The Ionic and Metabolic Response Mechanisms of Kochia scoparia in Response to Saline–Alkaline Stress
Abstract
1. Introduction
2. Materials and Methods
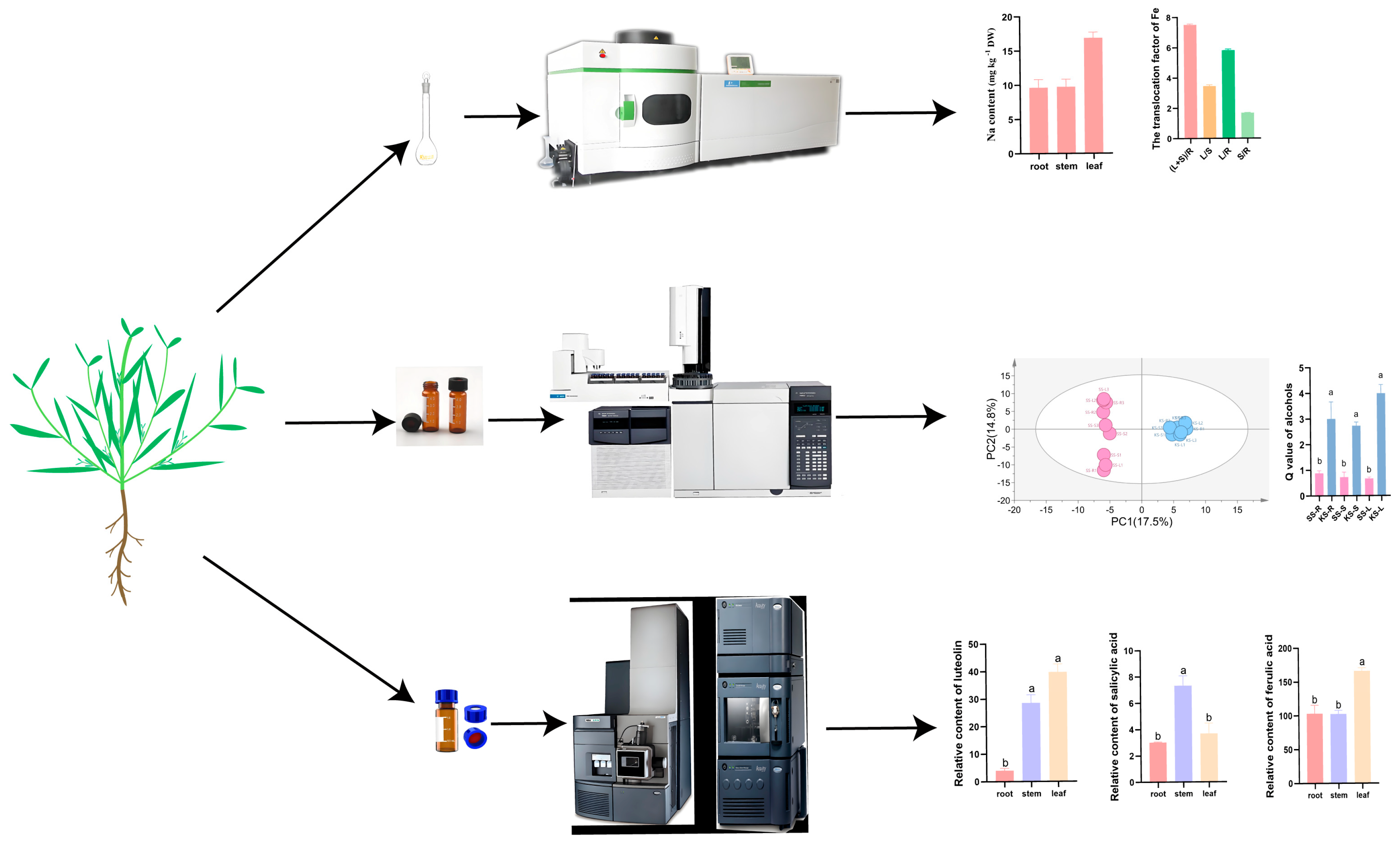
2.1. Plant Materials and Sample Collection
2.2. Element Detection in K. scoparia
2.3. Untargeted Metabolomics Detection in K. scoparia
2.4. Phenolic Compound Detection in K. scoparia
2.5. Statistical Analysis
3. Results
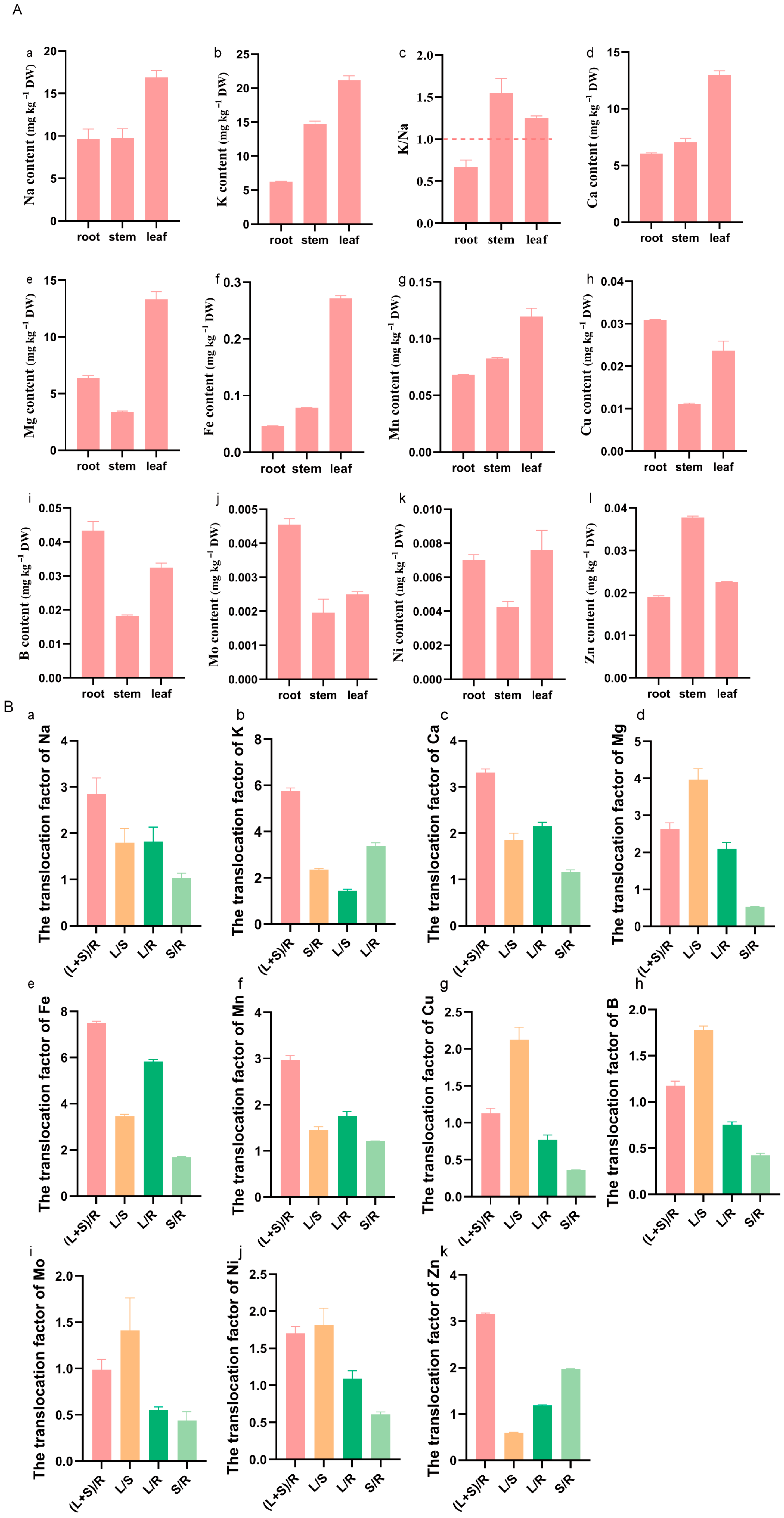
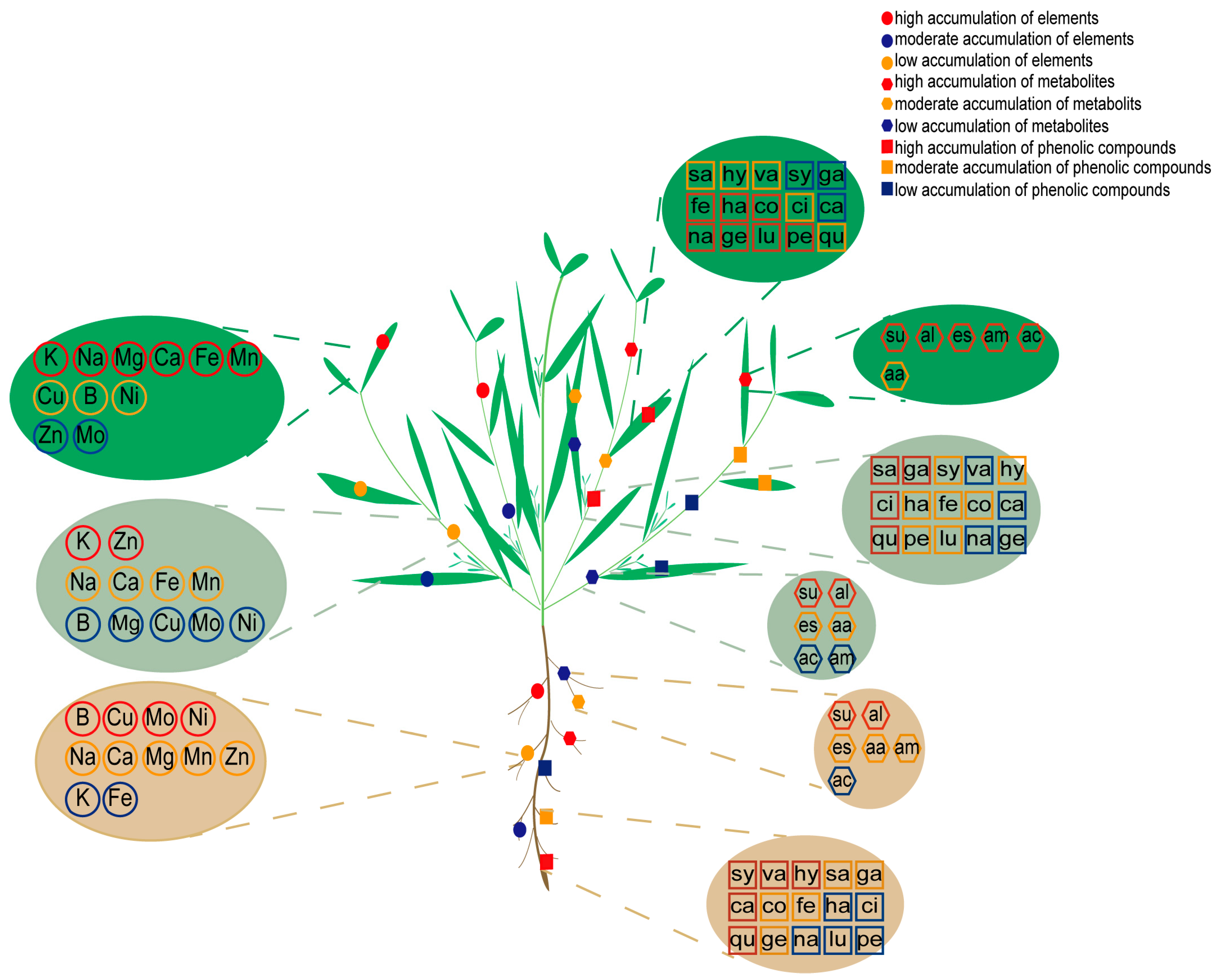
3.1. Element Accumulation and Translocation in K. scoparia
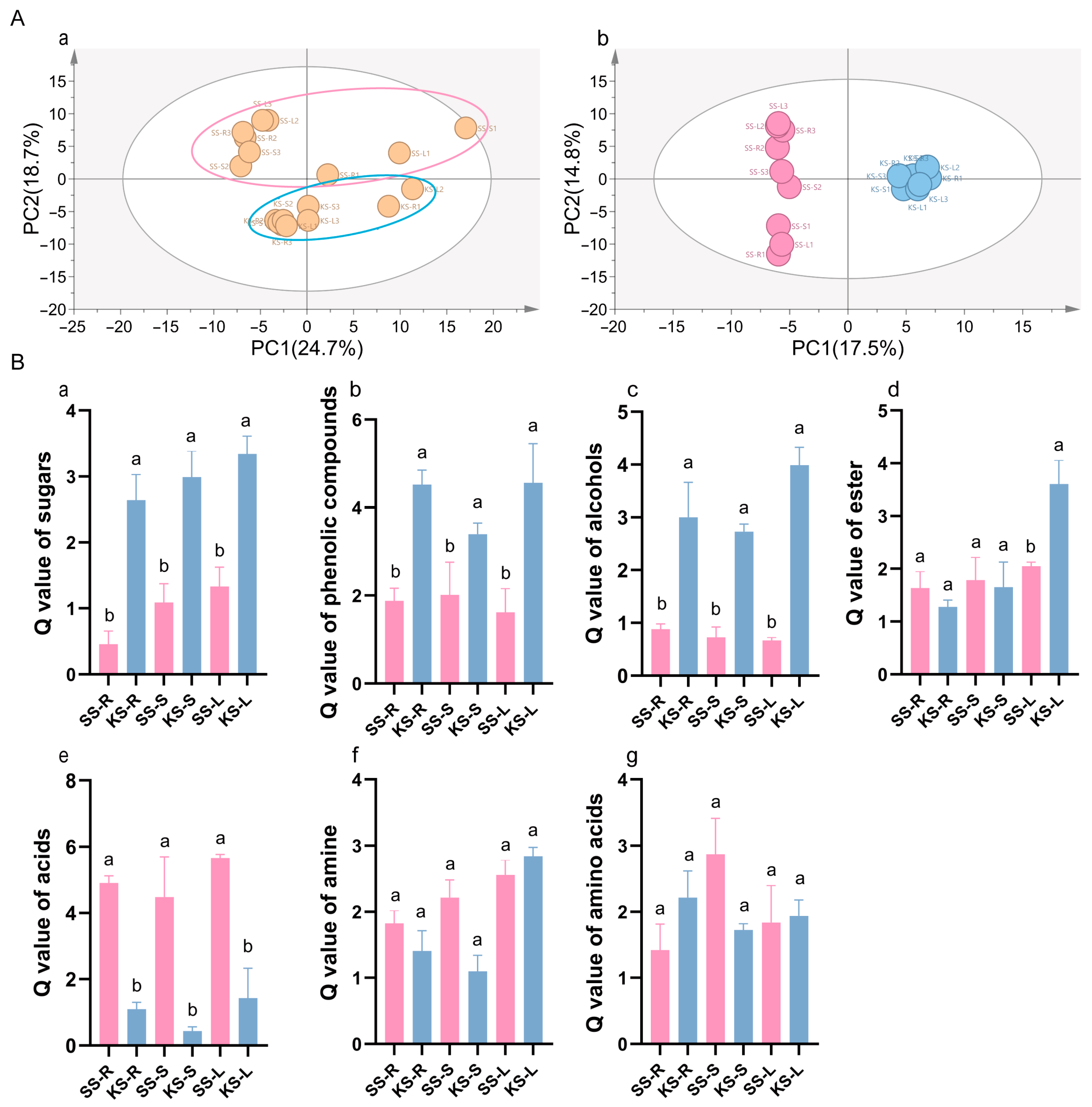
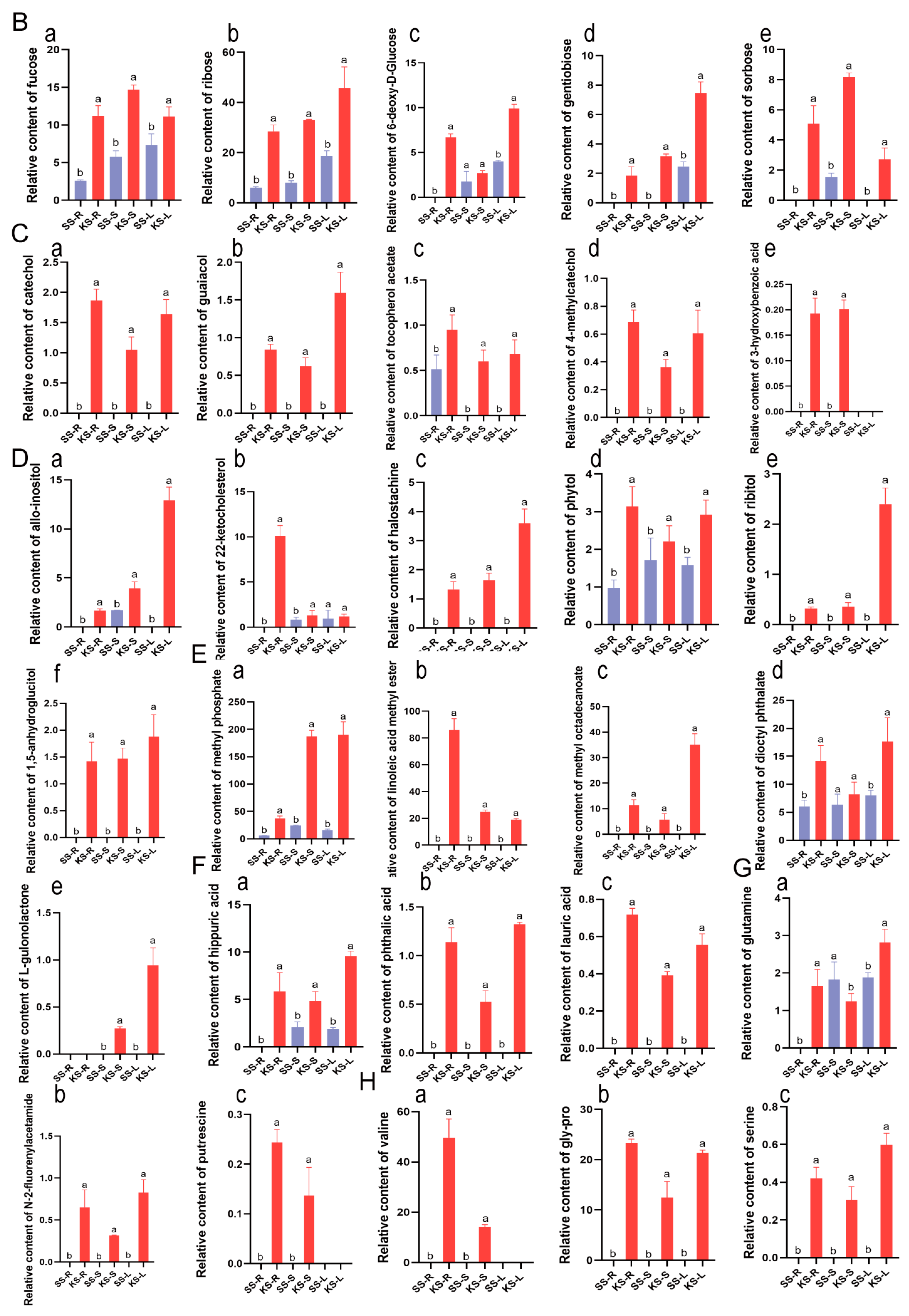
3.2. Metabolic Response of K. scoparia to Saline–Alkaline Stress
3.3. Phenolic Compounds Response of K. scoparia to Saline–Alkaline Stress
4. Discussion
4.1. The Response of Elements in K. scoparia to Saline–Alkaline Stress
4.2. The Response of Metabolites in K. scoparia to Saline–Alkaline Stress
4.3. The Response of Phenolic Compounds in K. scoparia to Saline–Alkaline Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pankaj; Devi, S.; Dhaka, P.; Kumari, G.; Satpal; Lakra, N.; Arya, S.S.; Ahlawat, Y.K.J.G.R. Enhancing salt stress tolerance of forage sorghum by foliar application of ortho-silicic acid. Grass Res. 2024, 4, e016. [Google Scholar] [CrossRef]
- Dissanayake, B.M.; Staudinger, C.; Ranathunge, K.; Munns, R.; Rupasinghe, T.W.; Taylor, N.L.; Millar, A.H.J.T.P.J. Metabolic adaptations leading to an enhanced lignification in wheat roots under salinity stress. Plant J. 2024, 119, 1800–1815. [Google Scholar] [CrossRef]
- Fang, H.; Fu, X.; Ge, H.; Jia, M.; Ji, J.; Zhao, Y.; Qu, Z.; Cui, Z.; Zhang, A.; Wang, Y.; et al. Genetic analysis and candidate gene identification of salt tolerance-related traits in maize. J. Integr. Agric. 2024, 23, 2196–2210. [Google Scholar] [CrossRef]
- Liang, X.; Li, J.; Yang, Y.; Jiang, C.; Guo, Y. Designing salt stress-resilient crops: Current progress and future challenges. J. Integr. Plant Biol. 2024, 66, 303–329. [Google Scholar] [CrossRef]
- Feng, H.; Du, Q.; Jiang, Y.; Jia, Y.; He, T.; Wang, Y.; Chapman, B.; Yu, J.; Zhang, H.; Gu, M.; et al. Hordeum I genome unlocks adaptive evolution and genetic potential for crop improvement. Nat. Plants 2025, 11, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, C.; Xuan, W.; An, H.; Tian, Y.; Wang, B.; Chi, W.; Chen, G.; Ge, Y.; Li, J.; et al. Genome-wide association studies identify OsWRKY53 as a key regulator of salt tolerance in rice. Nat. Commun. 2023, 14, 3550. [Google Scholar] [CrossRef]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.J.L.; et al. Salinity stress in potato: Understanding physiological, biochemical and molecular responses. Life 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Navada, S.; Vadstein, O.; Gaumet, F.; Tveten, A.-K.; Spanu, C.; Mikkelsen, Ø.; Kolarevic, J.J.W.R. Biofilms remember: Osmotic stress priming as a microbial management strategy for improving salinity acclimation in nitrifying biofilms. Water Res. 2020, 176, 115732. [Google Scholar] [CrossRef]
- Sahito, Z.A.; Benavides-Mendoza, A.; Cota-Ruiz, K. Plant responses to salt stress. Front. Media SA 2024, 15, 1475599. [Google Scholar]
- Zhao, C.; Jiang, W.; Zayed, O.; Liu, X.; Tang, K.; Nie, W.; Li, Y.; Xie, S.; Li, Y.; Long, T.J.N.S.R.; et al. The LRXs-RALFs-FER module controls plant growth and salt stress responses by modulating multiple plant hormones. Natl. Sci. Rev. 2021, 8, nwaa149. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innov. 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Gao, Y.; Ma, Q.; Wang, X.; Zhu, J.-K.; Li, W.; Wang, B.; Yuan, F. Global dynamics and cytokinin participation of salt gland development trajectory in recretohalophyte Limonium bicolor. Plant Physiol. 2024, 195, 2094–2110. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Liu, Y.-Q.; Duan, H.-R.; Yin, X.-X.; Cui, Y.-N.; Chai, W.-W.; Song, X.; Flowers, T.J.; Wang, S.-M. SsHKT1; 1 is coordinated with SsSOS1 and SsNHX1 to regulate Na+ homeostasis in Suaeda salsa under saline conditions. Plant Soil 2020, 449, 117–131. [Google Scholar] [CrossRef]
- Matsushita, N.; Matoh, T. Characterization of Na+ exclusion mechanisms of salt-tolerant reed plants in comparison with salt-sensitive rice plants. Physiol. Plant. 1991, 83, 170–176. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E.; Gaber, A.; Fetouh, M.; Mazrou, R. Chitosan nanoparticles effectively combat salinity stress by enhancing antioxidant activity and alkaloid biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef]
- Hounslow, E.; Evans, C.; Pandhal, J.; Sydney, T.; Couto, N.; Pham, T.; Gilmour, D.J.; Wright, P. Quantitative proteomic comparison of salt stress in Chlamydomonas reinhardtii and the snow alga Chlamydomonas nivalis reveals mechanisms for salt-triggered fatty acid accumulation via reallocation of carbon resources. Biotechnol. Biofuels 2021, 14, 121. [Google Scholar] [CrossRef]
- Jia, C.; Guo, B.; Wang, B.; Li, X.; Yang, T.; Li, N.; Wang, J.; Yu, Q. Integrated metabolomic and transcriptomic analysis reveals the role of phenylpropanoid biosynthesis pathway in tomato roots during salt stress. Front. Plant Sci. 2022, 13, 1023696. [Google Scholar] [CrossRef]
- Junze, R.; Yu, W.; Zhanpin, Z.; Ruibing, C. Biosynthesis and regulation of diterpenoids in medicinal plants. Chin. J. Nat. Med. 2022, 20, 761–772. [Google Scholar] [CrossRef]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef] [PubMed]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, Y.; Liu, G.; Yao, F.; Zhang, Y.; Yang, C.; Guo, H.; Liu, X.; Jin, C.; Luo, J. Natural variation in the OsbZIP18 promoter contributes to branched-chain amino acid levels in rice. New Phytol. 2020, 228, 1548–1558. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, R.; Zhang, L. Simple phenylpropanoids: Recent advances in biological activities, biosynthetic pathways, and microbial production. Nat. Prod. Rep. 2024, 41, 6–24. [Google Scholar] [CrossRef]
- Hua, Y.Q.; Zeng, Y.; Xu, J.; Le Xu, X. Naringenin alleviates nonalcoholic steatohepatitis in middle-aged Apoe−/− mice: Role of SIRT1. Phytomedicine 2021, 81, 153412. [Google Scholar] [CrossRef]
- Li, J.; Xiang, H.; Huang, C.; Lu, J. Pharmacological actions of myricetin in the nervous system: A comprehensive review of preclinical studies in animals and cell models. Front. Pharmacol. 2021, 12, 797298. [Google Scholar] [CrossRef]
- Fan, B.Y.; Jiang, X.; Li, Y.X.; Wang, W.L.; Yang, M.; Li, J.L.; Wang, A.D.; Chen, G.T. Chemistry and biological activity of resin glycosides from Convolvulaceae species. Med. Res. Rev. 2022, 42, 2025–2066. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Lu, X.; Wu, C.; Chen, J.; Chen, C.; Zhang, J.; Huang, C.; Cui, Z. A review for the pharmacological effects of paeoniflorin in the nervous system. Front. Pharmacol. 2022, 13, 898955. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Cai, L.; Wang, S.; Wang, J.; Chen, B. Baicalin prevents myocardial ischemia/reperfusion injury through inhibiting ACSL4 mediated ferroptosis. Front. Pharmacol. 2021, 12, 628988. [Google Scholar] [CrossRef]
- Chen, Y.; Dan, Z.; Li, S. GROWTH REGULATING FACTOR 7-mediated arbutin metabolism enhances rice salt tolerance. Plant Cell 2024, 36, 2834–2850. [Google Scholar] [CrossRef]
- Lu, M.; Riaz, M.; Tong, K.; Hao, W.; Yang, Y.; Zhao, X.; Wang, L.; Niu, Y.; Yan, L. Boron-induced phenylpropanoid metabolism, Na+/K+ homeostasis and antioxidant defense mechanisms in salt-stressed soybean seedlings. J. Hazard. Mater. 2025, 491, 138036. [Google Scholar] [CrossRef]
- Jia, Q.; Chen, Y.; Kong, D.; Fan, H.; Sun, S.; Liu, Y.; Fu, J.; Li, M.W.; Wong, F.L.; Li, Q.; et al. Soybean Inositol Polyphosphate 5-Phosphatase 8 Confers Salt Tolerance by Reducing Sodium Influx Through Inositol 1, 4, 5-Trisphosphate Signalling. Plant Cell Environ. 2025, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jin, Y.; Zhang, Z.; Cao, M.; Wei, G.; Guo, X.; Zhang, J.; Lu, X.; Tang, Z. Ionomic and metabolomic analyses reveal different response mechanisms to saline–alkali stress between Suaeda salsa community and Puccinellia tenuiflora community. Front. Plant Sci. 2021, 12, 774284. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lu, X.; Guo, X.; Xu, M.; Tang, Z. A source-sink model explains the difference in the metabolic mechanism of mechanical damage to young and senescing leaves in Catharanthus roseus. BMC Plant Biol. 2021, 21, 154. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, H.; Wei, G.; Guo, X.; Zhang, J.; Lu, X.; Tang, Z. Metabolic differences of two constructive species in saline-alkali grassland in China. BMC Plant Biol. 2022, 22, 53. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; You, C.; Wu, X.; Pan, D.; Lv, Z.; Li, T.; Zhang, D.; Shen, Z.; Zhang, X.; et al. Telomere-to-telomere genome of the allotetraploid legume Sesbania cannabina reveals transposon-driven subgenome divergence and mechanisms of alkaline stress tolerance. Sci. China Life Sci. 2024, 67, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhou, Y.; Liu, X.; Meng, F.; Xu, C.; Chen, M. Integrated transcriptomic and metabolomic analyses uncover the key pathways of Limonium bicolor in response to salt stress. Plant Biotechnol. J. 2025, 23, 715–730. [Google Scholar] [CrossRef]
- Guo, R.; Liu, L.; Li, J.; Qu, H.; Guo, W.; Zhang, L.; Yang, D.; Wang, R.; Guo, C. Metabolo-Transcriptomics Analyses Reveal Alfalfa Adaptation to Combined Saline-Alkali and Low-Temperature Stress in the Field. Plant Biotechnol. J. 2025, online ahead of print. [Google Scholar] [CrossRef]
- Li, K.-L.; Xue, H.; Tang, R.-J.; Luan, S. A calcium sensor kinase pathway interacts with the TOR complex to balance growth and salt tolerance in Arabidopsis. Plant Cell 2025, 37, koaf103. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, C.; Xue, Y.; Liu, X.; Chen, S.; Song, C.; Yang, Y.; Guo, Y. Calcium-activated 14–3-3 proteins as a molecular switch in salt stress tolerance. Nat. Commun. 2019, 10, 1199. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, X.; Wang, R.; Hao, F.; Zhang, H.; Zhang, Y.; Lin, G. Exogenous calcium alleviates oxidative stress caused by salt stress in peanut seedling roots by regulating the antioxidant enzyme system and flavonoid biosynthesis. Antioxidants 2024, 13, 233. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Han, X.; Chen, Z.; Li, M.; Jiang, L.; Zeng, J. Magnesium-doped carbon quantum dot nanomaterials alleviate salt stress in rice by scavenging reactive oxygen species to increase photosynthesis. Acs Nano 2024, 18, 31188–31203. [Google Scholar] [CrossRef]
- Cui, J.; Li, J.; Dai, C.; Li, L. Transcriptome and metabolome analyses revealed the response mechanism of sugar beet to salt stress of different durations. Int. J. Mol. Sci. 2022, 23, 9599. [Google Scholar] [CrossRef]
- Liu, J.; Gu, J.; Hu, J.; Ma, H.; Tao, Y.; Li, G.; Yue, L.; Li, Y.; Chen, L.; Cao, F. Use of Mn3O4 nanozyme to improve cotton salt tolerance. Plant Biotechnol. J. 2023, 21, 1935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Liang, X.; Zheng, J.; Lu, X.; Zhao, J.; Li, H.; Zhan, Y.; Teng, W.; Li, H.; et al. GmFER1, a soybean ferritin, enhances tolerance to salt stress and root rot disease and improves soybean yield. Plant Biotechnol. J. 2025, 23, 3094–3112. [Google Scholar] [CrossRef]
- Gao, D.; Ran, C.; Zhang, Y.; Wang, X.; Lu, S.; Geng, Y.; Guo, L.; Shao, X. Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions. Plant Physiol. Biochem. 2022, 185, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.N.; Rasheed, R.; Ashraf, M.Y.; Ashraf, M.A.; Hussain, I. Exogenously applied zinc and copper mitigate salinity effect in maize (Zea mays L.) by improving key physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2018, 25, 23883–23896. [Google Scholar] [CrossRef]
- Shah, I.H.; Sabir, I.A.; Rehman, A.; Hameed, M.K.; Albashar, G.; Manzoor, M.A.; Shakoor, A. Co-application of copper oxide nanoparticles and Trichoderma harzianum with physiological, enzymatic and ultrastructural responses for the mitigation of salt stress. Chemosphere 2023, 336, 139230. [Google Scholar] [CrossRef]
- Duan, X.; Yu, Y.; Zhang, Y.; Chen, C.; Duanmu, H.; Cao, L.; Sun, M.; Sun, X.; Zhu, Y. A potential efflux boron transporter gene GsBOR2, positively regulates Arabidopsis bicarbonate tolerance. Plant Sci. 2018, 274, 284–292. [Google Scholar] [CrossRef]
- Hua, Y.; Pei, M.; Song, H.; Liu, Y.; Zhou, T.; Chao, H.; Yue, C.; Huang, J.; Qin, G.; Feng, Y. Boron confers salt tolerance through facilitating BnaA2. HKT1-mediated root xylem Na+ unloading in rapeseed (Brassica napus L.). Plant J. 2024, 120, 1326–1342. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Yan, L.; Riaz, M.; Babar, S.; Hou, J.; Zhang, Y.; Jiang, C. Exogenous boron alleviates salt stress in cotton by maintaining cell wall structure and ion homeostasis. Plant Physiol. Biochem. 2023, 201, 107858. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Yang, X.; Tan, Q.; Yao, S.; Zhou, Y.; Wang, X.; Sun, X. Alterations of glycerolipidome induced by molybdenum conferred drought tolerance of wheat. J. Exp. Bot. 2020, 71, 5074–5086. [Google Scholar]
- Lilay, G.H.; Thiébaut, N.; du Mee, D.; Assunção, A.G.; Schjoerring, J.K.; Husted, S.; Persson, D.P. Linking the key physiological functions of essential micronutrients to their deficiency symptoms in plants. New Phytol. 2024, 242, 881–902. [Google Scholar] [CrossRef]
- Subhani, M.A.; Amjad, M.; Iqbal, M.M.; Murtaza, B.; Imran, M.; Naeem, M.A.; Abbas, G.; Andersen, M.N. Nickel toxicity pretreatment attenuates salt stress by activating antioxidative system and ion homeostasis in tomato (Solanum lycopersicon L.): An interplay from mild to severe stress. Environ. Geochem. Health 2023, 45, 227–246. [Google Scholar] [CrossRef]
- Dogan, Y.; Alam, P.; Sultan, H.; Sharma, R.; Soysal, S.; Baran, M.F.; Faizan, M. Zinc oxide nanoparticles for sustainable agriculture: A tool to combat salinity stress in rice (Oryza sativa) by modulating the nutritional profile and redox homeostasis mechanisms. J. Agric. Food Res. 2025, 19, 101598. [Google Scholar] [CrossRef]
- Dang, K.; Mu, J.; Tian, H.; Gao, D.; Zhou, H.; Guo, L.; Shao, X.; Geng, Y.; Zhang, Q. Zinc regulation of chlorophyll fluorescence and carbohydrate metabolism in saline-sodic stressed rice seedlings. BMC Plant Biol. 2024, 24, 464. [Google Scholar] [CrossRef]
- Jan, A.U.; Hadi, F.; Midrarullah; Nawaz, M.A.; Rahman, K. Potassium and zinc increase tolerance to salt stress in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2017, 116, 139–149. [Google Scholar] [CrossRef]
- Wang, W.; Feng, R.; Zhu, J.; Cao, Y.; Feng, J.; Zhao, Y.; Du, J.; Du, Y. Exogenous tryptophan increases soybean yield by enhancing sucrose-starch metabolism in leaves and seeds at the R6 stage under salt-alkali stress. BMC Plant Biol. 2025, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Radić, S.; Štefanić, P.P.; Lepeduš, H.; Roje, V.; Pevalek-Kozlina, B. Salt tolerance of Centaurea ragusina L. is associated with efficient osmotic adjustment and increased antioxidative capacity. Environ. Exp. Bot. 2013, 87, 39–48. [Google Scholar] [CrossRef]
- Shinta; Bentoy, K.M.Y.; Fauzia, A.N.; Nampei, M.; Linh, N.M.; Ueda, A. Physiological and Transcriptomic Insights into Mechanisms of Salt Tolerance in the Leaves and Roots of the Halophyte Suaeda japonica Makino Under High Salinity Stress. J. Plant Growth Regul. 2025, 1–19. [Google Scholar] [CrossRef]
- Wang, C.; Wei, X.; Wang, Y.; Wu, C.; Jiao, P.; Jiang, Z.; Liu, S.; Ma, Y.; Guan, S. Metabolomics and Transcriptomic Analysis Revealed the Response Mechanism of Maize to Saline-Alkali Stress. Plant Biotechnol. J. 2025, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Win, P.P.; Park, H.-H.; Kuk, Y.-I. Integrated Approach of Using Biostimulants for Improving Growth, Physiological Traits, and Tolerance to Abiotic Stressors in Rice and Soybean. Agronomy 2025, 15, 2265. [Google Scholar] [CrossRef]
- Shen, S.; Pan, L.; Li, J.; Wang, J.; Ahmad, I.; Liu, H.; Bai, Y.; Kang, B.; Yin, J.; Gao, Y.; et al. The Involvement of Amino Acid Metabolism in the Mechanisms of Salt Tolerance Adaptation in Medicago sativa and Medicago truncatula. Plants 2025, 14, 929. [Google Scholar] [CrossRef]
- Ji Biao, J.B.; Li Zan, L.Z.; Gu WanRong, G.W.; Li Jing, L.J.; Xie TengLong, X.T.; Wei Shi, W.S. Methyl jasmonate pretreatment promotes the growth and photosynthesis of maize seedlings under saline conditions by enhancing the antioxidant defense system. Int. J. Agric. Biol. 2018, 20, 1454–1462. [Google Scholar]
- Lu, J.; Xu, Y.; Wang, J.; Singer, S.D.; Chen, G. The role of triacylglycerol in plant stress response. Plants 2020, 9, 472. [Google Scholar] [CrossRef]
- Mueller, S.P.; Unger, M.; Guender, L.; Fekete, A.; Mueller, M.J. Phospholipid: Diacylglycerol acyltransferase-mediated triacylglyerol synthesis augments basal thermotolerance. Plant Physiol. 2017, 175, 486–497. [Google Scholar] [CrossRef]
- Mehrabi, S.S.; Sabokdast, M.; Bihamta, M.R.; Soorni, J.; Mirmazloum, I. Strigolactone-mediated amelioration of salinity stress in bread wheat: Insights from phytochemical and ion channels related genes expression analyses. Plant Stress 2024, 11, 100324. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Xu, C.; Wang, Q. WRKYs as regulatory hubs of secondary metabolic network: Diverse inducers and distinct responses. Plant Commun. 2025, 6, 101438. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Meng, Z.; Jia, Z.; Fu, F.; Jin, B.; Cao, F.; Wang, L. The LncNAT11–MYB11–F3′H/FLS module mediates flavonol biosynthesis to regulate salt stress tolerance in Ginkgo biloba. J. Exp. Bot. 2025, 76, 1179–1201. [Google Scholar] [CrossRef]
- Shim, Y.; Kim, B.; Choi, Y.; Cho, S.H.; Kim, Y.; Kim, S.H.; Yim, Y.; Kang, K.; Paek, N.C. Rice OsDof12 enhances tolerance to drought stress by activating the phenylpropanoid pathway. Plant J. 2025, 121, e17175. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Yang, Z.; Zhou, Y.; Huo, S.; Zhang, S.; Wu, D.; Shu, X.; Wang, Y. OsJRL negatively regulates rice cold tolerance via interfering phenylalanine metabolism and flavonoid biosynthesis. Plant Cell Environ. 2024, 47, 4071–4085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, C.; Guo, H.; Li, Y.; Shen, S.; Zhou, Q.; Li, C.; Wang, C.; Zhai, T.; Qu, L.; et al. Dissecting the genetic basis of UV-B responsive metabolites in rice. Genome Biol. 2024, 25, 234. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shang, C.; Duan, P.; Yang, J.; Wang, J.; Sui, D.; Chen, G.; Li, X.; Li, G.; Hu, S.; et al. The SlWRKY42–SlMYC2 module synergistically enhances tomato saline–alkali tolerance by activating the jasmonic acid signaling and spermidine biosynthesis pathway. J. Integr. Plant Biol. 2025, 67, 1254–1273. [Google Scholar] [CrossRef]
- Maslennikova, D.; Ivanov, S.; Petrova, S.; Burkhanova, G.; Maksimov, I.; Lastochkina, O. Components of the phenylpropanoid pathway in the implementation of the protective effect of sodium nitroprusside on wheat under salinity. Plants 2023, 12, 2123. [Google Scholar] [CrossRef]
- Mustafa, N.R.; Verpoorte, R. Chorismate derived C6C1 compounds in plants. Planta 2005, 222, 1–5. [Google Scholar] [CrossRef]
- Gong, F.; Yu, W.; Zeng, Q.; Dong, J.; Cao, K.; Xu, H.; Zhou, X. Rhododendron chrysanthum’s primary metabolites are converted to phenolics more quickly when exposed to UV-B radiation. Biomolecules 2023, 13, 1700. [Google Scholar] [CrossRef]
- Ma, J.; Saleem, M.H.; Ali, B.; Rasheed, R.; Ashraf, M.A.; Aziz, H.; Ercisli, S.; Riaz, S.; Elsharkawy, M.M.; Hussain, I.; et al. Impact of foliar application of syringic acid on tomato (Solanum lycopersicum L.) under heavy metal stress-insights into nutrient uptake, redox homeostasis, oxidative stress, and antioxidant defense. Front. Plant Sci. 2022, 13, 950120. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Kucukoduk, M. Upregulation of antioxidant enzymes by exogenous gallic acid contributes to the amelioration in Oryza sativa roots exposed to salt and osmotic stress. Environ. Sci. Pollut. Res. 2015, 22, 1487–1498. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Liu, A.; Wang, M.; Dong, J.; Yan, Z.; Wang, X.; Li, J.; Song, H. Foliar application of exogenous salicylic acid mitigates the detrimental effects caused by salt stress in sunflower seedlings. Ind. Crops Prod. 2024, 222, 119854. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.-M.; Sun, W.-J.; Wang, X.-J.; Bai, J.-G. Exogenous p-hydroxybenzoic acid regulates antioxidant enzyme activity and mitigates heat stress of cucumber leaves. Sci. Hortic. 2012, 148, 235–245. [Google Scholar] [CrossRef]
- Zheng, Y.; Cao, X.; Zhou, Y.; Ma, S.; Wang, Y.; Li, Z.; Zhao, D.; Yang, Y.; Zhang, H.; Meng, C.; et al. Purines enrich root-associated Pseudomonas and improve wild soybean growth under salt stress. Nat. Commun. 2024, 15, 3520. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, H.; Abbasi, G.H.; Jamil, M.; Malik, Z.; Ali, M.; Iqbal, R. Assessing the potential of exogenous caffeic acid application in boosting wheat (Triticum aestivum L.) crop productivity under salt stress. PLoS ONE 2021, 16, e0259222. [Google Scholar] [CrossRef]
- Minh, L.; Khang, D.; Ha, P.; Tuyen, P.; Minh, T.; Quan, N.; Xuan, T. Effects of salinity stress on growth and phenolics of rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2016, 57, 1–10. [Google Scholar] [CrossRef]
- Sepehry Javan, Z.; Razavi, S.M.; Khalofah, A.; Ghorbani, A. The ameliorating effects of cinnamic acid-based nanocomposite against salt stress in peppermint. Environ. Sci. Pollut. Res. 2024, 31, 45055–45073. [Google Scholar] [CrossRef] [PubMed]
- Colin, L.; Ruhnow, F.; Zhu, J.-K.; Zhao, C.; Zhao, Y.; Persson, S. The cell biology of primary cell walls during salt stress. Plant Cell 2023, 35, 201–217. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Mota, T.R.; Salatta, F.V.; Sinzker, R.C.; Končitíková, R.; Kopečný, D.; Simister, R.; Silva, M.; Goeminne, G.; Morreel, K. Cell wall remodeling under salt stress: Insights into changes in polysaccharides, feruloylation, lignification, and phenolic metabolism in maize. Plant Cell Environ. 2020, 43, 2172–2191. [Google Scholar] [CrossRef] [PubMed]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Fanti, F.; Oliva, E.; Benincasa, P. Salt-Induced Changes in the Phenolic Content of Melon F2 Offspring Sprouts Obtained from Fruit Deseeding. Foods 2025, 14, 2242. [Google Scholar] [CrossRef]
- Lv, X.; Zhu, L.; Ma, D.; Zhang, F.; Cai, Z.; Bai, H.; Hui, J.; Li, S.; Xu, X.; Li, M. Integrated metabolomics and transcriptomics analyses highlight the flavonoid compounds response to alkaline salt stress in Glycyrrhiza uralensis Leaves. J. Agric. Food Chem. 2024, 72, 5477–5490. [Google Scholar] [CrossRef]
- Ma, S.; Lv, L.; Meng, C.; Zhang, C.; Li, Y. Integrative analysis of the metabolome and transcriptome of Sorghum bicolor reveals dynamic changes in flavonoids accumulation under saline–alkali stress. J. Agric. Food Chem. 2020, 68, 14781–14789. [Google Scholar] [CrossRef]
- Jan, R.; Kim, N.; Lee, S.-H.; Khan, M.A.; Asaf, S.; Lubna; Park, J.-R.; Asif, S.; Lee, I.-J.; Kim, K.-M. Enhanced flavonoid accumulation reduces combined salt and heat stress through regulation of transcriptional and hormonal mechanisms. Front. Plant Sci. 2021, 12, 796956. [Google Scholar] [CrossRef]
- Su, L.; Lv, A.; Wen, W.; Fan, N.; You, X.; Gao, L.; Zhou, P.; Shi, F.; An, Y. MsMYB206–MsMYB450–MsHY5 complex regulates alfalfa tolerance to salt stress via regulating flavonoid biosynthesis during the day and night cycles. Plant J. 2025, 121, e17216. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Alp, F.N.; Kucukoduk, M.; Turkan, I. Naringenin induces tolerance to salt/osmotic stress through the regulation of nitrogen metabolism, cellular redox and ROS scavenging capacity in bean plants. Plant Physiol. Biochem. 2020, 157, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Ghitti, E.; Rolli, E.; Vergani, L.; Borin, S. Flavonoids influence key rhizocompetence traits for early root colonization and PCB degradation potential of Paraburkholderia xenovorans LB400. Front. Plant Sci. 2024, 15, 1325048. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Fang, H.; Chen, Q.; Zhang, Y. The Ionic and Metabolic Response Mechanisms of Kochia scoparia in Response to Saline–Alkaline Stress. Plants 2025, 14, 3540. https://doi.org/10.3390/plants14223540
Lu X, Fang H, Chen Q, Zhang Y. The Ionic and Metabolic Response Mechanisms of Kochia scoparia in Response to Saline–Alkaline Stress. Plants. 2025; 14(22):3540. https://doi.org/10.3390/plants14223540
Chicago/Turabian StyleLu, Xueyan, Hui Fang, Qi Chen, and Ying Zhang. 2025. "The Ionic and Metabolic Response Mechanisms of Kochia scoparia in Response to Saline–Alkaline Stress" Plants 14, no. 22: 3540. https://doi.org/10.3390/plants14223540
APA StyleLu, X., Fang, H., Chen, Q., & Zhang, Y. (2025). The Ionic and Metabolic Response Mechanisms of Kochia scoparia in Response to Saline–Alkaline Stress. Plants, 14(22), 3540. https://doi.org/10.3390/plants14223540






