Floristic Composition and Species Conservation Status in Three Polylepis (Rosaceae) Relict Forests in Peru
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Collection Method
2.3. Conservation Status
2.4. Statistical Analyses
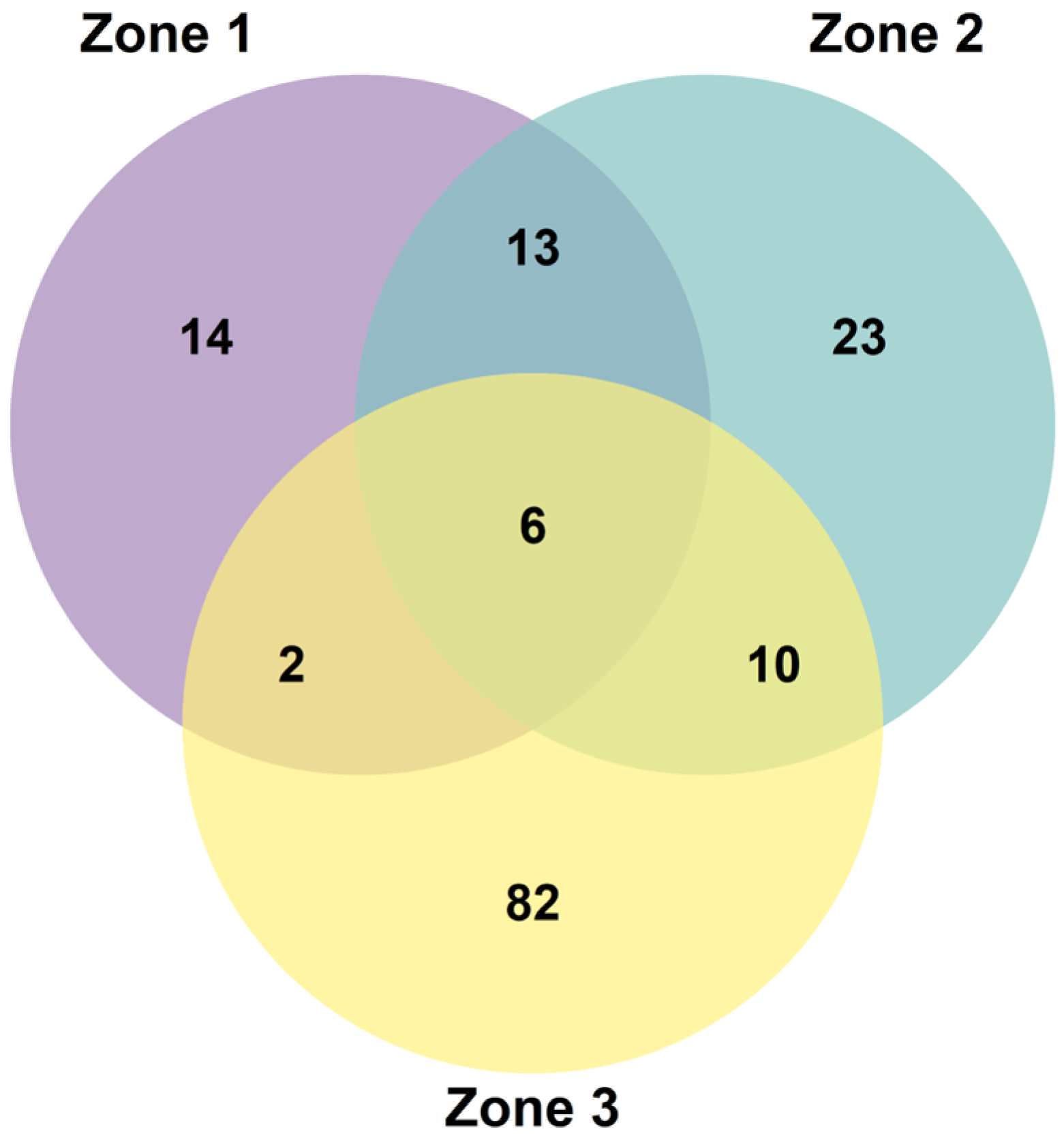
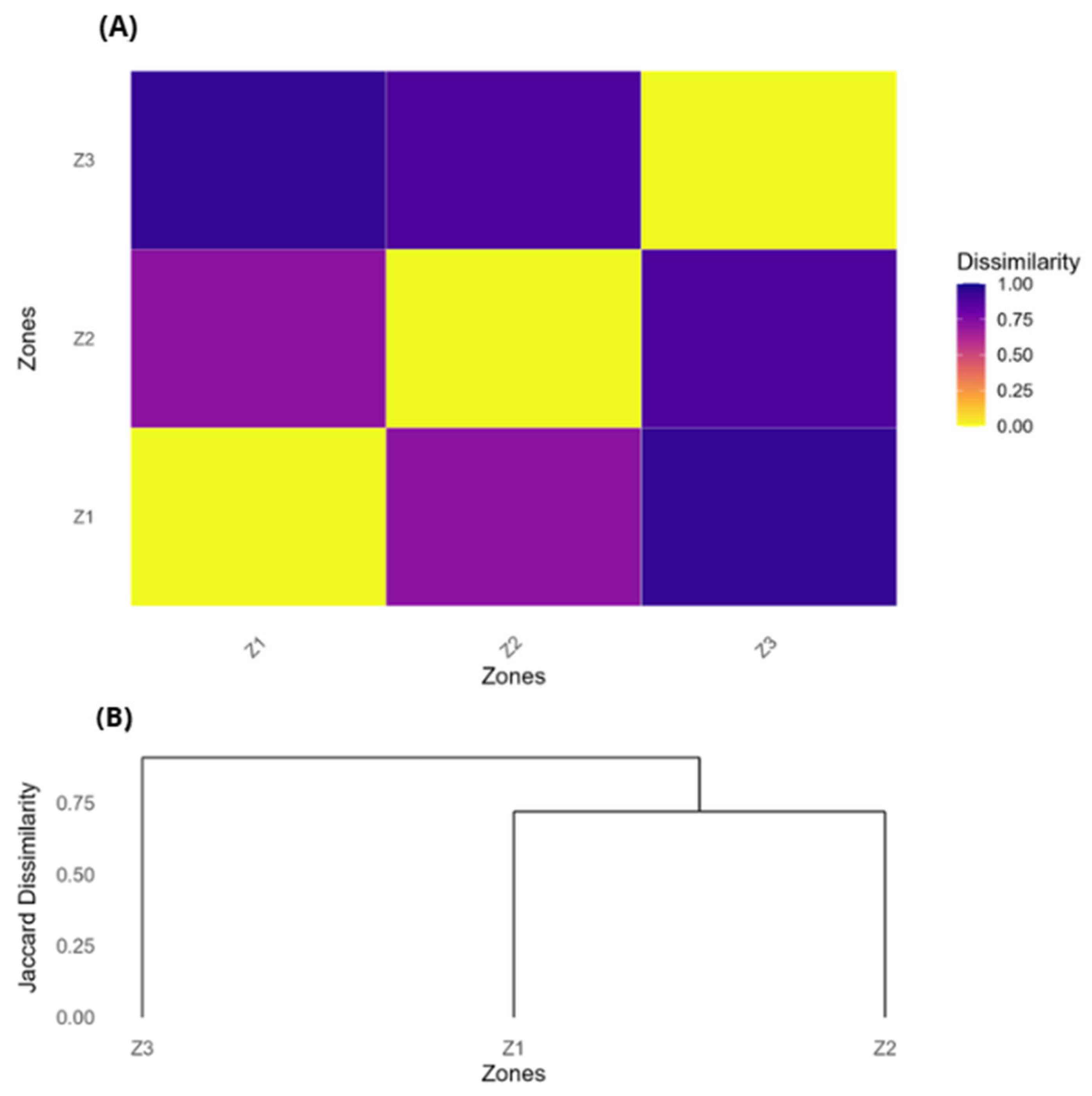
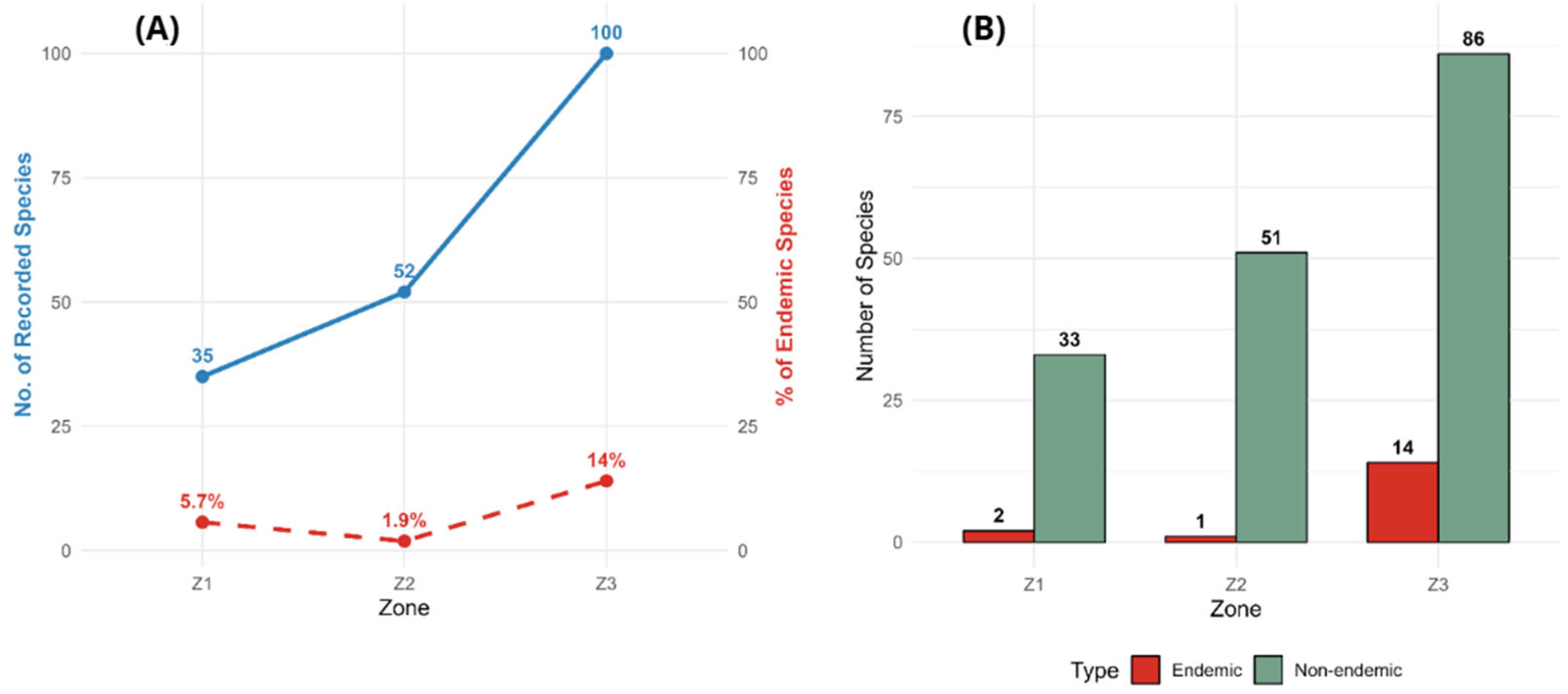
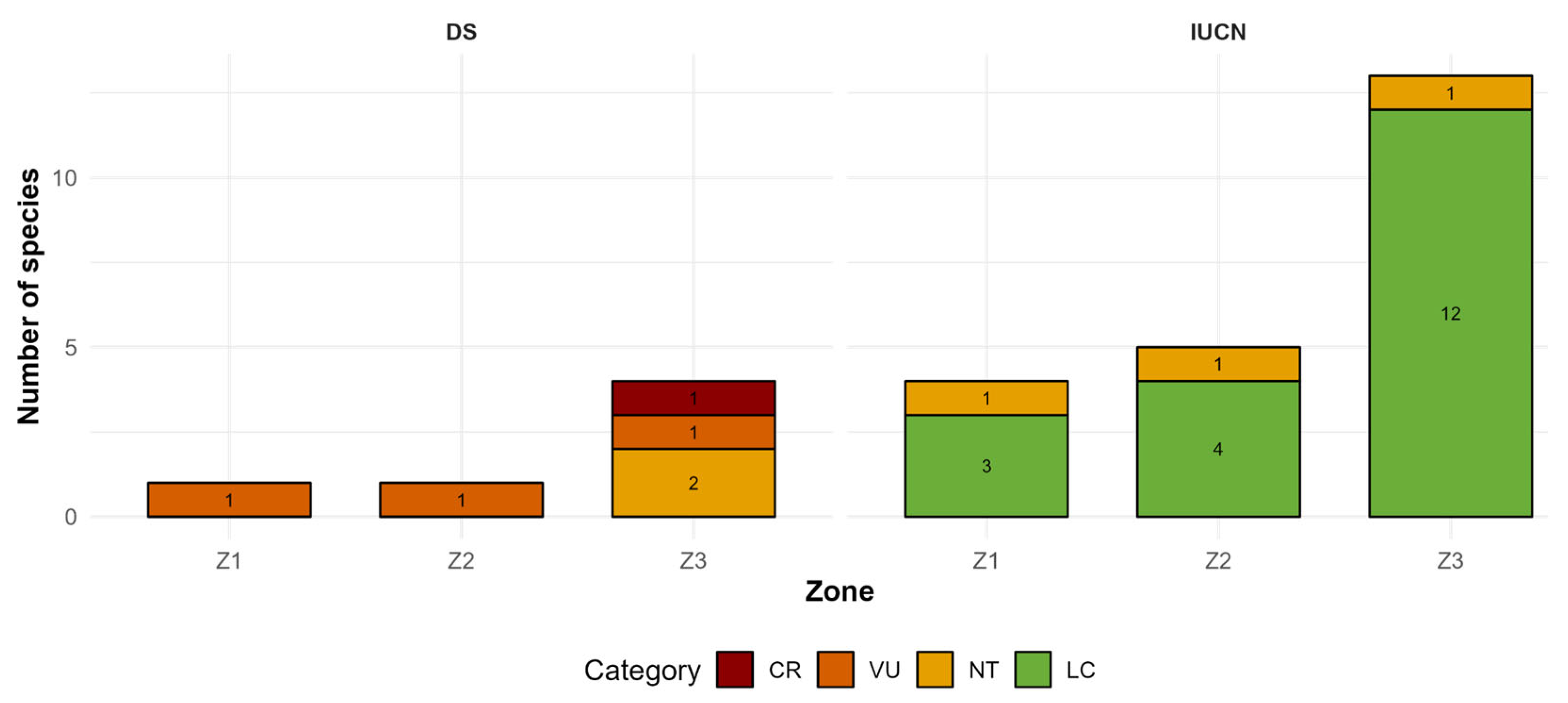
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramos, V.A. Anatomy and global context of the Andes: Main geologic features and the Andean orogenic cycle. In Backbone of the Americas: Shallow Subduction, Plateau Uplift, and Ridge and Terrane Collision; Mahlburg, S., Ramos, V.A., Dickison, W.R., Eds.; Geological Society of America: Boulder, CO, USA, 2009; pp. 31–65. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.A.; Zizka, A.; Bermúdez, M.A.; Meseguer, A.S.; Condamine, F.L.; Hoorn, C.; Hooghiemstra, H.; Pu, Y.; Bogarín, D.; Boschman, L.M.; et al. The Andes through time: Evolution and distribution of Andean floras. Trends Plant Sci. 2022, 27, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escobar, O.A.; Cámara-Leret, R.; Antonelli, A.; Bateman, R.; Bellot, S.; Chomicki, G.; Cleef, A.; Diazgranados, M.; Dodsworth, S.; Jaramillo, C.; et al. Mining threatens Colombian ecosystems. Science 2018, 359, 1475. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Wu, J.; Suppe, J. Southward propagation of Nazca subduction along the Andes. Nature 2019, 565, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Young, B.E. Distribución de las Especies Endémicas en la Vertiente Oriental de los Andes en Perú y Bolivia; NatureServe: Arlington, VA, USA, 2007. [Google Scholar]
- MINAM. Mapa Nacional de Ecosistemas del Perú; Ministerio del Ambiente: Lima, Peru, 2019.
- Ignazi, G.; Mathiasen, P.; Premoli, A.C. Gradientes climáticos modelan la diversidad genética en especies leñosas de amplia distribución: El caso de Nothofagus pumilio en los Andes del sur. Ecosistemas 2019, 28, 35–47. [Google Scholar] [CrossRef]
- Simpson, B.B. A Revision of the Genus Polylepis (Rosaceae: Sanguisorbeae); Smithsonian Institution Press: Washington, DC, USA, 1979. [Google Scholar] [CrossRef]
- Kessler, M.; Schmidt-lebuhn, A.N. Taxonomical and distributional notes on Polylepis (Rosaceae). Organ. Divers. Evol. 2006, 6, 67–69. [Google Scholar] [CrossRef]
- Kessler, M. Bosques de Polylepis. In Botánica Económica de los Andes Centrales; Morae, R.M., Øllgaard, B., Kvist, L.P., Borchsenius, F., Balslev, H., Eds.; Universidad Mayor de San Andrés: La Paz, Bolivia, 2006; pp. 110–120. [Google Scholar]
- Mendoza, W.; Cano, A. Diversidad del género Polylepis (Rosaceae, Sanguisorbeae) en los Andes peruanos. Rev. Peru. Biol. 2011, 18, 197–200. [Google Scholar] [CrossRef]
- Renison, D.; Cuyckens, G.A.E.; Pacheco, S.; Guzmán, G.F.; Grau, H.R.; Marcora, P.; Robledo, G.; Cingolani, A.M.; Dominguez, J.; Landi, M.; et al. Distribución y estado de conservación de las poblaciones de árboles y arbustos del género Polylepis (Rosaceae) en las montañas de Argentina. Ecol. Austral 2013, 23, 27–36. [Google Scholar] [CrossRef]
- Arévalo, R.; Recharte, J. Bosques de montaña: Ecosistemas relictos. In Islas en el Cielo; Recharte, J., Arévalo, R., Glave, M., Eds.; Instituto de la Montaña: Lima, Peru, 2003; pp. 11–19. [Google Scholar]
- Arnal, H.; Sampson, A.; Aucca, C.; Navarro, G.; Ferreira, W.; Romoleroux, K.; Caro, D.; Teich, I.; Torres, H.; Antezana, C.; et al. Mapa de Bosques Altiandinos de Polylepis Prioritarios para Conservación. In Una Contribución al Conocimiento de los Bosques Altoandinos de Polylepis: Distribución, Diversidad y Estado Actual de los Bosques Más Altos del Mundo; Arnal, H., Ed.; American Bird Conservancy–Comunidad Andina: Lima, Peru, 2007. [Google Scholar] [CrossRef]
- Kessler, M. Polylepis-Wälder Boliviens: Taxa, Ökologie, Verbreitung und Geschichte; Schweizerbart Science Publishers: Stuttgart, Germany, 1995. [Google Scholar]
- Bedoya-Canas, L.E.; López-Hernández, F.; Cortés, A.J. Climate change responses of high-elevation Polylepis forests. Forests 2024, 15, 811. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Coblentz, D.; Keating, P.L. Topographic controls on the distribution of tree islands in the high Andes of south-western Ecuador. J. Biogeogr. 2008, 35, 2026–2038. [Google Scholar] [CrossRef]
- Zutta, B.; Rundel, P. Modeled shifts in Polylepis species ranges in the Andes from the Last Glacial Maximum to the present. Forests 2017, 8, 232. [Google Scholar] [CrossRef]
- Ames-Martínez, F.N.; Quispe-Melgar, H.R.; Renison, D. Conservation status assessment of the highest forests in the world: Polylepis flavipila forests as a case study. Neotrop. Biodivers. 2021, 7, 160–169. [Google Scholar] [CrossRef]
- Quinteros-Gómez, Y.; Salinas-Inga, A.; Macedo-Bedoya, J.; Peralta-Alcantara, E.; La Rosa-Sánchez, M.; Gonzales, F.C.; Yamunaque, A.; Angeles-Alvarez, F.; Gómez-Ticerán, D.; Dávila, O.L.S. Noninvasive sonic tomography for the detection of internal defects in relict woodlands of Polylepis in Peru. Forests 2025, 16, 957. [Google Scholar] [CrossRef]
- Toivonen, J.M.; Gonzales-Inca, C.A.; Bader, M.Y.; Ruokolainen, K.; Kessler, M. Elevational shifts in the topographic position of Polylepis forest stands in the Andes of Southern Peru. Forests 2018, 9, 7. [Google Scholar] [CrossRef]
- Fjeldså, J.; Kessler, M. Conservación de la Biodiversidad de los Bosques de Polylepis de las Tierras Altas de Bolivia: Una Contribución al Manejo Sustantable en los Andes; DIVA Technical Report 11; Editorial FAN: Santa Cruz de la Sierra, Bolivia, 2004. [Google Scholar]
- Cuyckens, G.A.E.; Renison, D. Ecología y conservación de los bosques montanos de Polylepis: Una introducción al número especial. Ecol. Austral 2018, 28, 157–162. [Google Scholar] [CrossRef]
- Delgado, J.; León-Vargas, Y. Musgos (Bryophyta) de bosques de Polylepis sericea (Rosaceae) del Estado Mérida (Venezuela). Bol. Soc. Argent. Bot. 2017, 52, 295–313. [Google Scholar] [CrossRef]
- Trinidad, H.; Cano, A. Composición florística de los bosques de Polylepis Yauyinazo y Chaqsii-Chaqsii, Reserva Paisajística Nor Yauyos-Cochas, Lima. Rev. Peru. Biol. 2016, 23, 271–286. [Google Scholar] [CrossRef]
- Morales-Aranibar, L.F. Estado actual del bosque de Polylepis y su eficiencia en la captura de CO2 en la provincia Tarata, departamento de Tacna. Rev. Cienc. Desarro. 2015, 19, 36–43. [Google Scholar] [CrossRef]
- Montalvo, J.; Minga, D.; Verdugo, A.; López, J.; Guazhambo, D.; Pacheco, D.; Siddons, D.; Crespo, A.; Zárate, E. Características morfológico-funcionales, diversidad arbórea, tasa de crecimiento y de secuestro de carbono en especies y ecosistemas de Polylepis del sur de Ecuador. Ecol. Austral 2018, 28, 249–261. [Google Scholar] [CrossRef]
- Fjeldså, J.; Kessler, M. Conserving the Biological Diversity of Polylepis Woodlands of the Highlands of Peru and Bolivia: A Contribution to Sustainable Natural Resource Management in the Andes; NORDECO: Copenhagen, Denmark, 1996. [Google Scholar]
- Zutta, B.R.; Rundel, P.W.; Saatchi, S.; Casana, J.D.; Gauthier, P.G.; Soto, A.; Velazco, Y.; Buermann, W. Prediciendo la distribución de Polylepis: Bosques Andinos vulnerables y cada vez más importantes. Rev. Peru. Biol. 2012, 19, 205–212. [Google Scholar] [CrossRef]
- Chanove-Manrique, A.; Cárdenas-Pillco, B. Fragmentación del paisaje y pérdida de conectividad en los bosques de queñua (Polylepis) en Perú y su vulnerabilidad ante el cambio climático. Mader. Bosques 2024, 30, e3032593. [Google Scholar] [CrossRef]
- Boza Espinoza, T.E.; Kessler, M. A monograph of the genus Polylepis (Rosaceae). PhytoKeys 2022, 203, 1–274. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, Y.M. Etnobotánica y Revaloración de los Conocimientos Tradicionales de la Flora Medicinal en Cajatambo, Lima. Master’s Thesis, Pontificia Universidad Católica del Perú, Lima, Peru, 2009. [Google Scholar]
- Ceroni-Stuva, A.; Vilcapoma-Segovia, G. Composición florística y estado de conservación de plantas vasculares del distrito de Cajatambo/Lima/Perú. Ecol. Apl. 2020, 19, 132–146. [Google Scholar] [CrossRef]
- Servicio Nacional de Meteorología e Hidrología del Perú. Available online: https://www.senamhi.gob.pe/site/descarga-datos/ (accessed on 23 July 2025).
- World Flora Online. Available online: https://www.worldfloraonline.org/ (accessed on 13 August 2025).
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/resources/categories-and-criteria (accessed on 10 August 2025).
- Ministerio de Agricultura y Riego; Decreto Supremo, N. 043-2006-AG: Aprueban Categorización de Especies Amenazadas de Flora Silvestre; Ministerio de Agricultura y Riego: Lima, Peru, 2006.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 17 August 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; Available online: https://ggplot2.tidyverse.org/ (accessed on 18 August 2025).
- Kolde, R. Pheatmap: Pretty Heatmaps, version 1.0.12, R Foundation for Statistical Computing: Vienna, Austria, 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 18 August 2025).
- Galili, T. Dendextend: Extending ‘Dendrogram’ Functionality, version 1.17.1, R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: https://CRAN.R-project.org/package=dendextend (accessed on 17 August 2025).
- Koepcke, M. Birds of the western slope of the Andes of Peru. Am. Mus. Novit. 1961, 2028, 1–31. [Google Scholar]
- Servat, G.P.; Mendoza, W.; Ochoa, J.A. Flora y fauna de cuatro bosques de Polylepis (Rosaceae) en la Cordillera del Vilcanota (Cusco, Perú). Ecol. Apl. 2002, 1, 25–35. [Google Scholar] [CrossRef]
- Castro, A.; Flores, M. Caracterización de un bosque de queñual (Polylepis spp.) ubicado en el distrito de Huasta, Provincia de Bolognesi (Ancash, Perú). Ecol. Apl. 2015, 14, 1–9. [Google Scholar] [CrossRef]
- Pinos, J. Challenges and conservation implications of Polylepis woodlands in the Andean region: Defining actions for sustainable management. Hacquetia 2020, 19, 143–153. [Google Scholar] [CrossRef]
- Morales-Aranibar, L.F.; Rojas de la Puente, E.E.; Costa, J.F. Ecology of Polylepis spp. forests, and proposal for its conservation in the andean region of Tacna, Peru. In Environment. Technology. Resources, Proceedings of the 12th International Scientific and Practical Conference, Rezekne, Latvia, 20–22 June 2019; Rezekne Academy of Technologies: Rezekne, Latvia, 2019; pp. 180–186. [Google Scholar] [CrossRef]
- Bañares-de-Dios, G.; Macía, M.J.; Arellano, G.; Granzow-de la Cerda, Í.; Vega-Álvarez, J.; Salinas, N. Woody plant taxonomic, functional, and phylogenetic diversity decrease along elevational gradients in Andean tropical montane forests: Environmental filtering and arrival of temperate taxa. Plant Divers. 2024, 46, 491–501. [Google Scholar] [CrossRef]
- Aquino, W.; Condo, F.; Romero, J.; Yllaconza, R.; La Torre, M.I. Composición florística del distrito de Huarochirí, provincia de Huarochirí (Lima, Perú). Arnaldoa 2018, 25, 877–922. [Google Scholar] [CrossRef]
- Béjar, L. Flora de los bosques de Polylepis spp. en tres localidades del Valle Sagrado de los Incas. Undergraduate Thesis, Universidad Nacional de San Antonio Abad del Cusco, Cusco, Peru, 1996. [Google Scholar]
- Mendoza, W.; Roque, J. Diversidad de la Flora Vascular Asociada a los Bosques de Polylepis (Rosaceae) en los Andes Meridionales del Perú (Ayacucho): Implicancias Para su Conservación; Serie de Publicaciones de Flora y Fauna Silvestre; Instituto Nacional de Recursos Naturales (INRENA): Lima, Peru, 2007.
- ECOAN. Evaluación de la Biodiversidad de los Bosques de Polylepis en la Zona sur Oeste del Parque Nacional Otishi; Asociación Ecosistemas Andinos: Lima, Peru, 2007. [Google Scholar]
- Valencia, B.G.; Bush, M.B.; Coe, A.L.; Orren, E.; Gosling, W.D. Polylepis woodland dynamics during the last 20,000 years. J. Biogeogr. 2018, 45, 1019–1030. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Anderson, C.B.; Kryston, K.; Vaughn, N.; Knapp, D.E.; Bentley, L.P.; Shenkin, A.; Salinas, N.; Sinca, F.; et al. Scale dependence of canopy trait distributions along a tropical forest elevation gradient. N. Phytol. 2017, 214, 973–988. [Google Scholar] [CrossRef]
- Cuesta, F.; Carilla, J.; Llambí, L.D.; Muriel, P.; Lencinas, M.V.; Meneses, R.I.; Feeley, K.J.; Pauli, H.; Aguirre, N.; Beck, S.; et al. Compositional shifts of alpine plant communities across the high Andes. Glob. Ecol. Biogeogr. 2023, 32, 1591–1606. [Google Scholar] [CrossRef]
- Urquiaga-Flores, E.G.; Bader, M.Y.; Kessler, M. Contrasting topography-vegetation relationships at natural and human-influenced mountain treelines in the Peruvian Andes. Landsc. Ecol. 2024, 39, 12. [Google Scholar] [CrossRef]
- Malizia, A.; Blundo, C.; Carilla, J.; Acosta, O.O.; Cuesta, F.; Duque, A.; Aguirre, N.; Aguirre, Z.; Ataroff, M.; Baez, S.; et al. Elevation and latitude drives structure and tree species composition in Andean forests: Results from a large-scale plot network. PLoS ONE 2020, 15, e0231553. [Google Scholar] [CrossRef]
- Fadrique, B.; Báez, S.; Duque, Á.; Malizia, A.; Blundo, C.; Carilla, J.; Osinaga-Acosta, O.; Malizia, L.; Silman, M.; Farfán-Ríos, W.; et al. Widespread but heterogeneous responses of Andean forests to climate change. Nature 2018, 564, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Sklenář, P.; Romoleroux, K.; Muriel, P.; Jaramillo, R.; Bernardi, A.; Diazgranados, M.; Moret, P. Distribution changes in páramo plants from the equatorial high Andes in response to increasing temperature and humidity variation since 1880. Alp. Botany 2021, 131, 201–212. [Google Scholar] [CrossRef]
- Fuentes-Lillo, E.; Lembrechts, J.J.; Barros, A.; Aschero, V.; Bustamante, R.O.; Cavieres, L.A.; Clavel, J.; Herrera, I.; Jiménez, A.; Tecco, P.; et al. Going up the Andes: Patterns and drivers of non-native plant invasions across latitudinal and elevational gradients. Biodivers. Conserv. 2023, 32, 4199–4219. [Google Scholar] [CrossRef]
- Camacho, L.F.; Schwartz, N.; Avilés, L. Vegetation Structural Complexity Across Elevational Gradients: Insights From the Tropical Andes. J. Biogeogr. 2025, 52, e15102. [Google Scholar] [CrossRef]
- Funk, V.A.; Susanna, A.; Stuessy, T.F.; Bayer, R.J. (Eds.) Systematics, Evolution, and Biogeography of Compositae; International Association for Plant Taxonomy (IAPT): Vienna, Austria, 2009. [Google Scholar]
- Ulloa, C.U.; Acevedo-Rodríguez, P.; Beck, S.; Belgrano, M.J.; Bernal, R.; Berry, P.E.; Brako, L.; Celis, M.; Davidse, G.; Forzza, R.C.; et al. An integrated assessment of the vascular plant species of the Americas. Science 2017, 358, 1614–1617. [Google Scholar] [CrossRef]
- Gonzáles, P.; Cano, A.; Müller, J. An unusual new record of Baccharis (Asteraceae) from the Peruvian Andes and its relation with the northern limit of the dry puna. Acta Bot. Mex. 2019, 126, e1393. [Google Scholar] [CrossRef]
- Huamantupa-Chuquimaco, I.; Martinez Trujillo, Y.L.; Orosco Ucamayta, E. Valuation of the diversity of native plants and the cultural-archaeological richness as an integrative approach for a potential use in ecotourism in the Inter-Andean Valley of Cusco, Southern Peru. Diversity 2023, 15, 760. [Google Scholar] [CrossRef]
- Cano, A.; Mendoza, W.; Castillo, S.; Morales, M.; La Torre, M.I.; Aponte, H.; Delgado, A.; Valencia, N.; Vega, N. Flora y vegetación de suelos crioturbados y hábitats asociados en la Cordillera Blanca, Ancash, Perú. Rev. Peru. Biol. 2010, 17, 95–103. [Google Scholar] [CrossRef]
- Cano, A.; Delgado, A.; Mendoza, W.; Trinidad, H.; González, P.; La Torre, M.I.; Chanco, M.; Aponte, H.; Roque, J.; Valencia, N.; et al. Flora y vegetación de suelos crioturbados y hábitats asociados en los alrededores del abra Apacheta, Ayacucho–Huancavelica (Perú). Rev. Peru. Biol. 2011, 18, 169–178. [Google Scholar] [CrossRef]
- Paulino, E.; La Torre, M.; Huamán, L.M. Distribución Altitudinal de la Flora Fanerogámica del distrito de Oyón, Lima, Perú. Biologist 2015, 13, 21–33. [Google Scholar]
- Kahn, F.; Millán, B.; Cano, A.; La Torre, M.I.; Baldeón, S.; Beltrán, H.; Trinidad, H.; Castillo, S.; Machahua, M. Contribución a la flora altoandina del distrito de Oyón, región Lima, Perú. Rev. Peru. Biol. 2016, 23, 67–72. [Google Scholar] [CrossRef]
- Aquino, W.; La Torre, M.I.; Condo, F.; Romero, J.; Ramírez, J. Flora vascular del anexo de Marachanca del distrito de Matucana, provincia de Huarochirí, Lima, Perú. Biologist 2017, 15, 359–377. [Google Scholar] [CrossRef]
- Quinteros-Gómez, Y.; Macedo-Bedoya, J.; Santos-Linares, V.; Angeles-Alvarez, F.; Gómez-Ticerán, D.; Campos-De la Cruz, J.; Solis Sarmiento, J.; Salinas-Inga, A.; Valencia-Saavedra, Z. Floristic Diversity and Distribution Pattern along an Altitudinal Gradient in the Central Andes: A Case Study of Cajatambo, Peru. Plants 2024, 13, 3328. [Google Scholar] [CrossRef]
- Naiman, R.J.; Decamps, H.; Pollock, M. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 1993, 3, 209–212. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems, 3rd ed.; Springer Nature: Cham, Switzerland, 2021. [Google Scholar]
- Kessler, M.; Driesch, P. Causas e historia de la destrucción de bosques altoandinos en Bolivia. Ecol. Bolivia 1993, 21, 1–18. [Google Scholar]
- Ames, F.N.; Quispe, H.R.; Zuñiga, D.G.; Segovia, M.C.; Kessler, M. Bosques de Polylepis: Biodiversidad en la Región Central del Perú; Universidad Continental: Huancayo, Peru, 2019; Available online: https://hdl.handle.net/20.500.12394/5922 (accessed on 7 November 2024).
- Gareca, E.E.; Martinez, Y.Y.; Solís, C.; Aguirre, L.F. Efectos de los árboles exóticos y del ambiente materno sobre la producción de semillas, la germinación y el crecimiento inicial de Polylepis subtusalbida (Rosaceae) en el Parque Nacional Tunari, Bolivia. Ecol. Austral 2018, 28, 262–277. [Google Scholar] [CrossRef]
- Renison, D.; Morales, L.; Cuyckens, G.É.; Sevillano, C.S.; Cabrera, D.M. Ecología y conservación de los bosques y arbustales de Polylepis: ¿qué sabemos y qué ignoramos? Ecol. Austral 2018, 28, 163–174. [Google Scholar] [CrossRef]
- Delgado, J.; León-Vargas, Y. Floristic diversity in Polylepis sericea Wedd. (Rosaceae) forest fragments in the Andes of the Mérida, Venezuela. Acta Bot. Venez. 2023, 46, 43–66. [Google Scholar]
- Herzog, S.K.; Jørgensen, P.M.; Martínez Güingla, R.; Martius, C.; Anderson, E.P.; Hole, D.G.; Larsen, T.H.; Marengo, J.A.; Carrascal, D.R.; Tiessen, H. Efectos del Cambio Climático en la Biodiversidad de los Andes Tropicales: El Estado del Conocimiento Científico. Resumen Para Tomadores de Decisiones y Responsables de la Formulación de Políticas Públicas; Instituto Interamericano para la Investigación del Cambio Global (IAI): Montevideo, Uruguay, 2010. [Google Scholar]

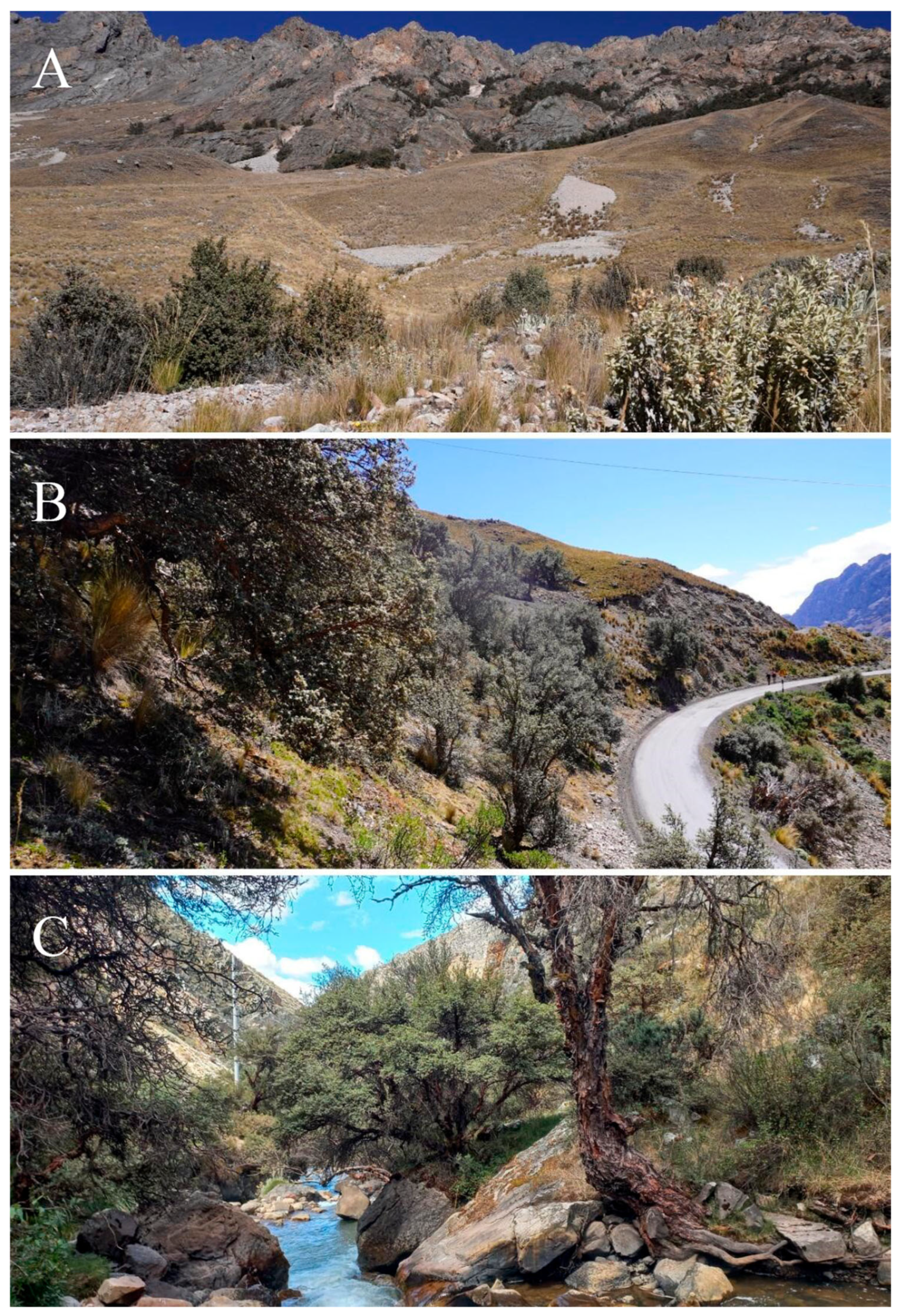
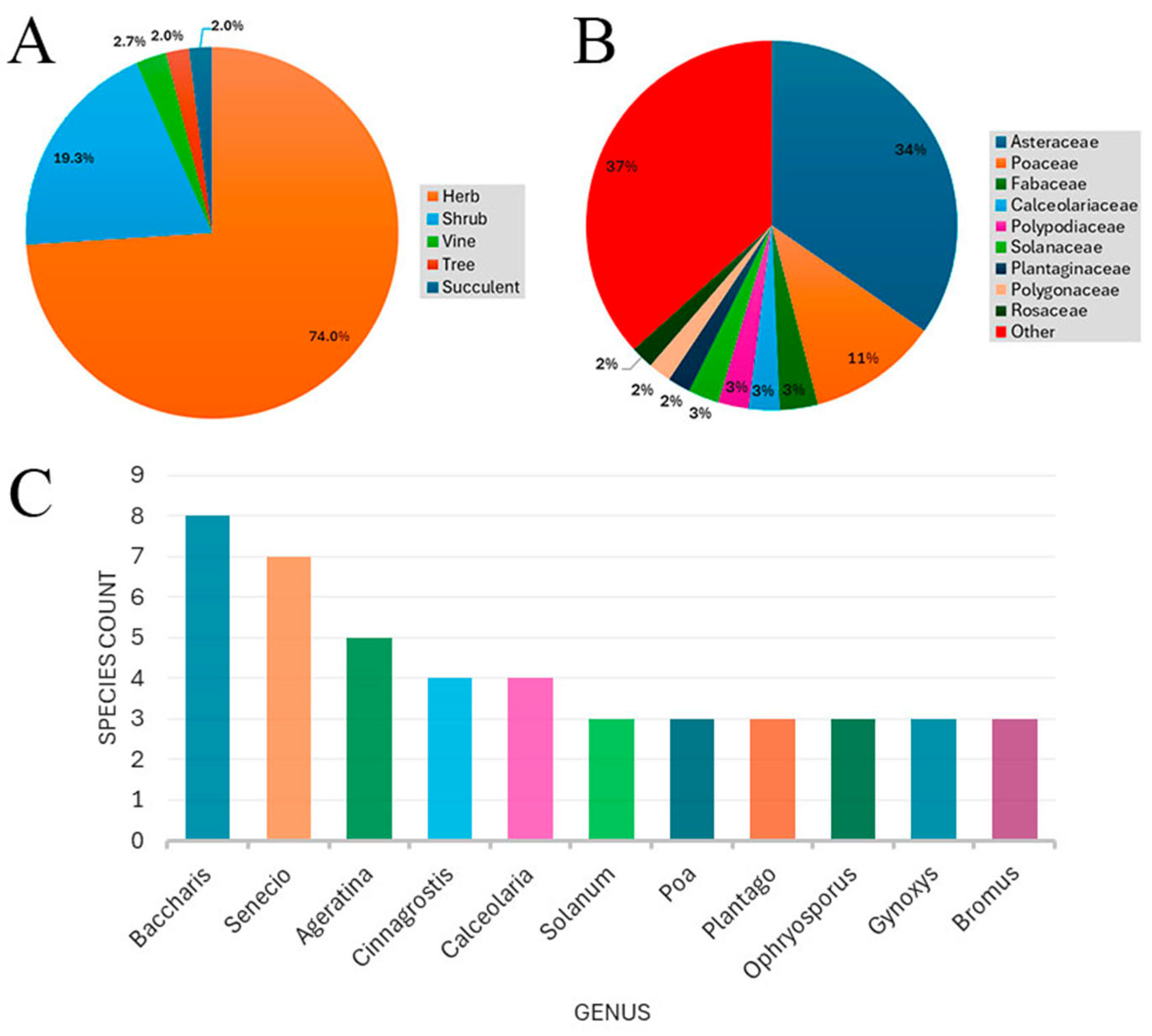
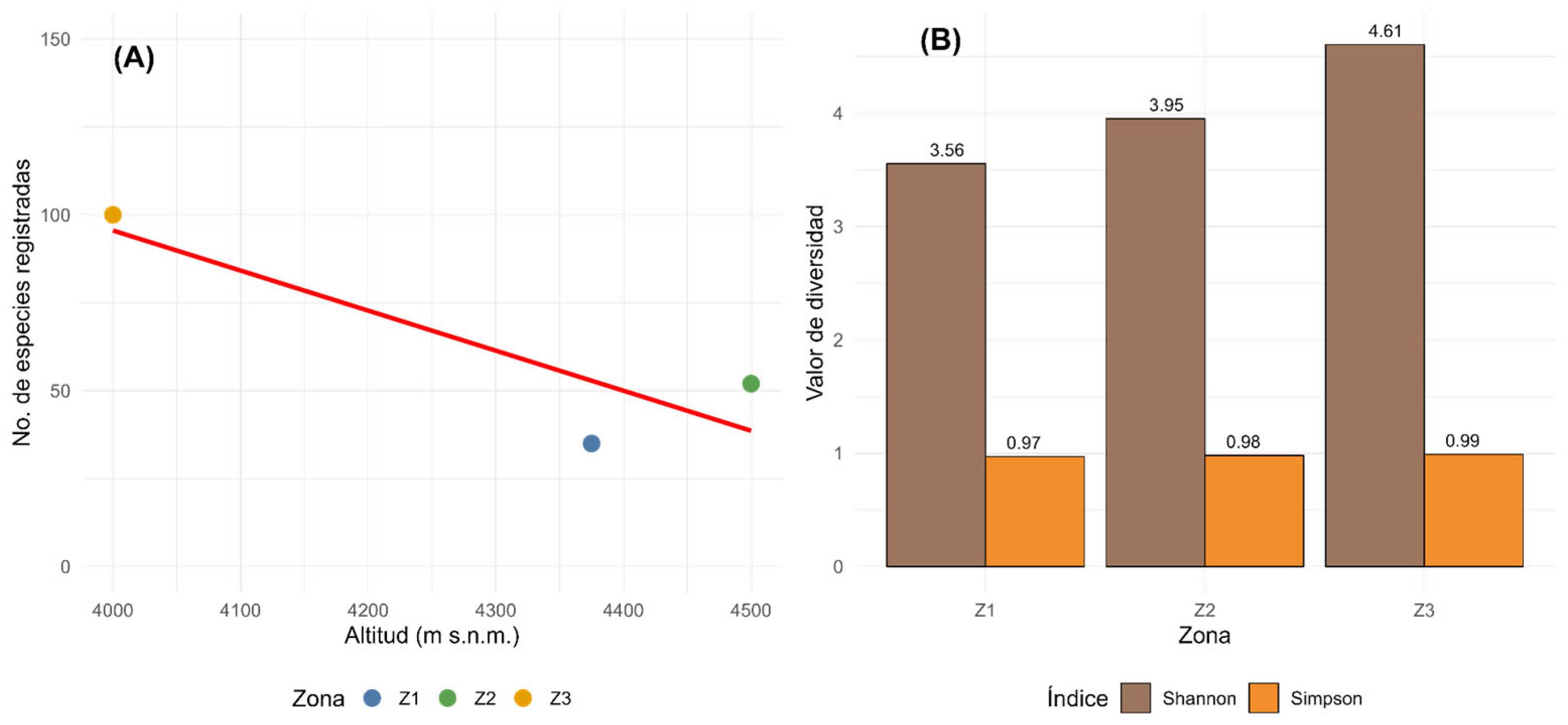

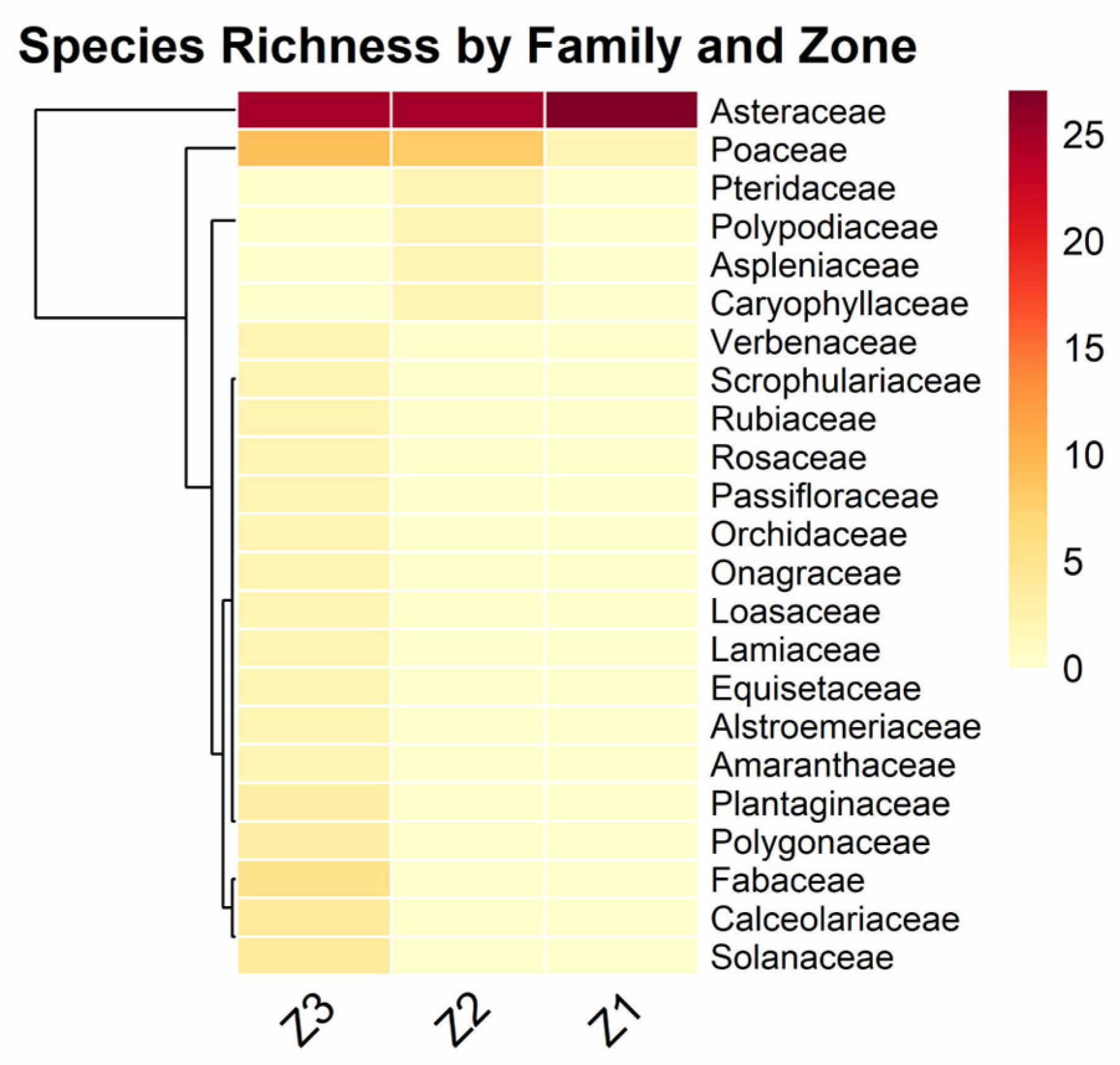
| Family | Species | Growth Habit | IUCN/SD * | Endemic | Zones |
|---|---|---|---|---|---|
| Alstroemeriaceae | Bomarea dulcis (Hook.) Beauverd | Vi | - | No | Z2, Z3 |
| Bomarea ovata (Cav.) Mirb. | Vi | - | No | Z3 | |
| Amaranthaceae | Alternanthera lanceolata (Benth.) Schinz | H | - | No | Z3 |
| Alternanthera macbridei Standl. | H | - | No | Z3 | |
| Aspleniaceae | Asplenium peruvianum Desv. | H | - | No | Z2 |
| Asplenium triphyllum C. Presl | H | - | No | Z2, Z3 | |
| Asteraceae | Achyrocline alata (Kunth) DC. | H | - | No | Z1, Z2, Z3 |
| Achyrophorus taraxacoides Walp. | H | - | No | Z1, Z2, Z3 | |
| Ageratina azangaroensis (Sch. Bip. ex Wedd.) R.M. King & H. Rob. | H | - | No | Z2, Z3 | |
| Ageratina glechonophylla (Less.) R.M. King & H. Rob. | H | - | No | Z1, Z3 | |
| Ageratina pentlandiana (DC.) R.M. King & H. Rob. | H | - | No | Z2 | |
| Ageratina sternbergiana (DC.) R.M. King & H. Rob. | H | - | No | Z2, Z3 | |
| Ageratina sp. | H | - | - | Z2 | |
| Aristeguietia discolor (DC.) R.M. King & H. Rob. | H | NT | No | Z3 | |
| Baccharis alpina Kunth | B | - | No | Z1, Z2 | |
| Baccharis caespitosa (Ruiz & Pav.) Pers. | B | - | No | Z1, Z2 | |
| Baccharis latifolia (Ruiz & Pav.) Pers. | B | LC | No | Z3 | |
| Baccharis nitida (Ruiz & Pav.) Pers. | B | LC | No | Z3 | |
| Baccharis salicina Torr. & A. Gray | B | - | No | Z3 | |
| Baccharis sp. | B | - | - | Z2 | |
| Baccharis tola Phil. | B | - | No | Z1, Z2 | |
| Baccharis tricuneata (L. f.) Pers. | B | - | No | Z2 | |
| Belloa sp. | H | - | - | Z1, Z2 | |
| Bidens andicola Kunth | H | - | No | Z2, Z3 | |
| Calendula officinalis L. | H | - | No | Z3 | |
| Chaptalia nutans (L.) Polák | H | - | No | Z1 | |
| Chersodoma antennaria (Wedd.) Cabrera | H | - | No | Z1, Z2 | |
| Conyza bonariensis (L.) Cronquist | H | - | No | Z3 | |
| Conyza canadensis (L.) Cronquist | H | - | No | Z1 | |
| Culcitium canescens Bonpl. | H | LC | No | Z1 | |
| Diplostephium sp. | H | - | - | Z1 | |
| Gnaphalium dombeyanum DC. | H | - | Yes | Z1, Z2, Z3 | |
| Gynoxys nitida Muschl. | B | - | Yes | Z1, Z3 | |
| Gynoxys oleifolia Muschl. | B | - | Yes | Z3 | |
| Gynoxys visoensis Cuatrec. | B | - | Yes | Z3 | |
| Hieracium sp. | H | - | - | Z1 | |
| Hypochaeris chillensis (Kunth) Britton | H | - | No | Z1 | |
| Laennecia artemisiifolia (Meyen & Walp.) G.L. Nesom | H | - | No | Z2 | |
| Asteraceae | Lasiocephalus sp. | H | - | - | Z1 |
| Loricaria ferruginea (Ruiz & Pav.) Wedd. | B | - | No | Z1 | |
| Onoseris odorata Hook. & Arn. | H | - | Yes | Z3 | |
| Ophryosporus chilca (Kunth) Hieron. | B | - | No | Z3 | |
| Ophryosporus heptanthus (Sch. Bip. ex Wedd.) R.M. King & H. Rob. | B | - | No | Z3 | |
| Ophryosporus piquerioides (DC.) Benth. ex Baker | B | - | No | Z1, Z2, Z3 | |
| Paranephelius ovatus A. Gray ex Wedd. | H | - | No | Z1, Z2 | |
| Rockhausenia nubigena Kunth | H | - | No | Z1, Z2 | |
| Senecio comosus Sch. Bip. | H | - | No | Z1, Z2, Z3 | |
| Senecio condimentarius Cabrera | H | LC | No | Z1, Z2 | |
| Senecio crassiflorus DC. | H | - | No | Z2 | |
| Senecio evacoides Sch. Bip. | H | - | No | Z2, Z3 | |
| Senecio hohenackeri Sch. Bip. | H | - | No | Z1 | |
| Senecio nutans Sch. Bip. | H | VU | No | Z3 | |
| Senecio sublutescens Cuatrec. | H | - | No | Z1 | |
| Sonchus asper (L.) Hill | H | - | No | Z3 | |
| Sonchus oleraceus L. | H | - | No | Z3 | |
| Taraxacum officinale F.H. Wigg. | H | LC | No | Z2 | |
| Werneria villosa A. Gray | H | - | No | Z1, Z2 | |
| Xenophyllum sp. | H | - | No | Z1 | |
| Berberidaceae | Berberis lutea Ruiz & Pav. | B | LC | No | Z3 |
| Brassicaceae | Weberbauera spathulifolia (A. Gray) O.E. Schulz | H | - | No | Z1, Z2 |
| Bromeliaceae | Puya alpestris (Poepp.) Gay | StH | - | No | Z3 |
| Cactaceae | Austrocylindropuntia floccosa (Salm-Dyck ex Winterfeld) F.Ritter | Suc | LC | No | Z1, Z2 |
| Calceolariaceae | Calceolaria glauca Ruiz & Pav. | H | - | Yes | Z3 |
| Calceolaria hispida Benth. | H | - | Yes | Z3 | |
| Calceolaria parvifolia Wedd. | H | - | No | Z3 | |
| Calceolaria sp. | H | - | - | Z3 | |
| Campanulaceae | Lobelia decurrens Cav. | H | - | No | Z3 |
| Caryophyllaceae | Stellaria weddellii Pedersen | H | - | No | Z2 |
| Paronychia andina A. Gray | H | - | No | Z2 | |
| Cyperaceae | Cyperus sp. | H | - | - | Z3 |
| Cystopteridaceae | Cystopteris fragilis (L.) Bernh. | H | LC | No | Z2 |
| Dryopteridaceae | Polystichum cochleatum (Klotzsch) Hieron. | H | - | No | Z2 |
| Polystichum orbiculatum (Desv.) J. Rémy & Fée | H | - | No | Z3 | |
| Ephedraceae | Ephedra americana Humb. & Bonpl. ex Willd. | H | LC/NT | No | Z3 |
| Equisetaceae | Equisetum bogotense Kunth | H | - | No | Z3 |
| Equisetum sp. | H | - | - | Z3 | |
| Ericaceae | Pernettya prostrata (Cav.) DC. | B | LC | No | Z3 |
| Fabaceae | Astragalus sp. | H | - | - | Z2, Z3 |
| Lupinus brachypremnon C.P. Sm. | B | - | Yes | Z3 | |
| Lupinus condensiflorus C.P. Sm. | B | - | Yes | Z3 | |
| Otholobium pubescens (Poir.) J.W. Grimes | B | - | No | Z3 | |
| Senna birostris (Dombey ex Vogel) H.S. Irwin & Barneby | B | - | Yes | Z3 | |
| Geraniaceae | Geranium sp. | H | - | - | Z3 |
| Juncaceae | Luzula racemosa Desv. | H | - | No | Z1, Z2, Z3 |
| Lamiaceae | Clinopodium sericeum (C. Presl ex Benth.) Govaerts | B | - | Yes | Z3 |
| Lepechinia meyenii (Walp.) Epling | H | - | No | Z3 | |
| Loasaceae | Caiophora cirsiifolia C. Presl | H | - | Yes | Z3 |
| Loasa sp. | H | - | - | Z3 | |
| Loranthaceae | Tristerix pubescens Kuijt | B | - | Yes | Z3 |
| Malvaceae | Malva sp. | H | - | - | Z3 |
| Melastomataceae | Brachyotum ledifolium (Desr.) Triana | B | - | No | Z3 |
| Myrtaceae | Eucalyptus globulus Labill. | T | LC | No | Z3 |
| Onagraceae | Oenothera rosea L’Hér. ex Aiton | H | - | No | Z3 |
| Oenothera laciniata Hill | H | - | No | Z3 | |
| Orchidaceae | Aa paleacea (Kunth) Rchb. f. | H | - | No | Z3 |
| Altensteinia fimbriata Kunth | H | - | No | Z3 | |
| Orobanchaceae | Castilleja sp. | H | - | - | Z1 |
| Neobartsia melampyroides (Kunth) Uribe-Convers & Tank | H | - | No | Z3 | |
| Oxalidaceae | Oxalis megalorrhiza Jacq. | H | - | No | Z3 |
| Passifloraceae | Passiflora mixta L. f. | Vi | - | No | Z3 |
| Passiflora trifoliata Cav. | Vi | - | No | Z3 | |
| Piperaceae | Peperomia microphylla Kunth | Suc | - | No | Z3 |
| Peperomia galioides Kunth | Suc | - | No | Z2 | |
| Plantaginaceae | Plantago australis Lam. | H | - | No | Z3 |
| Plantago lamprophylla Pilg. | H | - | No | Z3 | |
| Plantago sp. | H | - | - | Z3 | |
| Poaceae | Aciachne pulvinata Benth. | H | - | No | Z3 |
| Agrostis tolucensis Kunth | H | - | No | Z2, Z3 | |
| Bothriochloa sp. | H | - | - | Z3 | |
| Bromus catharticus Vahl | H | - | No | Z3 | |
| Bromus pitensis Kunth | H | - | No | Z2 | |
| Bromus sp. | H | - | - | Z3 | |
| Cinnagrostis heterophylla (Wedd.) P.M. Peterson, Soreng, Romasch. & Barberá | H | - | No | Z2 | |
| Cinnagrostis intermedia (J. Presl) P.M. Peterson, Soreng, Romasch. & Barberá | H | - | No | Z2 | |
| Cinnagrostis tarmensis (Pilg.) P.M. Peterson, Soreng, Romasch. & Barberá | H | - | No | Z1, Z2 | |
| Cinnagrostis vicunarum (Wedd.) P.M. Peterson, Soreng, Romasch. & Barberá | H | - | No | Z2 | |
| Festuca humilior Nees & Meyen | H | - | No | Z3 | |
| Festuca myuros L. | H | - | No | Z2 | |
| Jarava ichu Ruiz & Pav. | H | - | No | Z3 | |
| Muhlenbergia peruviana Meisn. | H | - | No | Z1 | |
| Poa annua L. | H | LC | No | Z3 | |
| Poa gilgiana Pilg. | H | - | No | Z3 | |
| Poa macusaniensis (E.H.L. Krause) Refulio | H | - | No | Z2 | |
| Polemoniaceae | Cantua buxifolia Juss. ex Lam. | B | LC/NT | No | Z3 |
| Polygalaceae | Monnina salicifolia Ruiz & Pav. | H | LC | Yes | Z3 |
| Polygonaceae | Muehlenbeckia volcanica (Benth.) Endl. | H | - | No | Z2, Z3 |
| Rumex crispus L. | H | - | No | Z3 | |
| Polygonaceae | Rumex obtusifolius L. | H | - | No | Z3 |
| Polypodiaceae | Campyloneurum angustifolium (Sw.) Fée | H | - | No | Z3 |
| Pleopeltis pycnocarpa (C. Chr.) A.R. Sm. | H | - | No | Z1 | |
| Polypodium sp. | H | - | - | Z2 | |
| Thelypteris sp. | H | - | - | Z2 | |
| Pteridaceae | Cheilanthes pruinata Kaulf. | H | - | No | Z2, Z3 |
| Cheilanthes pilosa Goldm. | H | - | No | Z2 | |
| Ranunculaceae | Ranunculus praemorsus Kunth ex DC. | H | - | No | Z3 |
| Rosaceae | Polylepis incana Kunth | T | LC/CR | No | Z3 |
| Polylepis weberbaueri Pilg. | T | NT/VU | No | Z1, Z2 | |
| Rubus sp. | B | - | - | Z3 | |
| Rubiaceae | Arcytophyllum sp. | H | - | - | Z3 |
| Galium hypocarpium (L.) Endl. ex Griseb. | H | - | No | Z3 | |
| Scrophulariaceae | Scrophulariaceae sp. | H | - | - | Z3 |
| Buddleja sp. | B | - | - | Z3 | |
| Solanaceae | Dunalia spinosa (Meyen) Dammer | B | - | No | Z3 |
| Solanum lanceolatum Cav. | H | LC | No | Z3 | |
| Solanum nitidum Ruiz & Pav. | H | LC | No | Z3 | |
| Solanum sp. | H | - | - | Z3 | |
| Urticaceae | Urtica magellanica Juss. ex Poir. | H | - | No | Z3 |
| Verbenaceae | Verbena litoralis Kunth | H | - | No | Z3 |
| Duranta sp. | B | - | - | Z3 | |
| Woodsiaceae | Woodsia sp. | H | - | - | Z2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinteros-Gómez, Y.; Macedo-Bedoya, J.; Anlas-Rosado, F.; Yangua-Evangelista, S.; Angeles-Alvarez, F.; Azurín-Sotelo, S.; La Rosa-Sánchez, M.; Gómez-Ticerán, D.; Jara-Peña, E.; Campos-De la Cruz, J.; et al. Floristic Composition and Species Conservation Status in Three Polylepis (Rosaceae) Relict Forests in Peru. Plants 2025, 14, 3537. https://doi.org/10.3390/plants14223537
Quinteros-Gómez Y, Macedo-Bedoya J, Anlas-Rosado F, Yangua-Evangelista S, Angeles-Alvarez F, Azurín-Sotelo S, La Rosa-Sánchez M, Gómez-Ticerán D, Jara-Peña E, Campos-De la Cruz J, et al. Floristic Composition and Species Conservation Status in Three Polylepis (Rosaceae) Relict Forests in Peru. Plants. 2025; 14(22):3537. https://doi.org/10.3390/plants14223537
Chicago/Turabian StyleQuinteros-Gómez, Yakov, Jehoshua Macedo-Bedoya, Flavia Anlas-Rosado, Sergio Yangua-Evangelista, Franco Angeles-Alvarez, Shirley Azurín-Sotelo, Marcel La Rosa-Sánchez, Doris Gómez-Ticerán, Enoc Jara-Peña, José Campos-De la Cruz, and et al. 2025. "Floristic Composition and Species Conservation Status in Three Polylepis (Rosaceae) Relict Forests in Peru" Plants 14, no. 22: 3537. https://doi.org/10.3390/plants14223537
APA StyleQuinteros-Gómez, Y., Macedo-Bedoya, J., Anlas-Rosado, F., Yangua-Evangelista, S., Angeles-Alvarez, F., Azurín-Sotelo, S., La Rosa-Sánchez, M., Gómez-Ticerán, D., Jara-Peña, E., Campos-De la Cruz, J., Padilla-Torres, B., & Fernández-De la Cruz, I. (2025). Floristic Composition and Species Conservation Status in Three Polylepis (Rosaceae) Relict Forests in Peru. Plants, 14(22), 3537. https://doi.org/10.3390/plants14223537






