Changes in Chlorophyll a Fluorescence in Ipomoea batatas (Convolvulaceae) Genotypes Under Attack by Bedellia somnulentella (Lepidoptera: Bedelliidae)
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Location and Conditions of the Experiments
4.2. Chlorophyll a Fluorescence
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sapakhova, Z.; Raissova, N.; Daurov, D.; Zhapar, K.; Daurova, A.; Zhigailov, A.; Zhambakin, K.; Shamekova, M. Sweet potato as a key crop for food security under the conditions of global climate change: A Review. Plants 2023, 12, 2516. [Google Scholar] [CrossRef]
- Shafiq, M.; Riaz, H.W.A.; Haider, M.S. Tuber crops: Sweet potatoes. In Vegetables, Tubers and Root Crops; Elsevier BV: Amsterdam, The Netherlands, 2024; pp. 537–543. [Google Scholar] [CrossRef]
- Echodu, R.; Odongo, B.; Mwanga, R.O.M.; Gibson, R.W.; Otieno, G. Farmers’ practices and their knowledge of biotic constraints to sweetpotato production in East Africa. Physiol. Mol. Plant Pathol. 2019, 105, 3–16. [Google Scholar] [CrossRef]
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Nascimento, S.; Silva, A.P.; Lima, V.R.; Oliveira, R.S. Carotene yield in sweet potato after potassium and phosphorus fertiliser application. Rev. Caatinga 2019, 32, 851–857. [Google Scholar] [CrossRef]
- Cartabiano-Leite, C.E.; Porcu, O.M.; de Casas, A.F. Sweet potato (Ipomoea batatas L. Lam) nutritional potential and social relevance: A review. History 2020, 11, 23–40. [Google Scholar] [CrossRef]
- Fleming, D.E.; Schiefer, T.L.; Bao, D. Insects associated with sweetpotato, Ipomoea batatas (L.), in Mississippi. Midsouth Entomol. 2009, 2, 11–16. [Google Scholar]
- Cabral, M.J.S.; Haseeb, M.; Soares, M.A. Major insect pests of sweet potatoes in Brazil and the United States, with information on crop production and regulatory pest management. Insects 2024, 15, 823. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.M.; Ferreira, E.A.; Nascimento, S.; Oliveira, A.P. Geographic distribution of the Ipomoea batatas (Convolvulaceae) pest, Bedellia somnulentella (Zeller) (Lepidoptera: Bedelliidae), in Minas Gerais state, Brazil. J. Plant Dis. Prot. 2021, 128, 617–621. [Google Scholar] [CrossRef]
- Santos, M.M.; Ferreira, E.A.; Oliveira, A.P.; Soares, M.A. First record of the sweet potato pest Bedellia somnulentella (Lepidoptera: Bedelliidae) in Brazil. Fla. Entomol. 2018, 101, 315–316. [Google Scholar] [CrossRef]
- Santadino, M.V.; Brentassi, M.E.; Fanello, D.D.; Coviella, C.E. First evidence of Thaumastocoris peregrinus (Heteroptera: Thaumastocoridae) feeding from mesophyll of Eucalyptus leaves. Environ. Entomol. 2017, 46, 251–257. [Google Scholar] [CrossRef]
- Souza, M.W.R.; Ferreira, E.A.; Santos, J.B.; Soares, M.A.; Castro, B.M.; Zanuncio, J.C. Fluorescence of chlorophyll a in transgenic maize with herbicide application and attacked by Spodoptera frugiperda (Lepidoptera: Noctuidae). Phytoparasitica 2020, 48, 567–573. [Google Scholar] [CrossRef]
- Sinclair, R.J.; Hughes, L. Leaf miners: The hidden herbivores. Austral Ecol. 2010, 35, 300–313. [Google Scholar] [CrossRef]
- Gonda-King, L.; Gómez, S.; Martin, J.L.; Orians, C.M.; Preisser, E.L. Tree responses to an invasive sap-feeding insect. Plant Ecol. 2014, 215, 297–304. [Google Scholar] [CrossRef]
- Li, Q.; Tan, W.; Xue, M.; Zhao, H. Dynamic changes in energy metabolism and electron transport of photosystem II in Nicotiana tabacum infested by nymphs of Bemisia tabaci (Middle East-Asia Minor 1). Arthropod-Plant Interact. 2018, 12, 505–515. [Google Scholar] [CrossRef]
- Wagner, D.L.; Defoliart, L.S.; Doak, P.; Schneiderheinze, J. Impact of epidermal leaf mining by the aspen leaf miner (Phyllocnistis populiella) on the growth, physiology, and leaf longevity of quaking aspen. Oecologia 2008, 157, 259–267. [Google Scholar] [CrossRef]
- Warabieda, W.; Borkowska, B. Chlorophyll α fluorescence as a diagnostic tool for assessment of apple resistance against two-spotted spider mite (Tetranychus urticae Koch). Electron. J. Polish Agric. Univ. Hortic. 2004, 7, 1. [Google Scholar]
- Gutsche, A.R.; Heng-Moss, T.M.; Higley, L.G.; Sarath, G.; Mornhinweg, D.W. Physiological responses of resistant and susceptible barley, Hordeum vulgare, to the Russian wheat aphid, Diurpahis noxia (Mordvilko). Arthropod-Plant Interact. 2009, 3, 233–240. [Google Scholar] [CrossRef]
- Gantner, M.; Michałek, W. Measurements of chlorophyll fluorescence as an auxiliary method in estimating susceptibility of cultivated hazel (Corylus L.) for filbert aphid (Myzocallis coryli Goetze). Acta Agrobot. 2010, 63, 189–195. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed]
- Schansker, G.; Tóth, S.Z.; Holzwarth, A.R.; Garab, G. Chlorophyll a fluorescence: Beyond the limits of the QA model. Photosynth. Res. 2014, 120, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Henriques, F.S. Leaf chlorophyll fluorescence: Background and fundamentals for plant biologists. Bot. Rev. 2009, 75, 249–270. [Google Scholar] [CrossRef]
- da Cruz, M.D.C.M.; de Siqueira, D.L.; Salomão, L.C.C.; Cecon, P.R. Fluorescência da clorofila a em folhas de tangerineira ‘Ponkan’ e limeira ácida ‘Tahiti’ submetidas ao estresse hídrico. Rev. Bras. Frutic. 2009, 31, 896–901. [Google Scholar] [CrossRef]
- Malavasi, U.C.; Malavasi, M.M. Quantifying abiotic stress of plants—Advantages and disadvantages of chlorophyll fluorescence. Ambiência Guarapuava 2013, 9, 421–432. [Google Scholar] [CrossRef]
- Chang, R.K.; Wang, Y.H.; Zhang, X.T.; Tang, G.C.; Wei, Y. The research of disease detection method of greenhouse cucumber leaf based on chlorophyll fluorescence analysis. Univers. J. Agric. Res. 2015, 3, 76–80. [Google Scholar] [CrossRef]
- Kmieć, K.; Rubinowska, K.; Michałek, W.; Sytykiewicz, H. The effect of galling aphids feeding on photosynthesis photochemistry of elm trees (Ulmus sp.). Photosynthetica 2018, 56, 989–997. [Google Scholar] [CrossRef]
- Neravathu, R. Feeding impact of Cisaberoptus kenyae Keifer (Acari: Eriophyidae) on photosynthetic efficiency and biochemical parameters of Mangifera indica L. Acarol. Stud. 2019, 1, 84–94. [Google Scholar]
- Zhang, B.; Zhou, L.; Zhou, X.; Bai, Y.; Zhan, M.; Chen, J.; Xu, C. Differential responses of leaf photosynthesis to insect and pathogen outbreaks: A global synthesis. Sci. Total Environ. 2022, 832, 155052. [Google Scholar] [CrossRef]
- Bueno, A.F.; Zechmann, B.J.; Hoback, W.W.; de Freitas Bueno, R.C.O.; Fernandes, O.A. Serpentine leafminer (Liriomyza trifolii) on potato (Solanum tuberosum): Field observations and plant photosynthetic responses to injury. Ciência Rural. 2007, 37, 1510–1517. [Google Scholar] [CrossRef]
- Pincebourde, S.; Frak, E.; Sinoquet, H.; Regnard, J.L.; Casas, J. Herbivory mitigation through increased water-use efficiency in a leaf-mining moth–apple tree relationship. Plant Cell Environ. 2006, 29, 2238–2247. [Google Scholar] [CrossRef]
- Golan, K.; Rubinowska, K.; Kmieć, K.; Kot, I.; Górska-Drabik, E.; Lagowska, B.; Michałek, W. Impact of scale insect infestation on the content of photosynthetic pigments and chlorophyll fluorescence in two host plant species. Arthropod-Plant Interact. 2015, 9, 55–65. [Google Scholar] [CrossRef]
- Ullah, M.I.; Arshad, M.; Ali, S.; Mehmood, N.; Khalid, S.; Afzal, M. Physiological characteristics of citrus plants infested with citrus leafminer, Phyllocnistis citrella (Lepidoptera: Gracillariidae). Int. J. Fruit. Sci. 2020, 20, S871–S883. [Google Scholar] [CrossRef]
- Du, J.; Wu, D.; Li, J.; Zhan, Q.; Huang, S.-C.; Huang, B.; Wang, X. Effects of aphid disoperation on photosynthetic performance and agronomic traits of different sorghum varieties. Pak. J. Bot. 2021, 53, 2275–2285. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Bounfour, M.; Tanigoshi, L.K.; Chen, C.; Cameron, S.J.; Klauer, S. Chlorophyll content and chlorophyll fluorescence in red raspberry leaves infested with Tetranychus urticae and Eotetranychus carpini borealis (Acari: Tetranychidae). Environ. Entomol. 2002, 31, 215–220. [Google Scholar] [CrossRef]
- Simpson, K.L.S.; Jackson, G.E.; Grace, J. The response of aphids to plant water stress—The case of Myzus persicae and Brassica oleracea var. capitata. Entomol. Exp. Appl. 2012, 142, 191–202. [Google Scholar] [CrossRef]
- Bolhar-Nordenkampf, H.R.; Long, S.P.; Baker, N.R.; Oquist, G.; Schreiber, U.; Lechner, E.G. Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: A review of current instrumentation. Funct. Ecol. 1989, 3, 497–514. [Google Scholar] [CrossRef]
- Barbagallo, R.P.; Oxborough, K.; Pallett, K.E.; Baker, N.R. Rapid, noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol. 2003, 132, 485–493. [Google Scholar] [CrossRef]
- Velikova, V.; Salerno, G.; Frati, F.; Peri, E.; Conti, E.; Colazza, S.; Loreto, F. Influence of feeding and oviposition by phytophagous pentatomids on photosynthesis of herbaceous plants. J. Chem. Ecol. 2010, 36, 629–641. [Google Scholar] [CrossRef]
- Ferreira, M.A.M.; Andrade, V.C.; Oliveira, A.J.; Ferreira, E.A.; Brito, O.G.; Silva, L.R. Physiological characterization of plant growth in sweet potato. Hortic. Bras. 2019, 37, 112–118. [Google Scholar] [CrossRef]
- Gilbert, M.; Grégoire, J.C. Visual, semi-quantitative assessments allow accurate estimates of leafminer population densities: An example comparing image processing and visual evaluation of damage by the horse chestnut leafminer Cameraria ohridella (Lep., Gracillariidae). J. Appl. Entomol. 2003, 127, 354–359. [Google Scholar] [CrossRef]
- Kasajima, I.; Suetsugu, N.; Wada, M.; Takahara, K. Collective calculation of actual values of non-photochemical quenching from their apparent values after chloroplast movement and photoinhibition. Am. J. Plant Sci. 2015, 6, 1792–1805. [Google Scholar] [CrossRef]
- Cantarutti, R.B.; Barros, N.D.; Martinez, H.E.P.; Novais, R.F. Avaliação da fertilidade do solo e recomendação de fertilizantes. In Fertilidade do Solo, 2nd ed.; Novais, R.F., Ed.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brasil, 2007; pp. 769–850. [Google Scholar]
- SAEG: Sistema para Análises Estatísticas e Genéticas; Versão 9.1; UFV: Viçosa, Brasil, 2007.
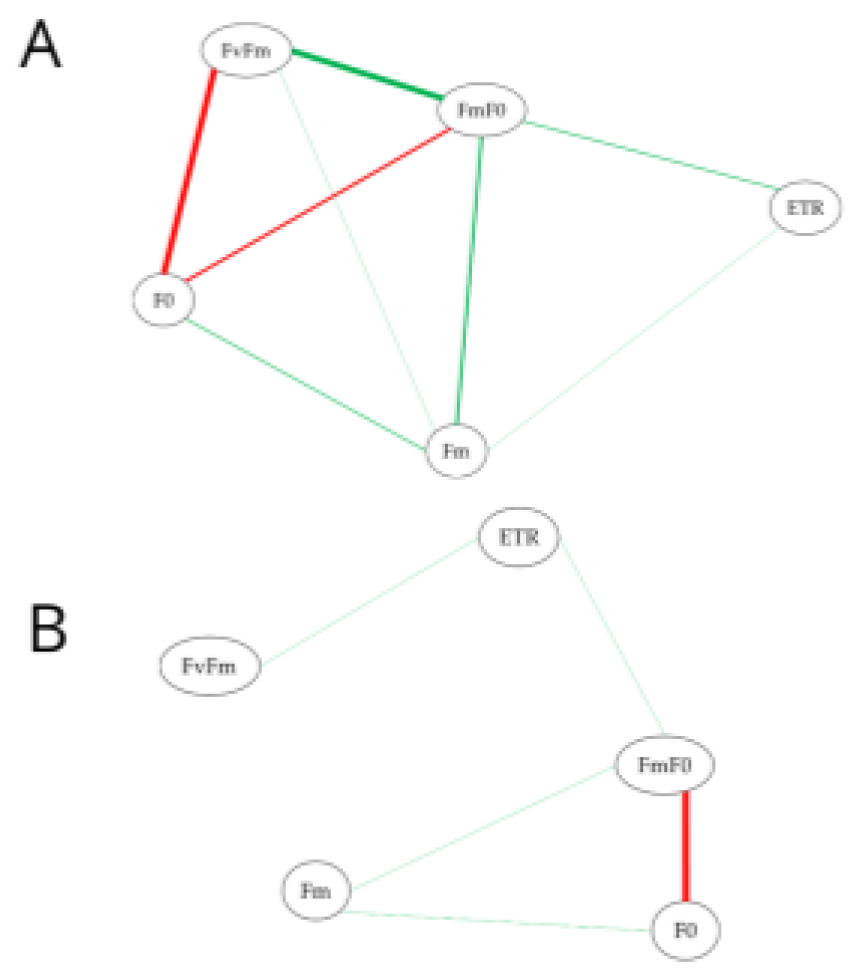
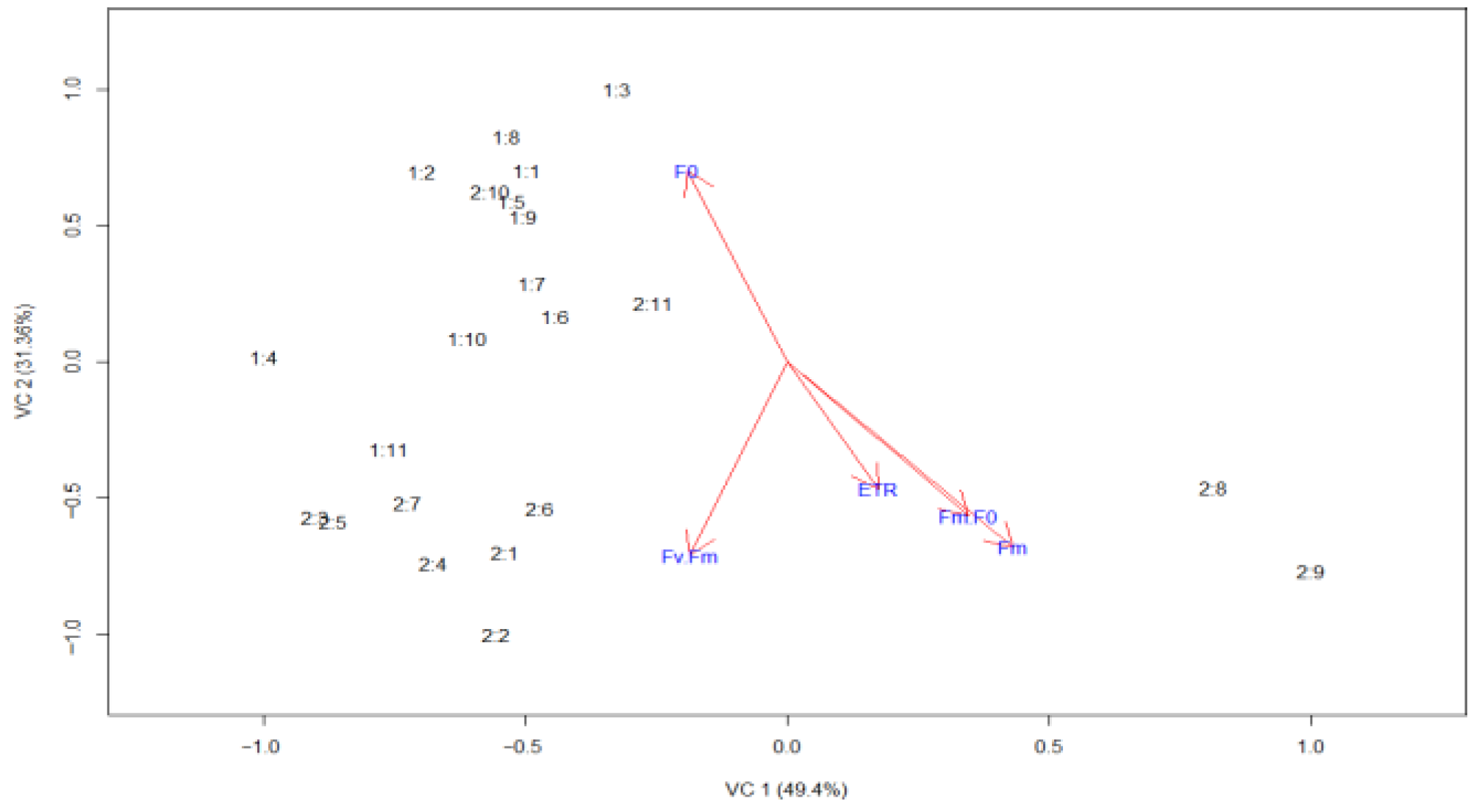

| Genotypes | Par. | Not Attacked | Attacked | CV(%) S | CV(%) C |
|---|---|---|---|---|---|
| UFVJM 01 | F0 | 39.20 ± 1.60 a | 23.90 ± 1.53 b | 12.88 | 20.25 |
| Fm | 93.50 ± 2.21 a | 68.50 ± 2.53 a | 31.16 | 11.70 | |
| Fm/F0 | 3.91 ± 0.42 b | 1.7 ± 0.82 a | 12.81 | 18. 23 | |
| Fv/Fm | 0.66 ± 0.03 a | 0.43 ± 0.02 b | 15.15 | 15.84 | |
| ETR | 20.84 ± 1.11 a | 18.66 ± 0.88 a | 16.95 | 15.03 | |
| UFVJM 02 | F0 | 41.90 ± 2.85 a | 25.60 ± 2.01 b | 21.51 | 24.86 |
| Fm | 109.30 ± 9.74 a | 76.20 ± 4.72 a | 28.18 | 19.60 | |
| Fm/F0 | 4.26 ± 0.52 b | 1.81 ± 0.78 a | 11.21 | 13.09 | |
| Fv/Fm | 0.72 ± 0.02 a | 0.45 ± 0.02 b | 10.12 | 18.66 | |
| ETR | 23.29 ± 1.31 a | 15.14 ± 1.12 b | 17.86 | 23.46 | |
| UFMG 03 | F0 | 42.10 ± 8.85 a | 22.00 ± 1.97 b | 21.43 | 28.35 |
| Fm | 93.30 ± 5.68 a | 66.3 ± 3.36 a | 19.26 | 16.06 | |
| Fm/F0 | 4.24 ± 0.54 b | 1.57 ± 0.62 a | 13.7 | 29.49 | |
| Fv/Fm | 0.72 ± 0.02 a | 0.35 ± 0.04 b | 12.64 | 38.52 | |
| ETR | 20.32 ± 0.84 a | 14.94 ± 0.81 a | 13.18 | 17.21 | |
| UFVJM 04 | F0 | 35.60 ± 1.84 a | 21.60 ± 1.10 b | 26.34 | 29.13 |
| Fm | 111.40 ± 3.33 a | 69.90 ± 2.97 b | 9.47 | 13.46 | |
| Fm/F0 | 5.15 ± 0.11 b | 1.96 ± 0.38 a | 13.78 | 19.38 | |
| Fv/Fm | 0.75 ± 0.03 a | 0.51 ± 0.02 b | 13.40 | 16.15 | |
| ETR | 19.38 ± 1.24 a | 13.37 ± 0.99 a | 20.26 | 23.54 | |
| UFVJM 08 | F0 | 38.10 ± 3.53 a | 23.90 ± 2.21 a | 29.37 | 29.29 |
| Fm | 108.60 ± 8.92 a | 82.10 ± 5.51 a | 25.99 | 21.26 | |
| Fm/F0 | 4.54 ± 0.65 b | 2.15 ± 0.73 a | 18.91 | 23.95 | |
| Fv/Fm | 0.73 ± 0.06 a | 0.53 ± 0.04 a | 28.63 | 27.52 | |
| ETR | 21.01 ± 1.72 a | 18.37 ± 0.86 a | 25.92 | 14.91 | |
| UFVJM 18 | F0 | 34.70 ± 2.78 a | 21.40 ± 2.05 a | 25.27 | 30.30 |
| Fm | 91.0 ± 5.85 a | 59.40 ± 3.08 b | 20.34 | 16.44 | |
| Fm/F0 | 4.25 ± 0.42 b | 1.71 ± 0.65 a | 13.05 | 18.01 | |
| Fv/Fm | 0.64 ± 0.03 a | 0.40 ± 0.05 a | 16.69 | 38.99 | |
| ETR | 17.75 ± 1.14 a | 13.96 ± 0.49 a | 20.42 | 11.13 | |
| UFVJM 91 | F0 | 36.80 ± 2.81 a | 20.90 ± 1.88 b | 24.16 | 28.57 |
| Fm | 90.10 ± 10.87 a | 68.0 ± 5.99 a | 38.18 | 27.82 | |
| Fm/F0 | 4.31 ± 0.54 b | 1.85 ± 0.73 a | 19.79 | 19.45 | |
| Fv/Fm | 0.68 ± 0.03 a | 0.43 ± 0.03 b | 13.96 | 26.62 | |
| ETR | 19.43 ± 0.83 a | 16.35 ± 1.27 a | 13.64 | 24.69 | |
| UFVJM 291 | F0 | 41.3 ± 2.53 a | 24.90 ± 3.94 a | 19.44 | 50.14 |
| Fm | 110.20 ± 6.10 a | 70.60 ± 2.62 b | 17.52 | 11.78 | |
| Fm/F0 | 4.43 ± 0.64 b | 1.71 ± 0.69 a | 17.34 | 21.35 | |
| Fv/Fm | 0.51 ± 0.01 a | 0.41 ± 0.02 a | 11.70 | 20.49 | |
| ETR | 20.20 ± 1.59 a | 16.26 ± 0.98 a | 24.93 | 19.23 | |
| UFVJM 526 | F0 | 39.50 ± 2.23 a | 21.30 ± 2.51 b | 17.87 | 37.37 |
| Fm | 119.70 ± 4.10 a | 71.30 ± 4.35 b | 13.20 | 19.30 | |
| Fm/F0 | 5.62 ± 1.02 b | 1.81 ± 7.38 a | 21.67 | 36.42 | |
| Fv/Fm | 0.55 ± 0.02 a | 0.42 ± 0.03 a | 11.60 | 27.01 | |
| ETR | 19.68 ± 0.63 a | 15.31 ± 0.69 b | 10.87 | 14,42 | |
| Brazlândia branca | F0 | 34.10 ± 1.10 a | 17.60 ± 2.33 b | 18.52 | 42.03 |
| Fm | 95.50 ± 7.81 a | 66.90 ± 4.59 a | 25.87 | 21.72 | |
| Fm/F0 | 5.43 ± 0.67 b | 1.96 ± 0.91 a | 20.87 | 30.35 | |
| Fv/Fm | 0.69 ± 0.03 a | 0.48 ± 0.03 a | 17.25 | 20.33 | |
| ETR | 23.79 ± 1.28 a | 17.93 ± 0.64 a | 17.07 | 11.35 | |
| Rubissol | F0 | 30.40 ± 1.67 a | 16.10 ± 1.51 b | 17.28 | 29.78 |
| Fm | 100.60 ± 6.91 a | 69.30 ± 2.09 a | 21.74 | 9.57 | |
| Fm/F0 | 6.25 ± 0.56 b | 2.28 ± 0.78 a | 10.99 | 34.21 | |
| Fv/Fm | 0.69 ± 0.02 a | 0.52 ± 0.02 a | 10.00 | 16.83 | |
| ETR | 22.35 ± 1.48 a | 17.14 ± 0.72 a | 21.07 | 13.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, M.J.S.; Pinheiro, R.A.; Silva, I.M.; Ngamgna, W.S.B.; Schmiele, M.; Leite, G.L.D.; Haseeb, M.; Soares, M.A. Changes in Chlorophyll a Fluorescence in Ipomoea batatas (Convolvulaceae) Genotypes Under Attack by Bedellia somnulentella (Lepidoptera: Bedelliidae). Plants 2025, 14, 3529. https://doi.org/10.3390/plants14223529
Cabral MJS, Pinheiro RA, Silva IM, Ngamgna WSB, Schmiele M, Leite GLD, Haseeb M, Soares MA. Changes in Chlorophyll a Fluorescence in Ipomoea batatas (Convolvulaceae) Genotypes Under Attack by Bedellia somnulentella (Lepidoptera: Bedelliidae). Plants. 2025; 14(22):3529. https://doi.org/10.3390/plants14223529
Chicago/Turabian StyleCabral, Maria J. S., Rodrigo A. Pinheiro, Isabel M. Silva, William S. B. Ngamgna, Marcio Schmiele, Germano L. Demolin Leite, Muhammad Haseeb, and Marcus A. Soares. 2025. "Changes in Chlorophyll a Fluorescence in Ipomoea batatas (Convolvulaceae) Genotypes Under Attack by Bedellia somnulentella (Lepidoptera: Bedelliidae)" Plants 14, no. 22: 3529. https://doi.org/10.3390/plants14223529
APA StyleCabral, M. J. S., Pinheiro, R. A., Silva, I. M., Ngamgna, W. S. B., Schmiele, M., Leite, G. L. D., Haseeb, M., & Soares, M. A. (2025). Changes in Chlorophyll a Fluorescence in Ipomoea batatas (Convolvulaceae) Genotypes Under Attack by Bedellia somnulentella (Lepidoptera: Bedelliidae). Plants, 14(22), 3529. https://doi.org/10.3390/plants14223529








