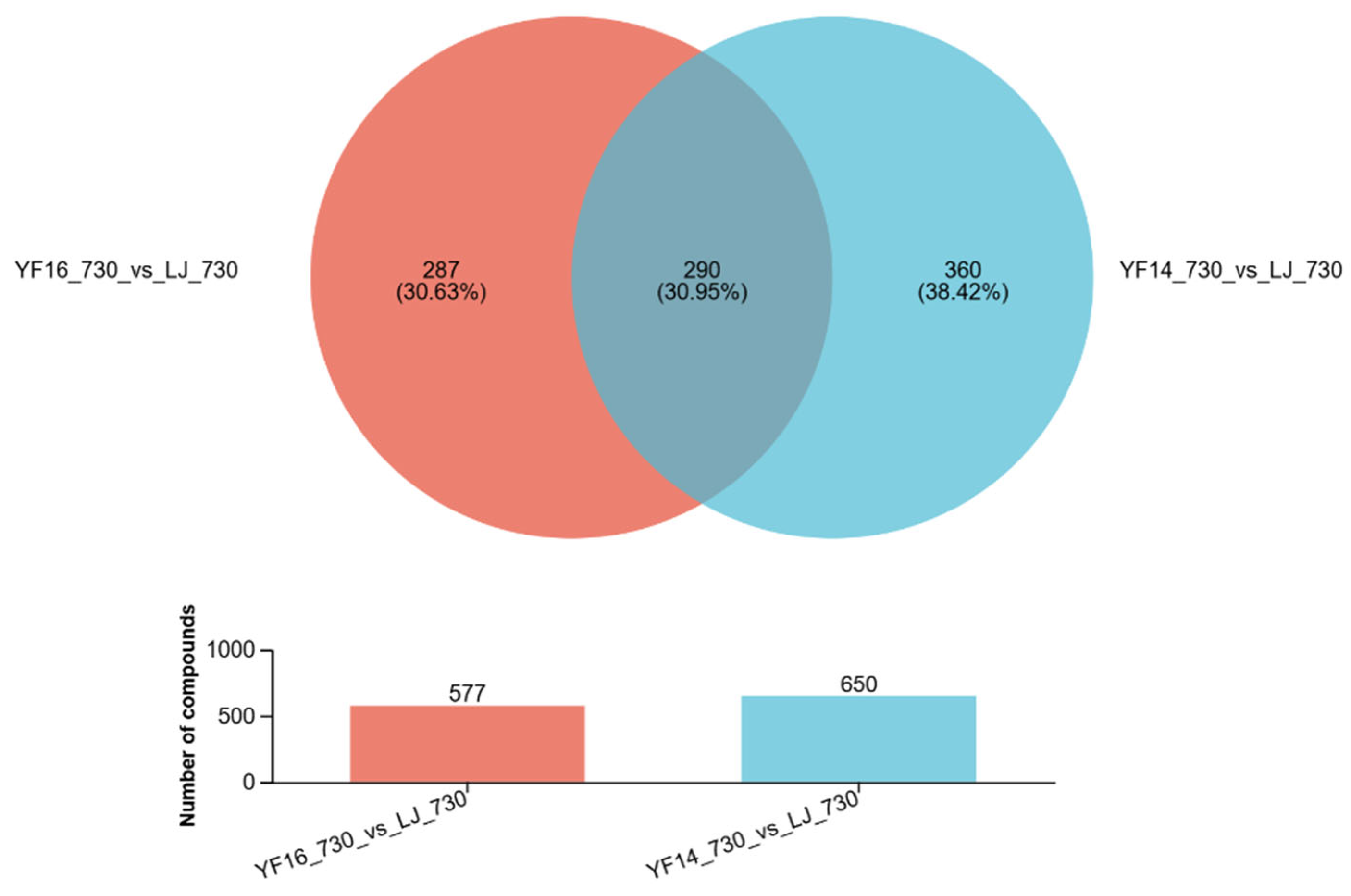
2.4. Untargeted Metabolomics Contents Identification of LJ43 Exposed Under 3 Different Lights
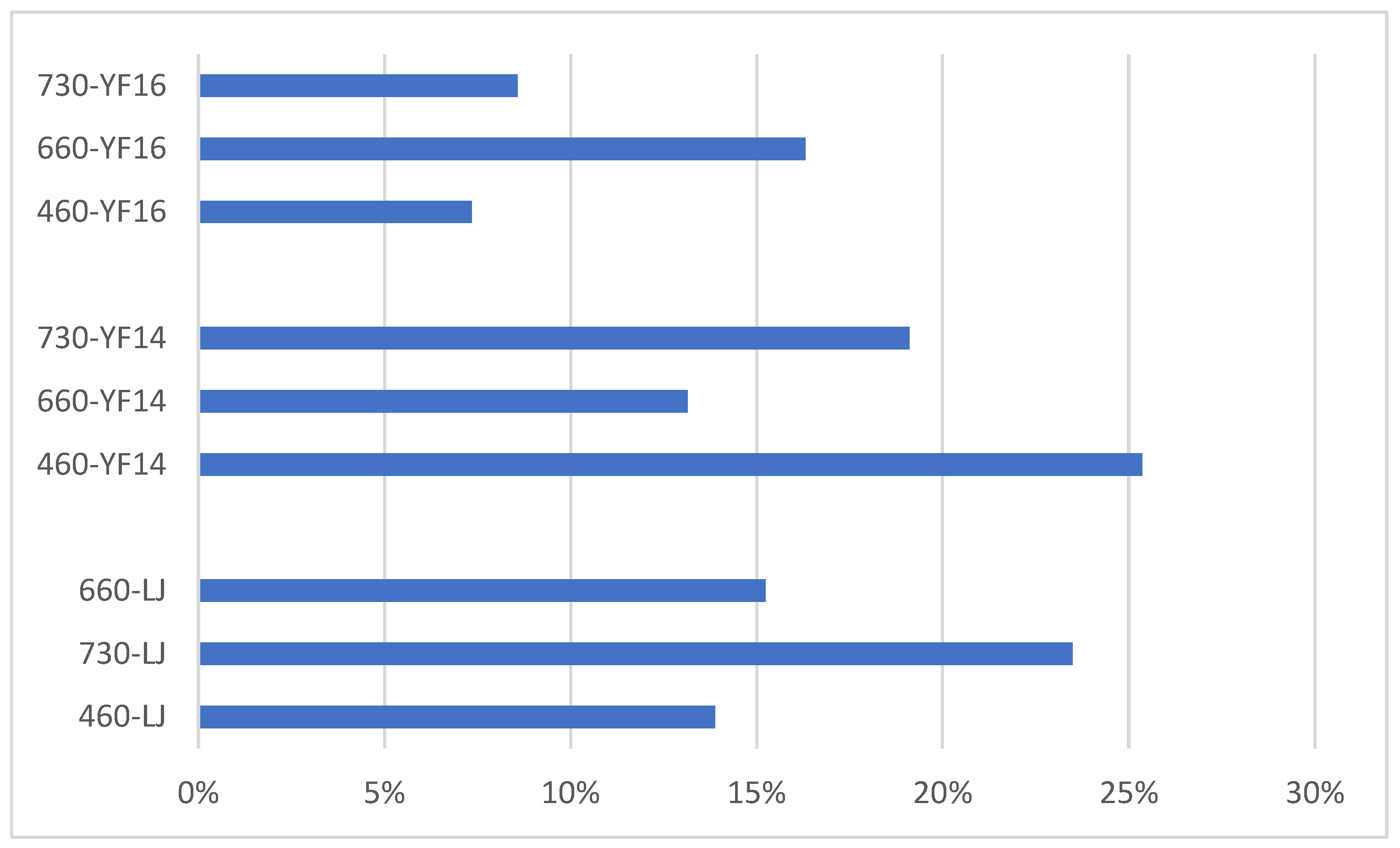
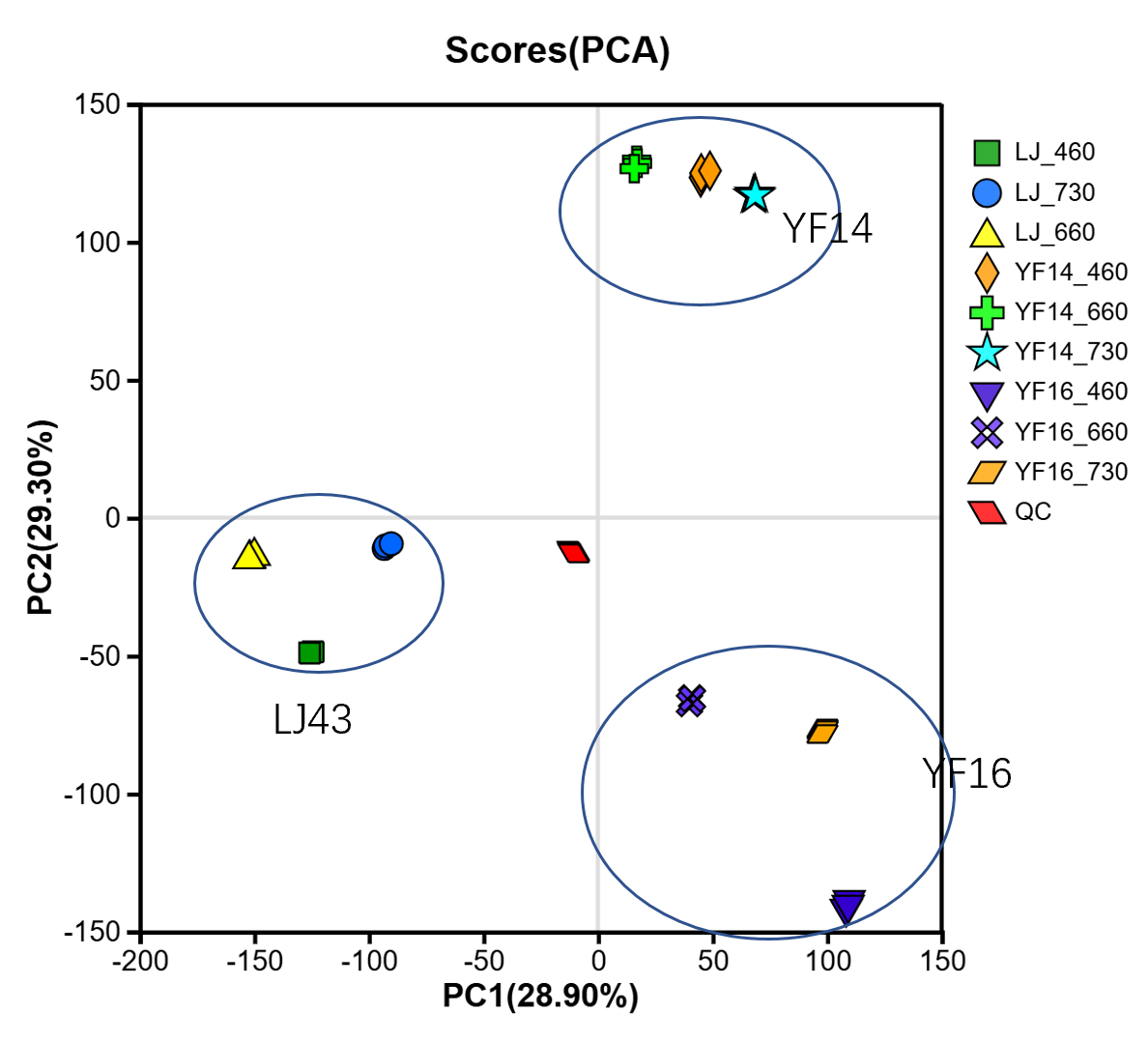
2.5. Untargeted Metabolomics Contents Identification of Other Tea Cultivars Exposed Under 3 Different Lights
3. Discussion
3.1. Optimal Tissue Culture Materials for Reducing Browning Rates
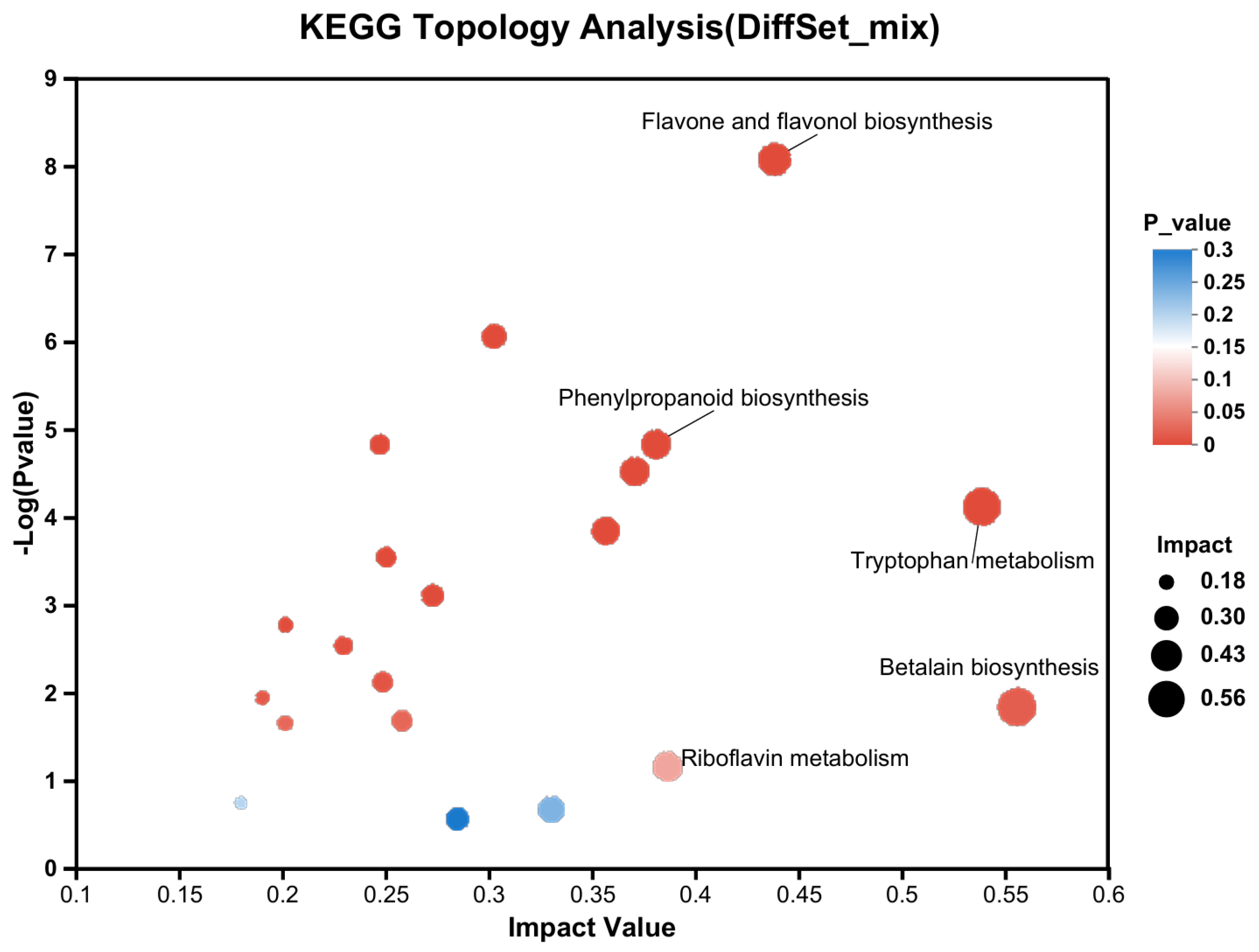
3.2. The Mechanism of Transient Light Quality Modulation on Browning Inhibition in Tea Plant Tissue Cultural
3.3. Different Wavelength Light Treatment Conditions on Different Tea Cultivars
4. Materials and Methods
4.1. Plant Material
4.2. Determination of Amino Acid and Polyphenols
4.3. Determination of Untargeted Metabolomics
4.4. Tissue Culture and Browning Rate Statistics
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Activated carbon |
| PVP | polyvinyl pyrrolidone (PVP) |
| LJ43 | Longjing 43 |
| YF14 | Yunfeng14 |
| YF16 | Yunfeng16 |
| AA | Amino acid |
| PP | Polyphenols |
| PP/AA | Phenol-Ammonia Ratio |
References
- Fu, X.; Chen, Y.; Mei, X.; Katsuno, T.; Kobayashi, E.; Dong, F.; Watanabe, N.; Yang, Z. Regulation of formation of volatile compounds of tea (Camellia sinensis) leaves by single light wavelength. Sci. Rep. 2015, 5, 16858. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, Y.; Xu, Y.; Tang, H.; Chen, K.; Shi, J.; Ren, Z.; Wu, S.; Xia, D.; Zheng, Y. Unveiling the Molecular Mechanisms of Browning in Camellia hainanica Callus through Transcriptomic and Metabolomic Analysis. Int. J. Mol. Sci. 2024, 25, 11021. [Google Scholar] [CrossRef]
- Su, Y.; Wei, M.; Guo, Q.; Huang, J.; Zhao, K.; Huang, J. Investigating the relationships between callus browning in Isatis indigotica Fortune, total phenol content, and PPO and POD activities. Plant Cell Tissue Organ Cult. 2023, 155, 175–182. [Google Scholar] [CrossRef]
- Levai, L.; Ngone, M.; Monono, E.; Ngale, J. Effect of Photoperiod and Ascorbic Acid Concentration on Degree of Browning, Shoot Regeneration and Survival of Plantain (Musa ssp.) Cultivars Growing in vitro. Eur. J. Appl. Sci. 2023, 11, 175–185. [Google Scholar]
- Jin, J.; Chen, Y.; Cai, J.; Litang, L.; Zeng, X.; Li, J.; Asghar, S.; Li, Y. Establishment of an efficient regeneration system of ‘ZiKui’ tea with hypocotyl as explants. Sci. Rep. 2024, 14, 11603. [Google Scholar] [CrossRef]
- Krishna, H.; Sairam, R.K.; Singh, S.K.; Patel, V.B.; Sharma, R.R.; Grover, M.; Nain, L.; Sachdev, A. Mango explant browning: Effect of ontogenic age, mycorrhization and pre-treatments. Sci. Hortic. 2008, 118, 132–138. [Google Scholar] [CrossRef]
- Liu, C.; Fan, H.; Zhang, J.; Wu, J.; Zhou, M.; Cao, F.; Tao, G.; Zhou, X. Combating browning: Mechanisms and management strategies in in vitro culture of economic woody plants. For. Res. 2024, 4, e032. [Google Scholar] [CrossRef]
- Gemechu, E.C.; Amante, G. Control of browning in Plant Tissue Culture: A Review. J. Sci. Innov. Res. 2021, 10, 89–93. [Google Scholar] [CrossRef]
- Permadi, N.; Akbari, S.; Prismantoro, D.; Indriyani, N.; Nurzaman, M.; Alhasnawi, A.; Doni, F.; Julaeha, E. Traditional and next-generation methods for browning control in plant tissue culture: Current insights and future directions. Curr. Plant Biol. 2024, 38, 100339. [Google Scholar] [CrossRef]
- Narang, A.; Kaur, S.; Shukla, A. Seasonal variation in contamination and browning of Acacia nilotica nodal explants in vitro. J. Appl. Nat. Sci. 2024, 1, 16. [Google Scholar] [CrossRef]
- Yang, J.; Bao, J.; Lu, X.; Zhang, X.; Tian, P.; Shi, X.; Li, S.; Ma, S. Transcriptomic analysis of the effects of melatonin on genes potentially related to the browning of broccoli (Brassica oleracea L. var. italica Planch) hairy roots. Plant Growth Regul. 2022, 98, 557–567. [Google Scholar] [CrossRef]
- Xia, H.; Chao, Y.; Nie, J.; Yang, H.; Ji, W.; Xu, G.; Zhu, H.; Jin, S.; Zhu, X. The Effect of Anti-browning Agent Activated Carbon and Polyvinyl Pyrrolidone on the Rooting of Embryo Seedlings of “FengDan” and Its Transcriptome Analysis. Front. Plant Sci. 2022, 13, 832619. [Google Scholar]
- Aghayeh, R.N.; Abedy, B.; Balandari, A.; Samiei, L.; Tehranifar, A. The first successful report: Control of browning problem in in vitro culture of Iranian Seedless Barberry, a medicinally important species. Erwerbs-Obstbau 2021, 63, 319–329. [Google Scholar] [CrossRef]
- Lainé, E.; David, A. Regeneration of plants from leaf explants of micropropagated clonal Eucalyptus grandis. Plant Cell Rep. 1994, 13, 473–477. [Google Scholar] [CrossRef]
- Ochoa-Alejo, N.; Ramirez-Malagon, R. In vitro chili pepper biotechnology. Vitr. Cell. Dev. Biol. Plant 2001, 37, 701–729. [Google Scholar] [CrossRef]
- Dobránszki, J.; Teixeira, J. Micropropagation of apple—A review. Biotechnol. Adv. 2010, 28, 462–488. [Google Scholar] [CrossRef]
- Ginting, N.; Restiani, R.; Prasetyaningsih, A. Effect of Ascorbic Acid, Activated Charcoal and Dark Incubation on Browning Intensity of Saurauia bracteosa In Vitro Culture. Biosaintifika J. Biol. Biol. Educ. 2023, 15, 401–411. [Google Scholar] [CrossRef]
- Liu, C.; Fan, X.; Qin, B.; Li, A.; Zhang, L. Low temperature and dark culture inhibited explant browning and promoted in vitro regeneration of Juglans mandshurica. Vitr. Cell. Dev. Biol. Plant 2024, 60, 487–495. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H. Effects of browning inhibition on phenolic substance content and related enzyme activity for in vitro propagation of Paeonia ostii var. lishizhenii. Acta Agr. Boreali-Occident. Sin. 2021, 30, 152–159. [Google Scholar]
- Dalal, M.A.; Sharma, B.B.; Rao, M.S. Studies on stock plant treatment and initiation culture mode in control of oxidative browning in in vitro cultures of grapevine. Sci. Hortic. 1992, 51, 35–41. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; She, G.; Zhang, X.; Jordan, B.; Chen, Q. Metabolite profiling and transcriptomic analyses reveal an essential role of UVR8-mediated signal transduction pathway in regulating flavonoid biosynthesis in tea plants (Camellia sinensis) in response to shading. BMC Plant Biol. 2018, 18, 233. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, J.; Chen, Z.; Qiao, X.; Huang, H.; Cui, B. Comparative proteomics reveals the physiological differences between winter tender shoots and spring tender shoots of a novel tea (Camellia sinensis L.) cultivar evergrowing in winter. BMC Plant Biol. 2017, 17, 206. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Maatta-Riihinen, K.; Karenlampi, S.; Hohtola, A. Activation of flavonoid biosynthesis by solar radiation in bilberry (Vaccinium myrtillus L.) leaves. Planta 2004, 218, 721–728. [Google Scholar] [PubMed]
- Perez-Lopez, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.; Muñoz-Rueda, A. Show more Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef]
- Re, G.A.; Piluzza, G.; Sanna, F.; Molinu, M.G.; Sulas, L. Polyphenolic composition and antioxidant capacity of legume-based swards are affected by light intensity in a Mediterranean agroforestry system. J. Sci. Food Agric. 2019, 99, 191–198. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, X.; Mei, X.; Zhou, Y.; Cheng, S.; Zeng, L.; Dong, F.; Yang, Z. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteom. 2017, 157, 10–17. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Li, J.; Zhou, X.; Xiao, Y.; Liao, Y.; Tang, J.; Dong, F.; Zeng, L. Effects of temperature and light on quality-related metabolites in tea [Camellia sinensis (L.) Kuntze] leaves. Food Res. Int. 2022, 161, 111882. [Google Scholar] [CrossRef]
- Xiang, P.; Zhu, Q.; Tukhvatshin, M.; Cheng, B.; Tan, M.; Liu, J.; Wang, X.; Huang, J.; Gao, S.; Lin, D.; et al. Light control of catechin accumulation is mediated by photosynthetic capacity in tea plant (Camellia sinensis). BMC Plant Biol. 2021, 21, 478. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, K.; Tang, Q.; Zeng, L.; Wu, Z. Light Intensity Regulates Low-Temperature Adaptability of Tea Plant through ROS Stress and Developmental Programs. Int. J. Mol. Sci. 2023, 24, 9852. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, X.; Yang, J.; Yuan, Q.; Yang, S.; Fu, W.; Zhang, X.; Li, Y.; Shen, Z.; Jiang, J. Effects of Photoperiod and Light Quality on Germination and Growth of Camellia sinensis ‘HuangKui’. Plants 2024, 13, 1782. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, Y.; Yang, M. Effects of composite LED light on root growth and antioxidant capacity of Cunninghamia lanceolata tissue culture seedlings. Sci. Rep. 2019, 9, 9766. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, H.; Yan, L. Embryonic callus induction of Tilia miqueliana. J. Jiangsu For. Sci. Technol. 2020, 47, 18–21. [Google Scholar]
- GB/T 8314-2013; Tea—Determination of Free Amino Acids Content. State Standardization Administration: Beijing, China, 2013.
- Ye, Y.; Yan, J.; Cui, J.; Mao, S.; Li, M.; Liao, X.; Tong, H. Dynamic changes in amino acids, catechins, caffeine and gallic acid in green tea during withering. J. Food Compos. Anal. 2018, 66, 98–108. [Google Scholar] [CrossRef]
- GB/T 8313-2018; Tea—Determination of Tea Polyphenols and Catechins Content. State Standardization Administration: Beijing, China, 2018.
- Yu, C.; Hao, D.; Chu, Q.; Wang, T.; Liu, S.; Lan, T.; Wang, F.; Pan, P. A one adsorbent QuEChERS method coupled with LC-MS/MS for simultaneous determination of 10 organophosphorus pesticide residues in tea. Food Chem. 2020, 321, 126657. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 16, e12. [Google Scholar] [CrossRef]

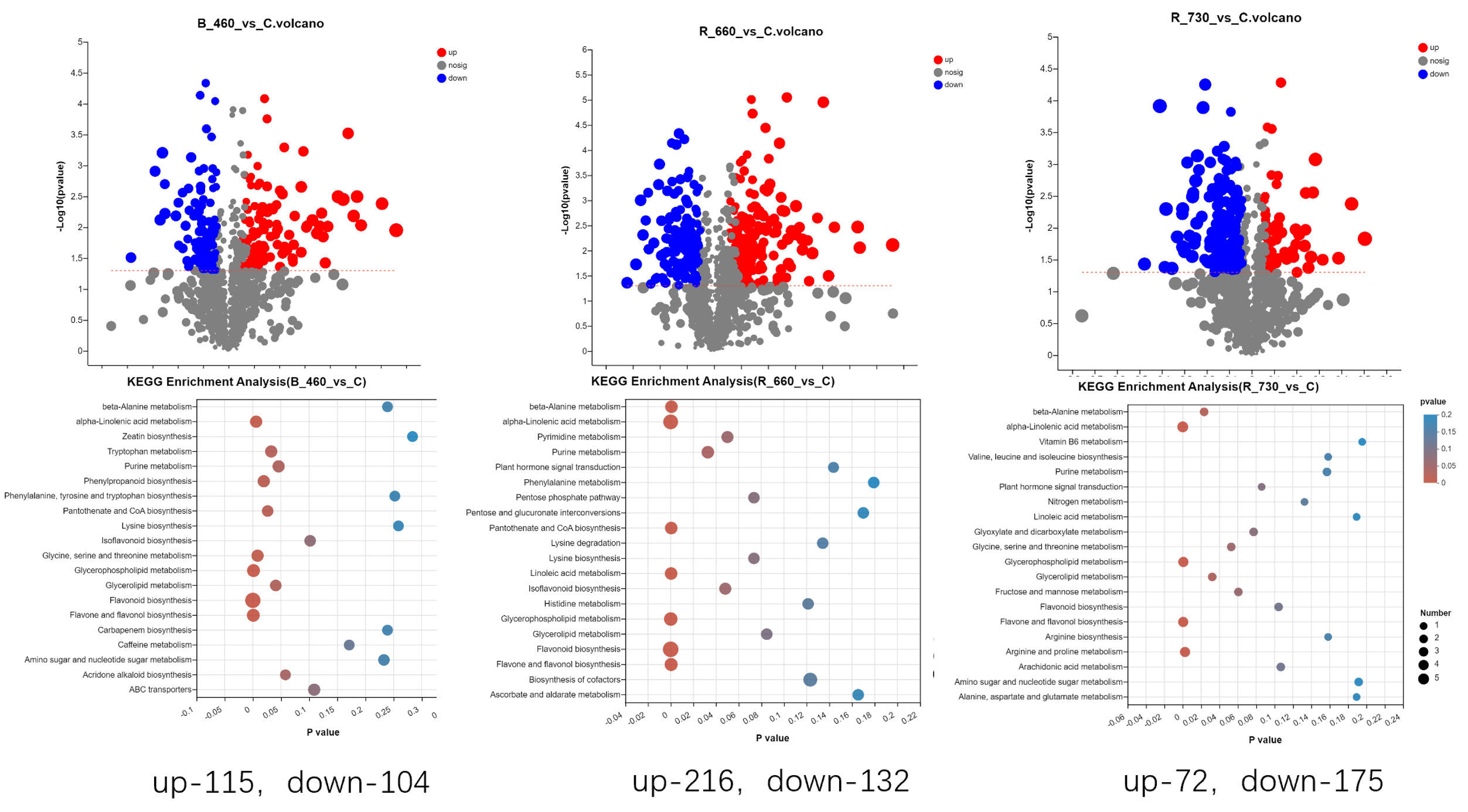
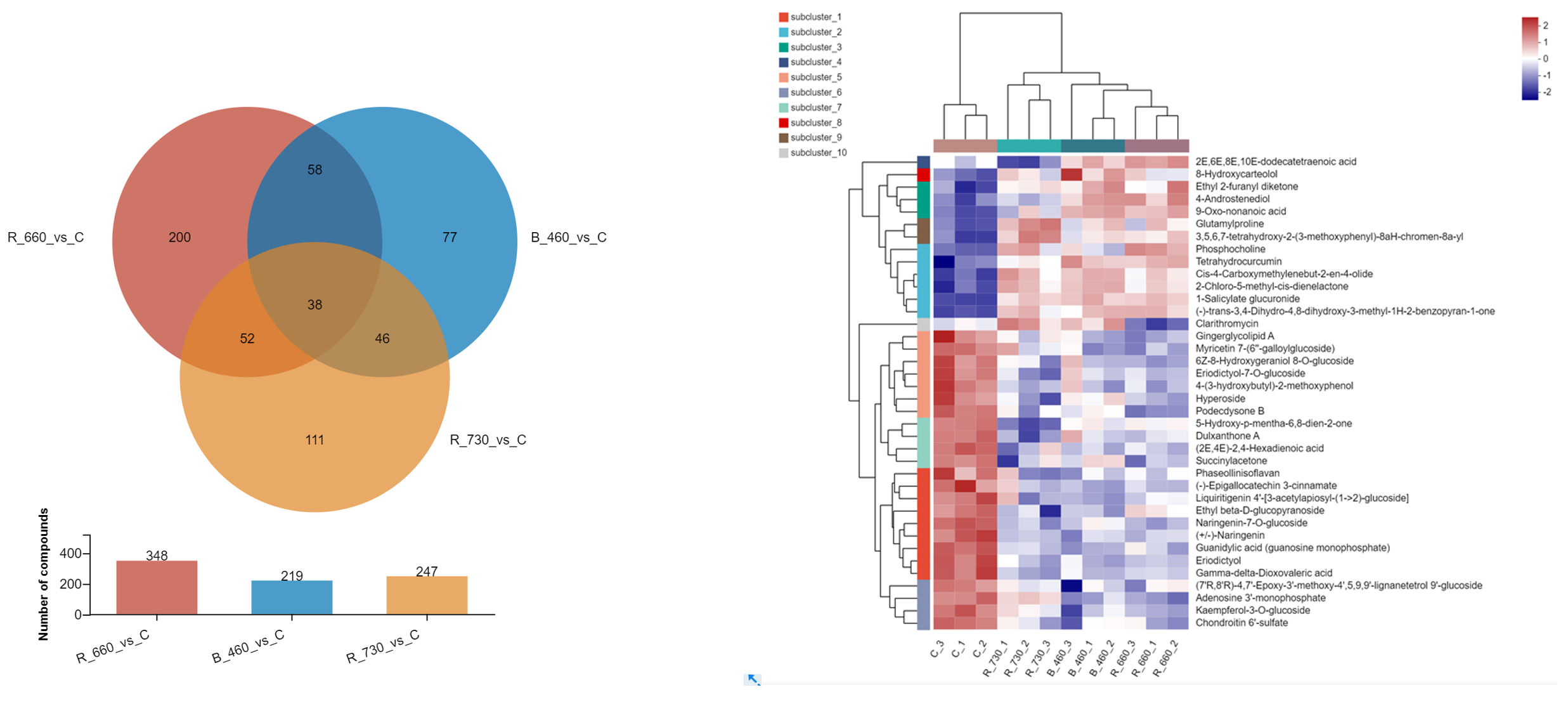
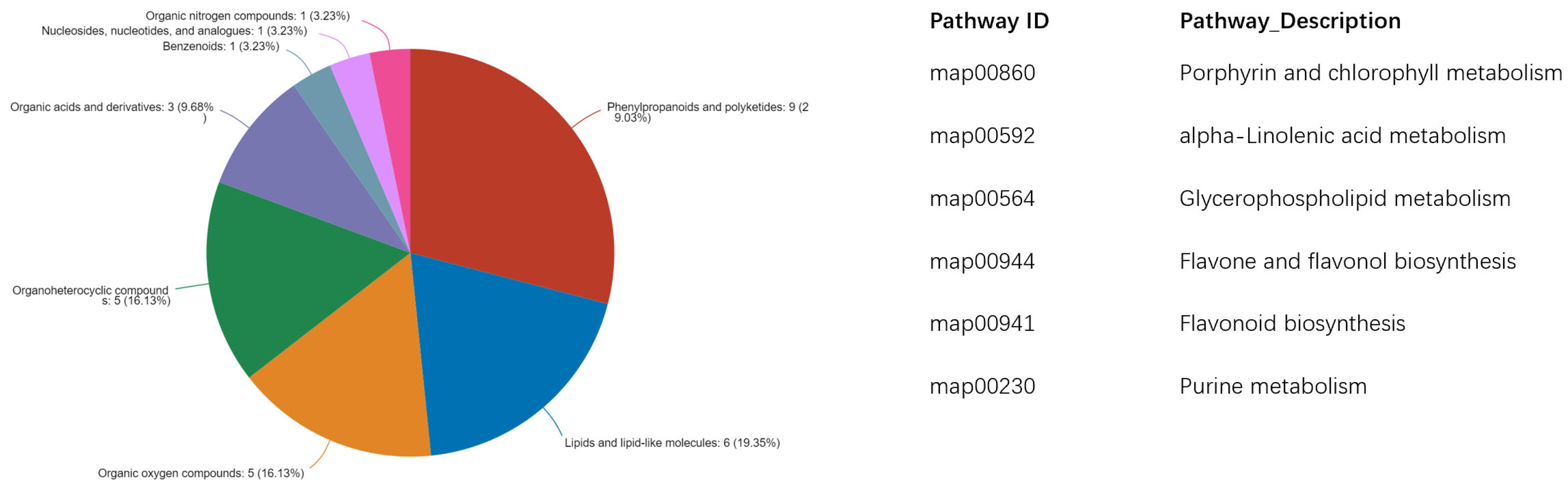

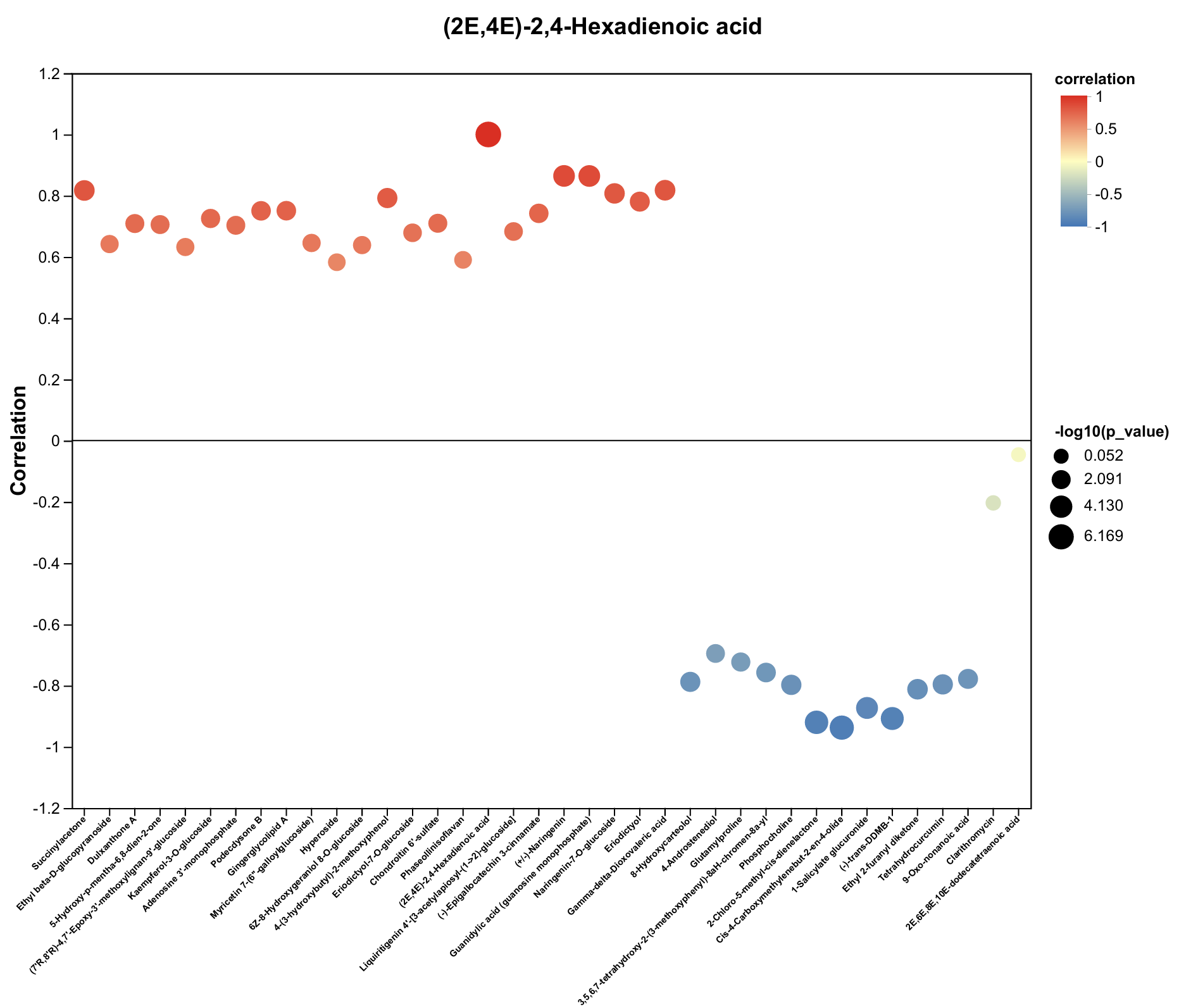
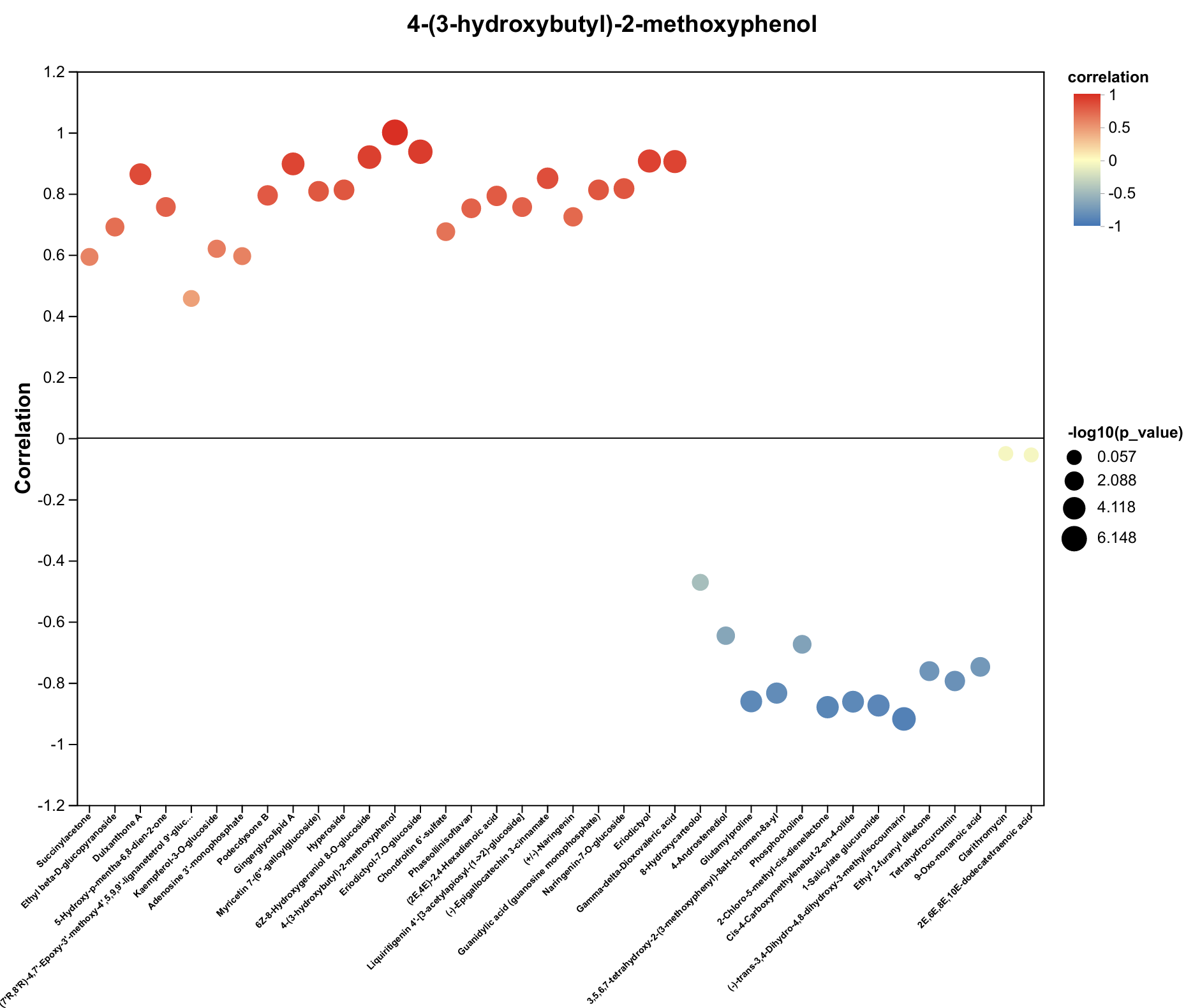
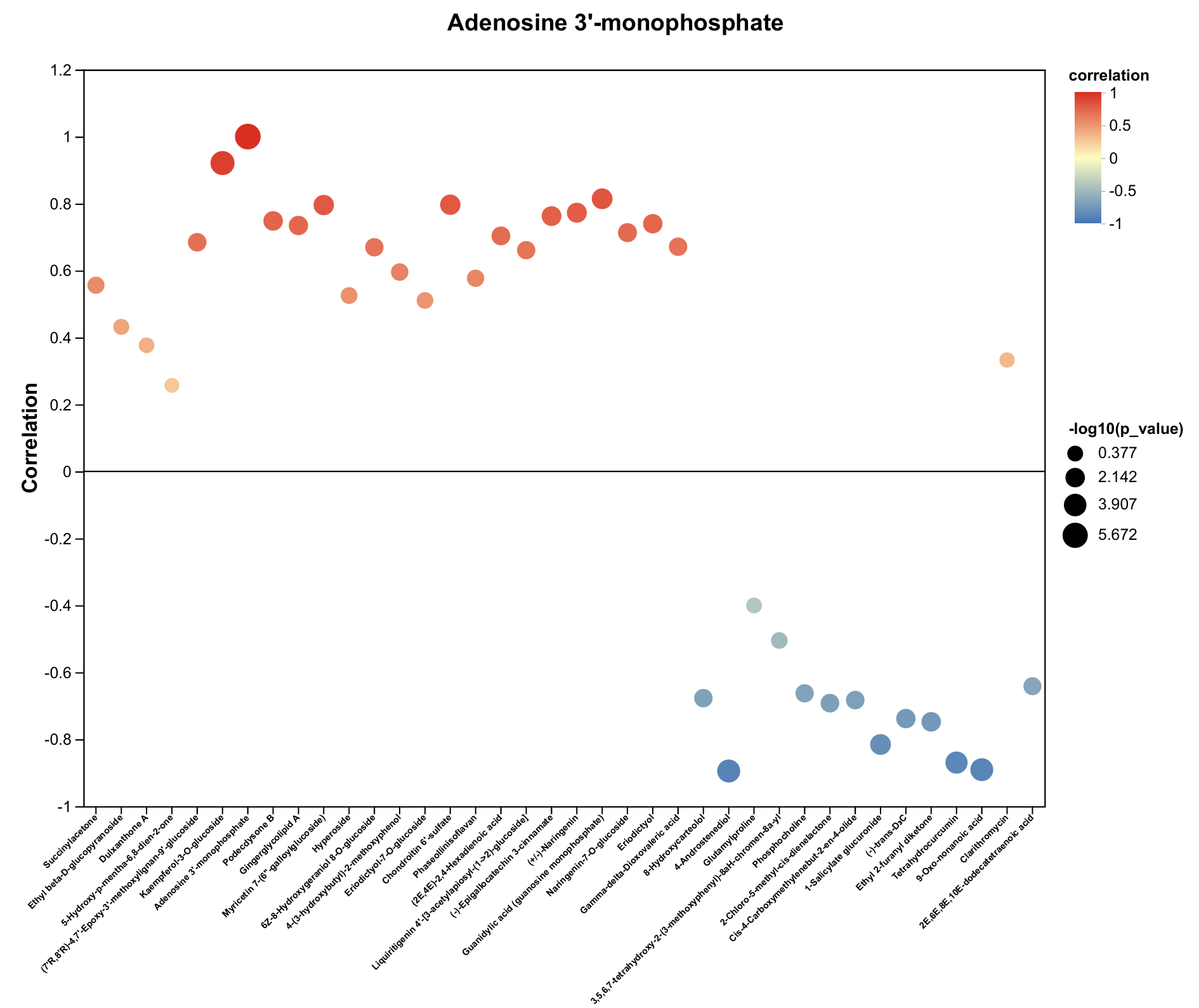
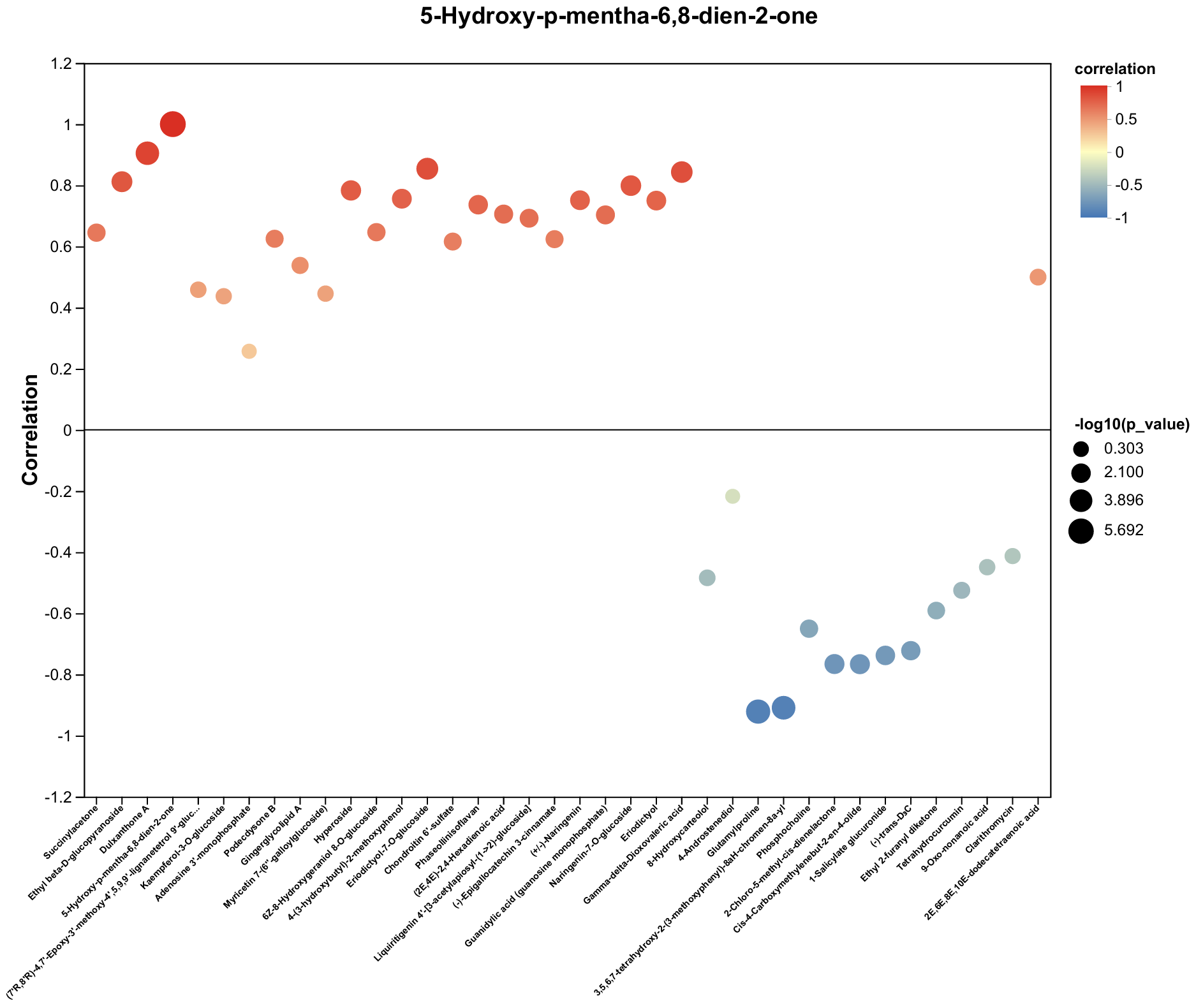
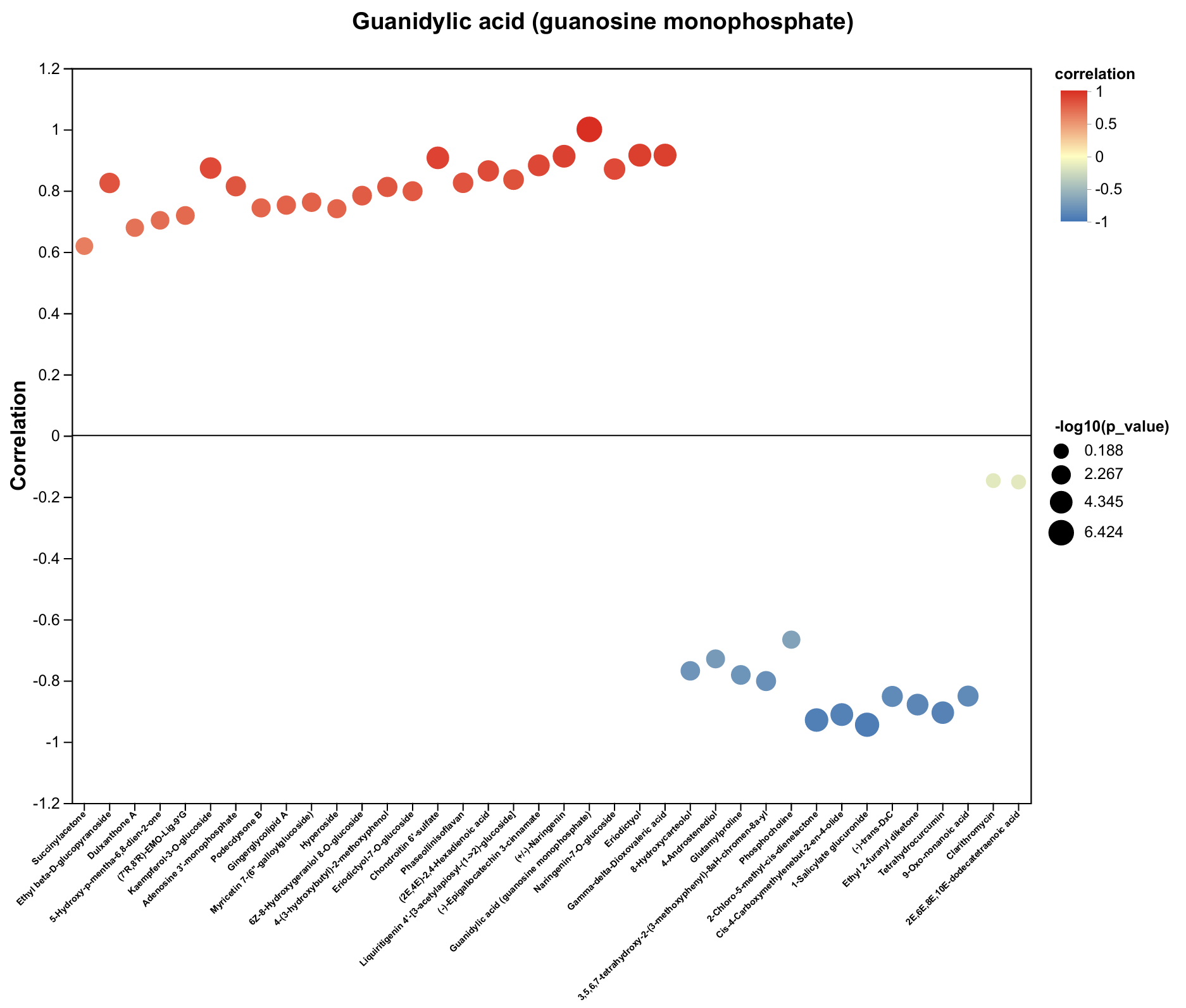
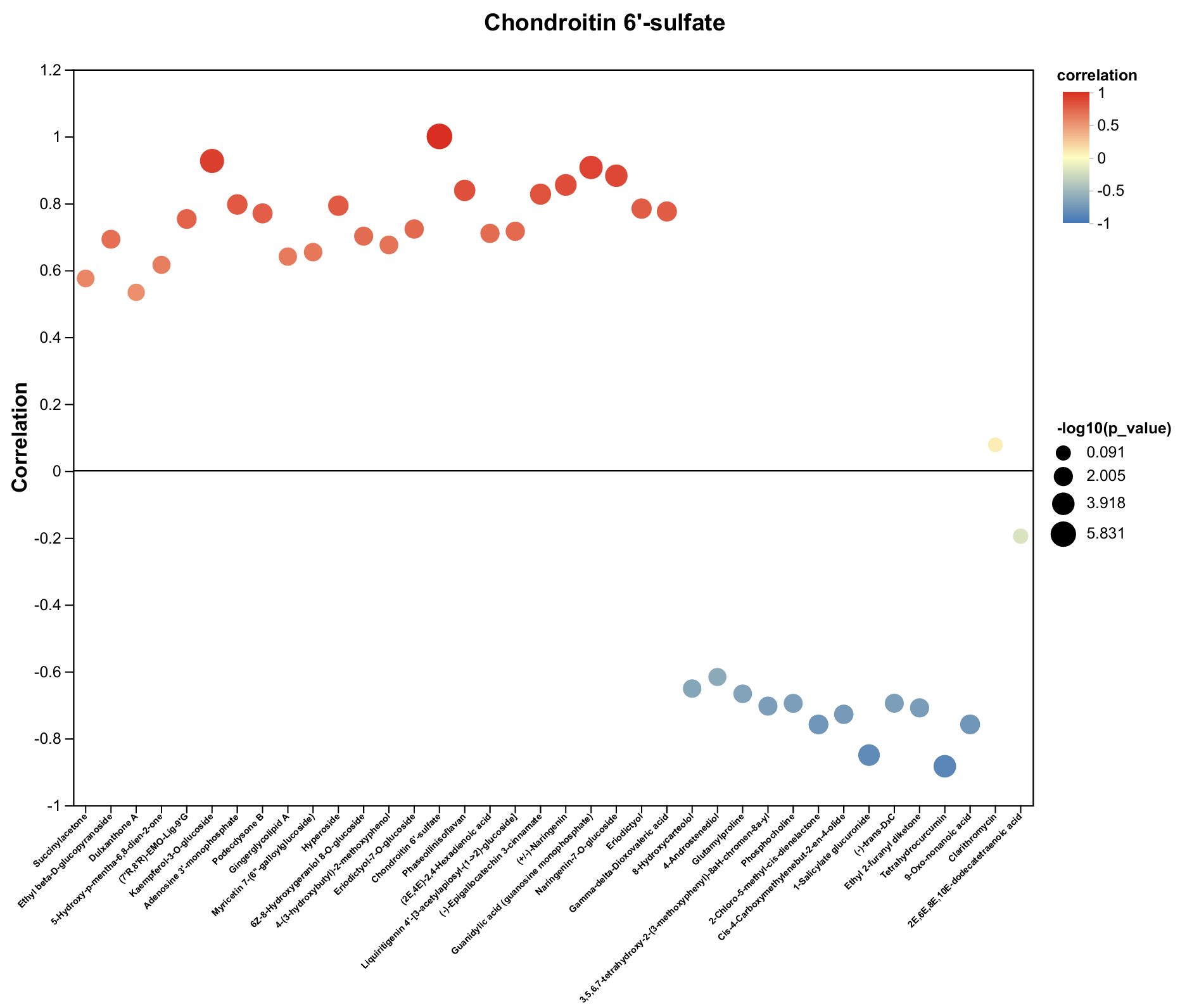
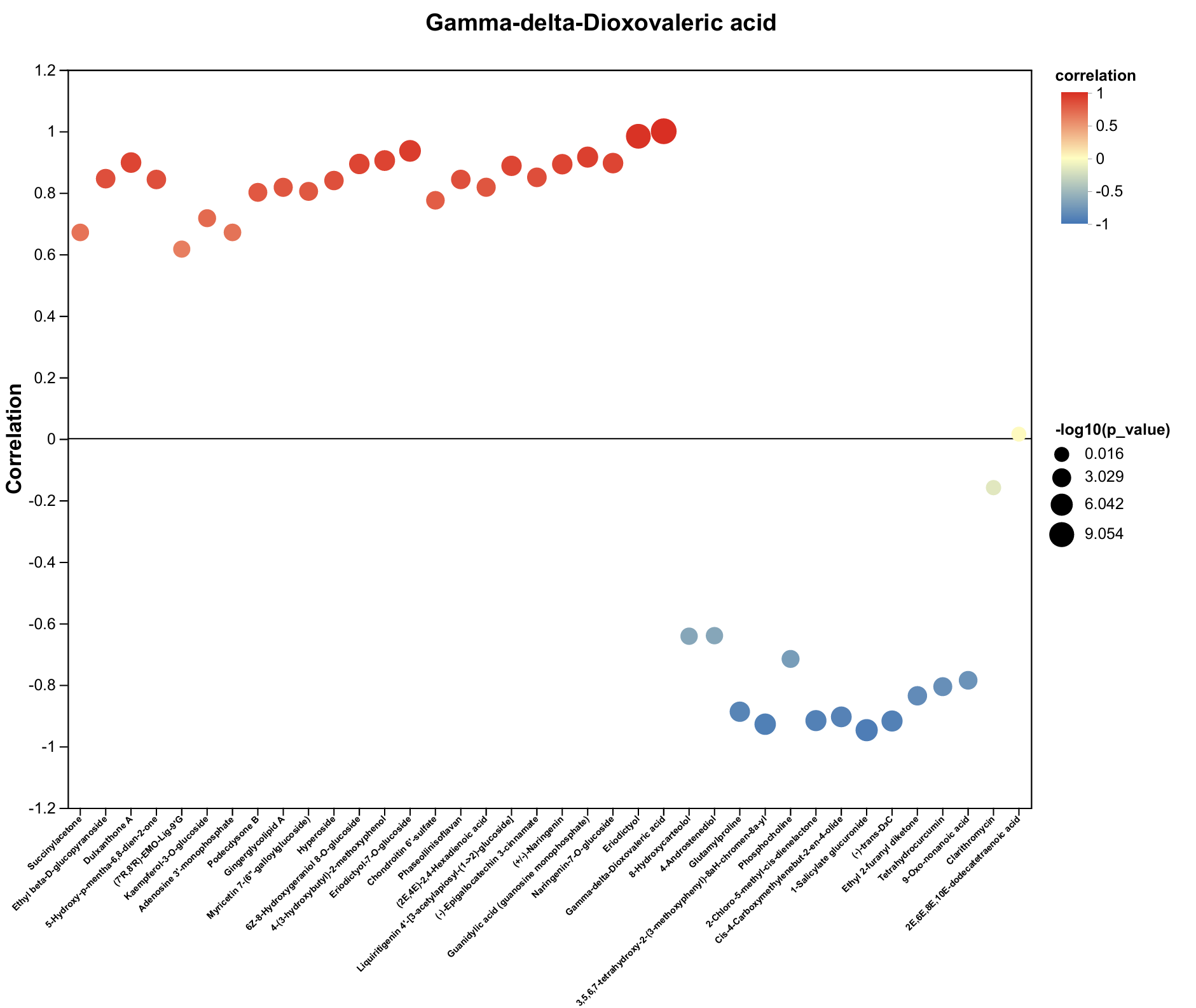
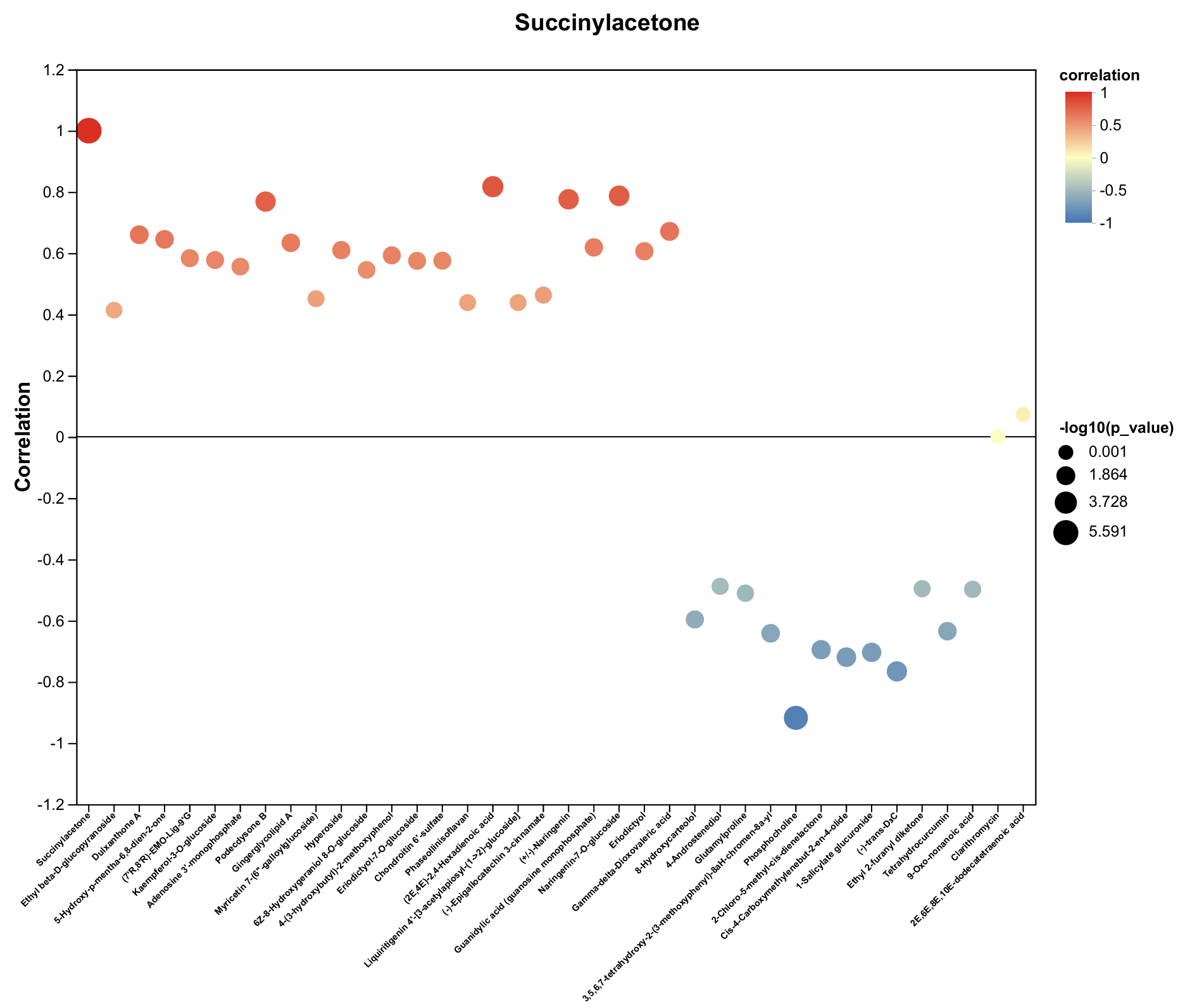


| Metabolite | Decrease Ratio Compared to the Control Group (%) | ||
|---|---|---|---|
| B_460 | R_660 | R_730 | |
| Gingerglycolipid A | −54% | −61% | −43% |
| 6Z-8-Hydroxygeraniol 8-O-glucoside | −44% | −59% | −47% |
| Kaempferol-3-O-glucoside | −63% | −64% | −42% |
| (2E,4E)-2,4-Hexadienoic acid | −45% | −43% | −38% |
| 4-(3-hydroxybutyl)-2-methoxyphenol | −36% | −41% | −37% |
| Dulxanthone A | −62% | −76% | −85% |
| Podecdysone B | −45% | −65% | −46% |
| Adenosine 3′-monophosphate | −62% | −67% | −28% |
| Hyperoside | −56% | −77% | −76% |
| 5-Hydroxy-p-mentha-6,8-dien-2-one | −48% | −47% | −73% |
| Liquiritigenin 4′-[3-acetylapiosyl-(1->2)-glucoside] | −72% | −62% | −58% |
| Myricetin 7-(6″-galloylglucoside) | −83% | −87% | −63% |
| (−)-Epigallocatechin 3-cinnamate | −85% | −84% | −69% |
| Guanidylic acid (guanosine monophosphate) | −66% | −63% | −47% |
| Chondroitin 6′-sulfate | −66% | −70% | −50% |
| (7′R,8′R)-4,7′-Epoxy-3′-methoxy-4′,5,9,9′-lignanetetrol 9′-glucoside | −79% | −72% | −56% |
| Eriodictyol | −78% | −80% | −70% |
| Phaseollinisoflavan | −74% | −70% | −60% |
| (+/−)-Naringenin | −75% | −74% | −64% |
| Gamma-delta-Dioxovaleric acid | −83% | −84% | −82% |
| Eriodictyol-7-O-glucoside | −78% | −88% | −86% |
| Naringenin-7-O-glucoside | −69% | −79% | −68% |
| Succinylacetone | −60% | −78% | −63% |
| Ethyl beta-D-glucopyranoside | −92% | −79% | −90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Huang, H.; Zhao, Y. Metabolomics Reveals the Mechanism of Browning Inhibition by Transient Light Quality in Tea Plant Tissue Culture. Plants 2025, 14, 3539. https://doi.org/10.3390/plants14223539
Ding Y, Huang H, Zhao Y. Metabolomics Reveals the Mechanism of Browning Inhibition by Transient Light Quality in Tea Plant Tissue Culture. Plants. 2025; 14(22):3539. https://doi.org/10.3390/plants14223539
Chicago/Turabian StyleDing, Yi, Haitao Huang, and Yun Zhao. 2025. "Metabolomics Reveals the Mechanism of Browning Inhibition by Transient Light Quality in Tea Plant Tissue Culture" Plants 14, no. 22: 3539. https://doi.org/10.3390/plants14223539
APA StyleDing, Y., Huang, H., & Zhao, Y. (2025). Metabolomics Reveals the Mechanism of Browning Inhibition by Transient Light Quality in Tea Plant Tissue Culture. Plants, 14(22), 3539. https://doi.org/10.3390/plants14223539





