Foliar Application of Iron Nanoparticles Improves Chinese Cabbage Growth
Abstract
1. Introduction
2. Results
2.1. Characterization of Two Kinds of Iron−Based Nanomaterials
2.2. Effects of Different Iron−Based Nanomaterials on the Growth Indices of Chinese Cabbage
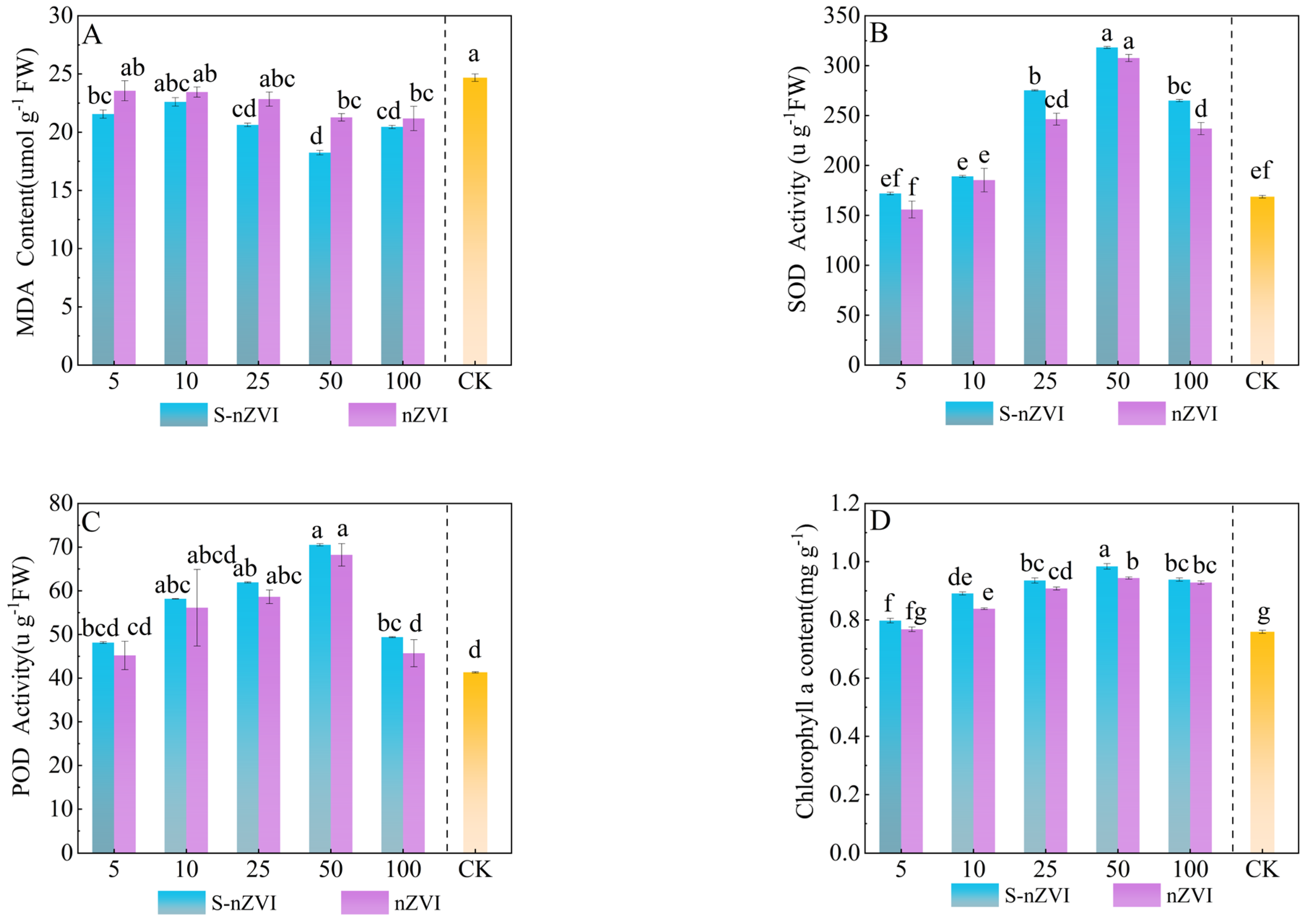
2.3. Effects of Different Iron−Based Nanomaterials on Enzyme Activity and Content
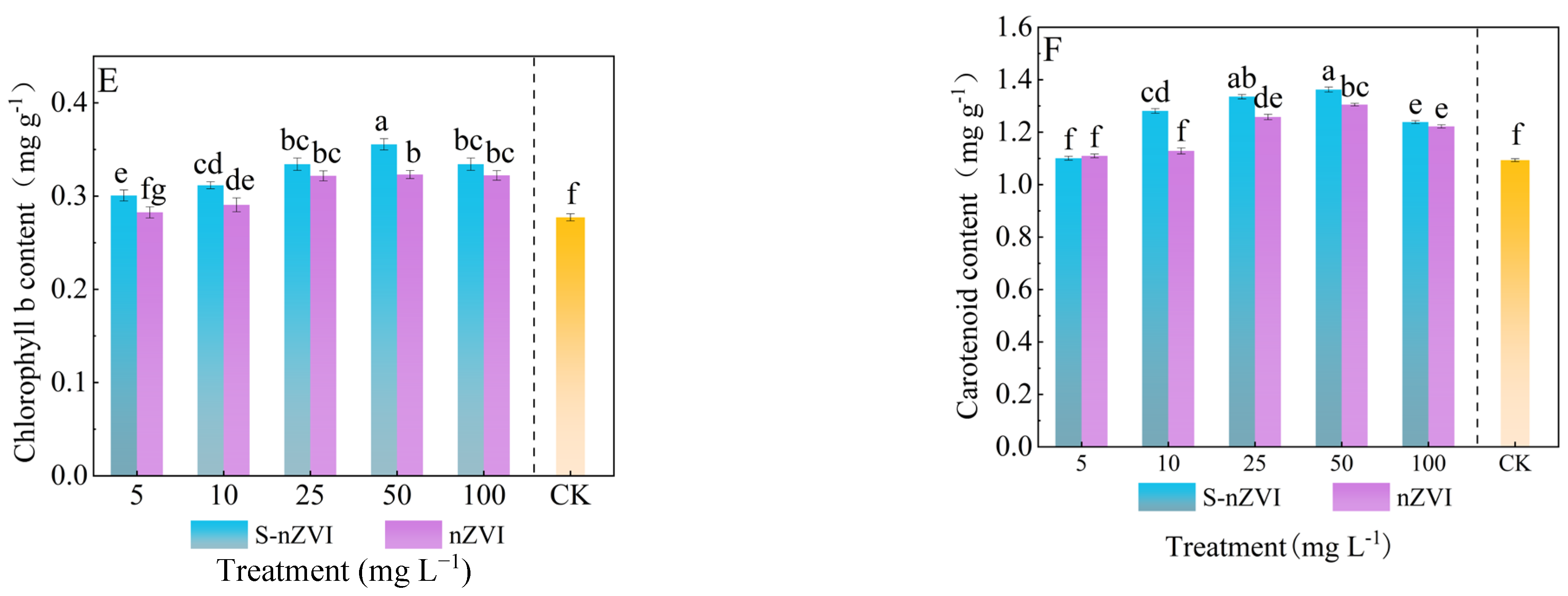
2.4. Effects of Different Iron−Based Nanomaterials on Photosynthetic Pigments and Parameters
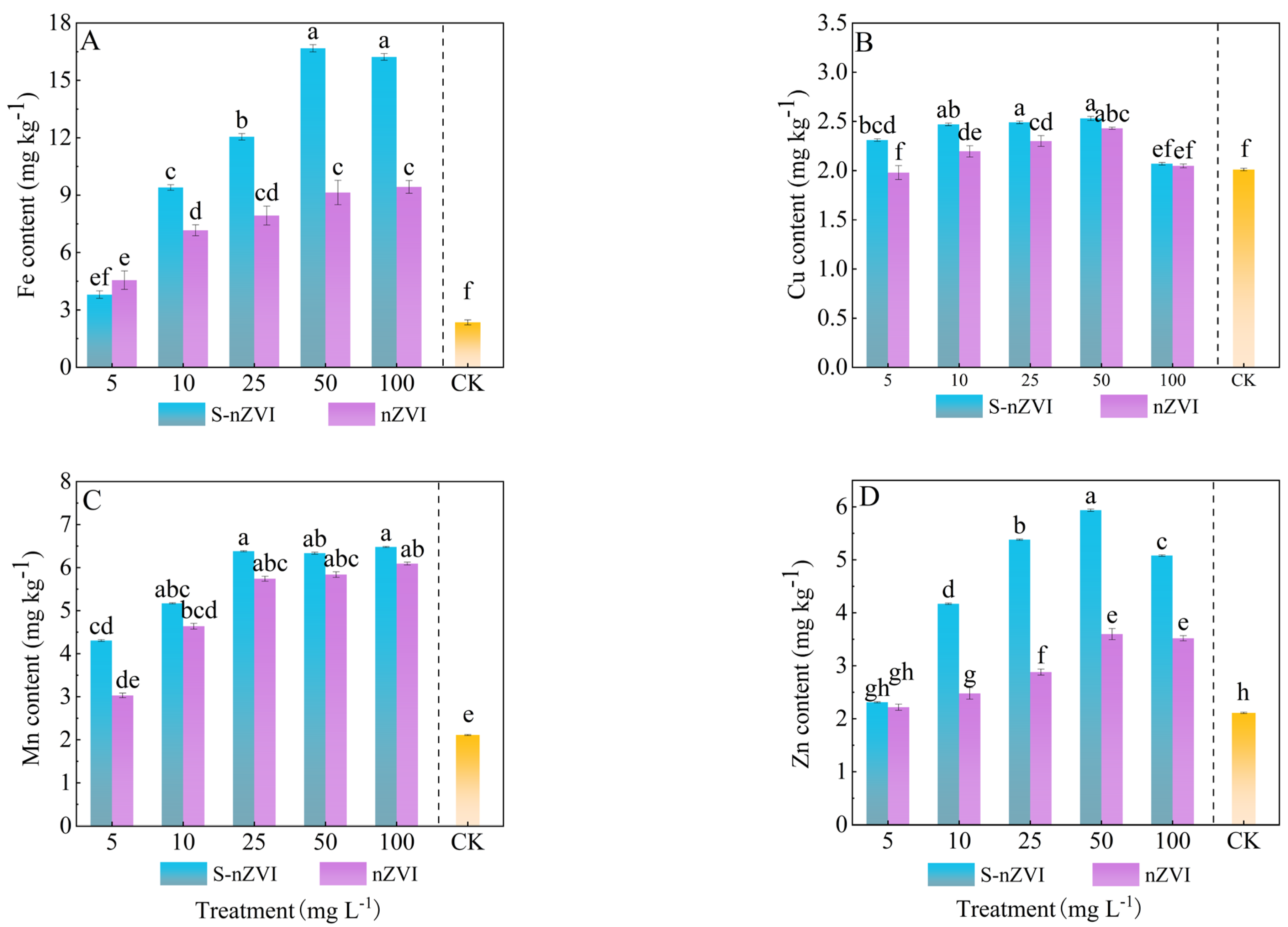
2.5. Effects of Different Iron−Based Nanomaterials on Trace Elements
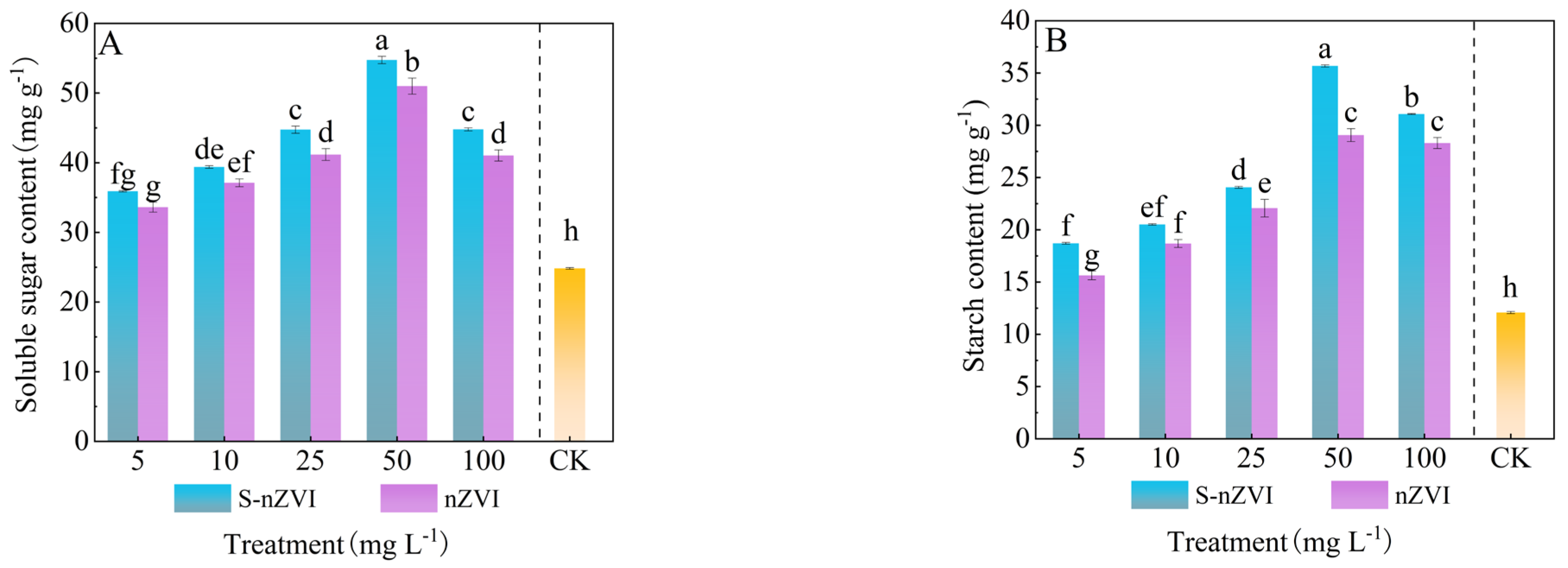
2.6. Effects of Different Concentrations of S−nZVI on Starch and Soluble Sugar Content
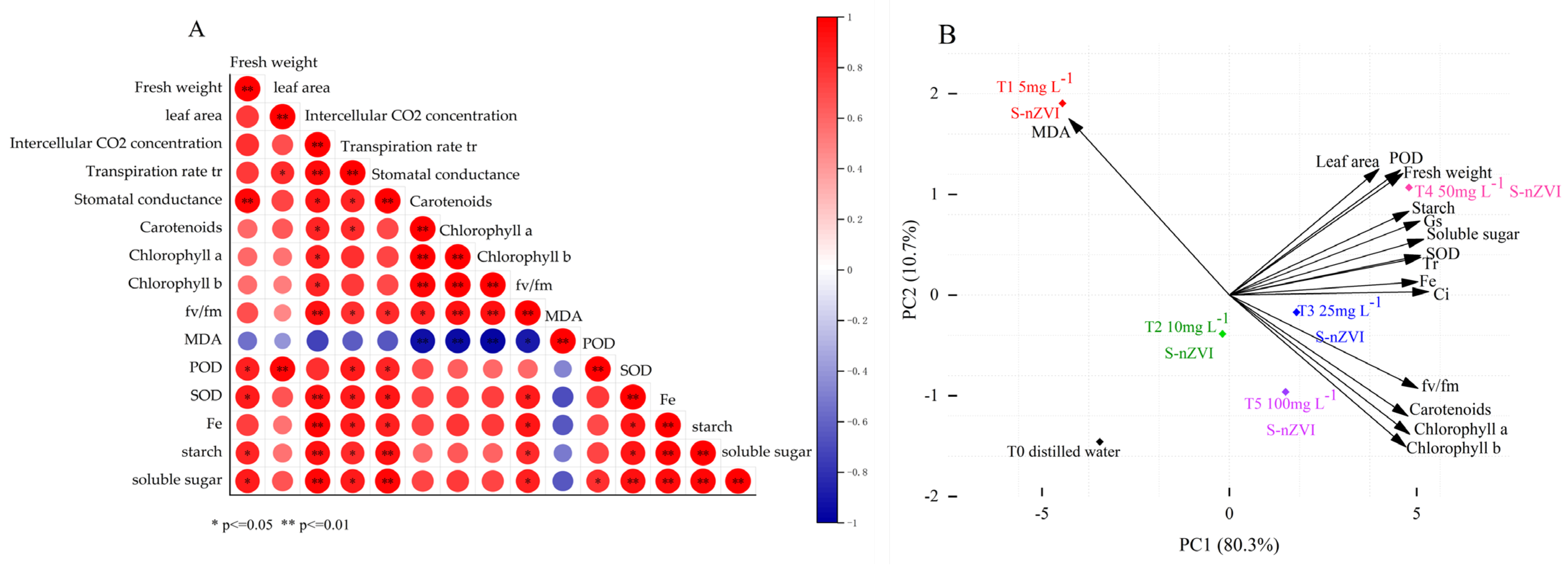
2.7. PCA and Correlation Analysis
3. Discussion
3.1. Effects of Different Iron−Based Nanomaterials on Growth and Photosynthetic Characteristics of Chinese Cabbage
3.2. Effects of Different Iron−Based Nanomaterials on the Physiological and Antioxidant Properties of Chinese Cabbage
4. Materials and Methods
4.1. Preparation of Experimental Materials and Sulfide Nano Zero−Valent Iron
4.2. Characterization of nZVI and S−nZVI and Preparation of Their Suspensions
4.3. Experimental Design
4.4. Analytical Methods
4.4.1. Plant Morphological Index Measurement for Chinese Cabbage
4.4.2. Measurement of Photosynthetic Pigments and Fluorescence Parameters
4.4.3. Gas Exchange Parameters for Chinese Cabbage Leaves
4.4.4. Measurement of Nutrient Element in Chinese Cabbage Leaves
4.4.5. Measurement of Enzyme Activity and Content
4.4.6. Measurement of Starch and Soluble Sugar Content
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Zhu, H.; Lan, P. Research Progress of Iron Homeostasis Regulation in Strategy I Plants. Soils 2021, 53, 1101–1106. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Li, H.; Qu, X.; Yang, S.; Chang, X. Bioaccumulation and Toxicity of13 C-Skeleton Labeled Graphene Oxide in Wheat. Environ. Sci. Technol. 2017, 51, 10146–10153. [Google Scholar] [CrossRef] [PubMed]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and Threats of Contamination on Aquatic Ecosystems; Qadri, H., Bhat, R.A., Hakeem, K.R., Eds.; Springer International Publishing AG: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Hakeem, R.; Rehman, R.U. Lead toxicity alters the antioxidant defense machinery and modulate the biomarkers in Tartary buckwheat plants. Int. Biodeterior. Biodegrad. 2020, 151, 104992. [Google Scholar] [CrossRef]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil-microorganism-plant system: Their role in iron availability. Eur. J. Soil. Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- De Souza, A.; Govea-Alcaide, E.; Masunaga, S.H.; Fajardo-Rosabal, L.; Effenberger, F.; Rossi, L.M.; Jardim, R.F. Impact of Fe3O4 nanoparticle on nutrient accumulation in common bean plants grown in soil. SN Appl. Sci. 2019, 1, 308. [Google Scholar] [CrossRef]
- Tan, H.L.; Lim, Y.C.; Ng, L.Y.; Lim, Y.P. Plant-mediated synthesis of iron nanoparticles for environmental application: Mini review. Mater. Today Proc. 2023, 87, 6. [Google Scholar] [CrossRef]
- Kornarzyński, K.; Sujak, A.; Czernel, G.; Wicek, D. Effect of Fe3O4 nanoparticles on germination of seeds and concentration of elements in Helianthus annuus L. under constant magnetic field. Sci. Rep. 2020, 10, 8068. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Luo, X.; Feng, Y. Effects of nano-Fe3O4 on lettuce growth and soil bacterial community structure. J. Appl. Ecol. 2017, 28, 3003–3010. [Google Scholar]
- Li, M.; Adeel, M.; Peng, Z.; Yukui, R. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2020, 269, 116134. [Google Scholar] [CrossRef]
- Yoon, H.; Kang, Y.; Chang, Y.; Kim, J. Effects of Zerovalent Iron Nanoparticles on Photosynthesis and Biochemical Adaptation of Soil-Grown Arabidopsis thaliana. Nanomaterials 2019, 9, 1543. [Google Scholar] [CrossRef]
- Ghost, I.; Mukherjee, A.; Mukherjee, A. In planta genotoxicity of nZVI: Influence of colloidal stability on uptake, DNA damage, oxidative stress and cell death. Mutagenesis 2017, 32, 371–387. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Zhang, Y.; Spielman-Sun, E.; Unrine, J.M.; Thieme, J.; Li, J.; Lombi, E.; Bland, G.; Lowry, G.V. Nanoparticle Size and Coating Chemistry Control Foliar Uptake Pathways, Translocation, and Leaf-to-Rhizosphere Transport in Wheat. Acs Nano 2019, 13, 5291–5305. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yue, L.; Wang, C.; Luo, X.; Zhang, C.; Zhao, X.; Wu, F.; White, J.C.; Wang, Z.; Xing, B. Foliar Application with Iron Oxide Nanomaterials Stimulate Nitrogen Fixation, Yield, and Nutritional Quality of Soybean. Acs Nano 2022, 16, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Z. Recent advances in nano-enabled agriculture for improving plant performance. Crop J. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Qin, X. Study on the Effect and Impact of Nano-Zero-Valent Iron-Enhanced Ryegrass on Remediation of Lead-Contaminated Sediment. Master’s Thesis, Hunan University, Changsha, China, 2018. [Google Scholar]
- Yang, Y. Effects and Mechanisms of Coupling of Tufted Mycorrhizal Fungi with Nano Zero-Valent Iron on Inter-Root Processes and Yield Formation in Dryland Maize (Zea mays). Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2023. [Google Scholar]
- Du, J.; Liu, B.; Zhao, T.; Xu, X.; Lin, H.; Ji, Y.; Li, Y.; Li, Z.; Lu, C.; Li, P.; et al. Silica nanoparticles protect rice against biotic and abiotic stresses. J. Nanobiotechnology 2022, 20, 197. [Google Scholar] [CrossRef]
- Masoumi, Z.; Haghighi, M.; Mozafarian, M. Effects of foliar spraying with melatonin and chitosan Nano-encapsulated melatonin on tomato (Lycopersicon esculentum L. cv. Falcato) plants under salinity stress. BMC Plant Biol. 2024, 24, 961. [Google Scholar] [CrossRef]
- Sepehrzadegan, Z.; Alizadeh, O. Investigation of the growth bacteria and Nano iron on the chlorophyll and some nutrients triticale. Rev. Agrogeoambiental 2021, 13, 97–106. [Google Scholar] [CrossRef]
- Li, H.F.; Wang, Y.; Yuan, Q.H.; Zhao, G.Q. The Impacts of Lead Stress on the Growth of Forage Grasses and Their Enzyme Activities. Seed 2014, 33, 1–7. [Google Scholar]
- Labille, J.; Catalano, R.; Slomberg, D.; Motellier, S.; Pinsino, A.; Hennebert, P.; Santaella, C.; Bartolomei, V. Assessing Sunscreen Lifecycle to Minimize Environmental Risk Posed by Nanoparticulate UV-Filters—A Review for Safer-by-Design Products. Front. Environ. Sci. 2020, 8, 101, Correction in Front. Environ. Sci. 2020, 8, 597861. [Google Scholar]
- Shi, J.; Liu, Y.; Wang, Y.; Yang, Z. Effects of Nitrogen Application on Fluorescence Characteristics of Cucumber in Fruit Growth Stage under High Temperature Stress. Acta Agric. Boreali-Sin. 2022, 37, 84–95. [Google Scholar]
- Abbas, J.; Mehrnaz, H. Foliar-applied nanoscale zero-valent iron (nZVI) and iron oxide (Fe3O4) induce differential responses in growth, physiology, antioxidative defense and biochemical indices in Leonurus cardiaca L. Environ. Res. 2022, 215, 114254. [Google Scholar]
- Xingli, X.U.; Zexin, J.; Weiming, H.E.; Xinglong, W.; Xiuxia, C. Effects of different day/night warming on the photosynthetic characteristics and chlorophyll fluorescence parameters of Sinocalycanthus chinensis seedlings. Ecol. Lett. 2012, 32, 6343–6353. [Google Scholar]
- Yangyang, M.A.; Chenchi, Z.; Xuesong, C.; Zhenyu, W. Mechanistic study on the effect of foliar-applied, iron-based nanomaterials on the growth of soybean. J. Agric. Resour. Environ. 2022, 39, 139–148. [Google Scholar]
- Qi, X.Y.; Wang, W.L.; Hu, S.Q.; Liu, M.Y.; Zheng, C.S.; Sun, X.Z. Effects of exogenous melatonin on photosynthesis and physiological characteristics of chry-santhemum seedlings under high temperature stress. J. Appl. Ecol. 2021, 32, 2496–2504. [Google Scholar]
- Haslett, B.S.; Reid, R.J.; Rengel, Z. Zinc Mobility in Wheat: Uptake and Distribution of Zinc Applied to Leaves or Roots. Ann. Bot. 2001, 87, 379–386. [Google Scholar] [CrossRef]
- Yang, B.; Xiong, Z.; Wang, J.; Xu, X.; Huang, Q.; Shen, Q. Mitigating net global warming potential and greenhouse gas intensities by substituting chemical nitrogen fertilizers with organic fertilization strategies in rice–wheat annual rotation systems in China: A 3-year field experiment. Ecol. Eng. 2015, 81, 289–297. [Google Scholar] [CrossRef]
- Tai, F.; Wang, S.; Liang, B.; Li, Y.; Wu, J.; Fan, C.; Hu, X.; Wang, H.; He, R.; Wang, W. Quaternary ammonium iminofullerenes improve root growth of oxidative-stress maize through ASA-GSH cycle modulating redox homeostasis of roots and ROS-mediated root-hair elongation. J. Nanobiotechnology 2022, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiao, X.; Song, X.; Ding, J.; Bai, P.; Li, J. Effect of VPD and potassium interactions on nutrient uptake and photosynthetic properties of tomato under high temperature. Agric. Res. Arid Areas 2020, 38, 30–38. [Google Scholar]
- Ghafariyan, M.H.; Malakouti, M.J.; Dadpour, M.R.; Stroeve, P.; Mahmoudi, M. Effects of magnetite nanoparticles on soybean chlorophyll. Environ. Sci. Technol. 2013, 47, 10645–10652. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses—A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef]
- Lu, A.; Li, Y.; Ding, H.; Xu, X.; Li, Y.; Ren, G.; Liang, J.; Liu, Y.; Hong, H.; Chen, N.; et al. Photoelectric conversion on Earth’s surface via widespread Fe- and Mn-mineral coatings. Proc. Natl. Acad. Sci. USA 2019, 116, 9741–9746. [Google Scholar] [CrossRef]
- Ma, X.; Gurung, A.; Deng, Y. Phytotoxicity and uptake of nanoscale zero-valent iron (nZVI) by two plant species. Sci. Total Environ. 2013, 443, 844–849. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Chang, D.; Lin, S.; Feng, Y.; Li, J.; Shi, H. Biochemical, Physiological and Transcriptomic Comparison between Burley and Flue-Cured Tobacco Seedlings in Relation to Carbohydrates and Nitrate Content. Molecules 2017, 22, 2126. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, P.; Zhang, X.; Liu, Y.; Feng, S.; Guo, D.; Nadezhda, T.; Song, Z.; Dang, X. Multi-Wall Carbon Nanotubes Promote the Growth of Maize (Zea mays) by Regulating Carbon and Nitrogen Metabolism in Leaves. J. Agric. Food Chem. 2021, 69, 4981–4991. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yuan, S.; Zhuang, J.; Wan, Y.; Wang, Q.; Zhang, J.; Li, H. Effect of selenium on the uptake kinetics and accumulation of and oxidative stress induced by cadmium in Brassica chinensis. Ecotoxicol. Environ. Saf. 2018, 162, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, S.; Zhao, J.; Wang, F.; Du, Y.; Zou, S.; Li, H.; Wen, D.; Huang, Y. Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2017, 133, 1–11. [Google Scholar]
- Wang, H.; Kou, X.; Pei, Z.; Xiao, J.Q.; Shan, X.; Xing, B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 2011, 5, 30–42. [Google Scholar] [CrossRef]
- Zhao, L.; Bai, T.; Wei, H.; Gardea-Torresdey, J.L.; Keller, A.; White, J.C. Nanobiotechnology-based strategies for enhanced crop stress resilience. Nat. Food 2022, 3, 829–836. [Google Scholar] [CrossRef]
- Xiao, S.; Jin, Z.; Dong, H.; Xiao, J.; Li, Y.; Li, L.; Li, R.; Chen, J.; Tian, R.; Xie, Q. A comparative study on the physicochemical properties, reactivity and long-term performance of sulfidized nanoscale zerovalent iron synthesized with different kinds of sulfur precursors and procedures in simulated groundwater. Water Res. 2022, 212, 118097. [Google Scholar] [CrossRef]
- Wintermans, J.F.; de Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Zhao, F.; McGrath, S.P.; Crosland, A.R. Comparison of three wet digestion methods for the determination of plant sulphur by inductively coupled plasma atomic emission spectroscopy (ICP-AES). Commun. Soil Sci. Plant Anal. 1994, 25, 407–418. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Method. Enzymol. 1955, 2, 764–775. [Google Scholar]
- Fales, F. The assimilation and degradation of carbohydrates by yeast cells. J. Biol. Chem. 1951, 193, 113–124. [Google Scholar] [CrossRef]
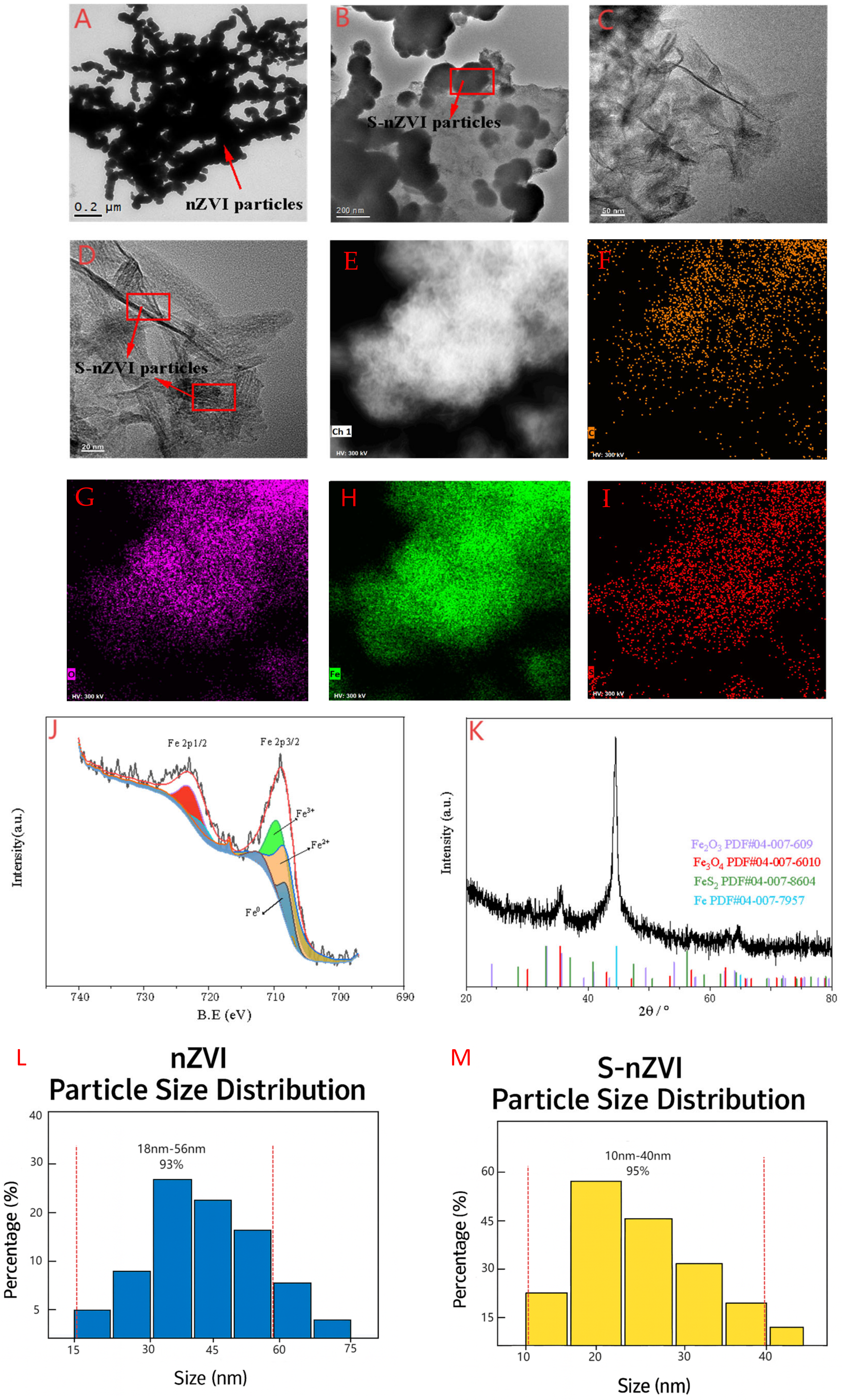
| NMs | Particle Size | Zeta Potential |
|---|---|---|
| S−nZVI | 31.0 ± 17.7 | −2.34 ± 0.17 |
| nZVI | 44.4 ± 22.3 | −0.61 ± 0.11 |
| Iron−Based Nanomaterials | Treatment | Leaf Area (cm2) | Plant Height (cm) | Number of Blades | Fresh Weight of Overground (g) | Dry Weight of Overground (g) |
|---|---|---|---|---|---|---|
| S−nZVI | T0 | 27.04 ± 0.01 g | 7.74 ± 0.48 d | 4.51 ± 0.09 f | 1.47 ± 0.01 f | 0.14 ± 0.02 c |
| T1 | 28.50 ± 0.11 e | 9.89 ± 0.08 ab | 5.27 ± 0.08 e | 1.81 ± 0.11 def | 0.15 ± 0.01 c | |
| T2 | 31.13 ± 0.03 c | 10.51 ± 0.09 ab | 7.35 ± 0.18 cd | 1.97 ± 0.14 def | 0.34 ± 0.03 abc | |
| T3 | 32.34 ± 0.04 b | 10.27 ± 0.13 ab | 7.92 ± 0.15 abc | 2.99 ± 0.37 bc | 0.39 ± 0.06 ab | |
| T4 | 33.56 ± 0.02 a | 10.55 ± 0.15 a | 8.44 ± 0.14 a | 5.30 ± 0.09 a | 0.55 ± 0.02 a | |
| T5 | 27.62 ± 0.05 fg | 10.46 ± 0.18 ab | 7.92 ± 0.15 abc | 2.47 ± 0.18 cd | 0.29 ± 0.02 bc | |
| nZVI | T0 | 27.04 ± 0.01 g | 7.74 ± 0.48 d | 4.51 ± 0.09 f | 1.47 ± 0.08 f | 0.14 ± 0.02 c |
| T1 | 27.72 ± 0.11 f | 7.93 ± 0.05 d | 4.78 ± 0.05 ef | 1.54 ± 0.03 f | 0.14 ± 0.02 c | |
| T2 | 29.83 ± 0.15 d | 8.52 ± 0.08 cd | 7.04 ± 0.19 d | 1.63 ± 0.02 ef | 0.28 ± 0.11 bc | |
| T3 | 31.12 ± 0.32 c | 9.41 ± 0.09 bc | 7.33 ± 0.07 cd | 2.43 ± 0.07 cd | 0.36 ± 0.01 b | |
| T4 | 32.67 ± 0.08 b | 10.27 ± 0.11 ab | 8.03 ± 0.09 ab | 3.57 ± 0.11 b | 0.40 ± 0.05 b | |
| T5 | 27.30 ± 0.23 fg | 10.43 ± 0.13 ab | 7.65 ± 0.12 bcd | 2.31 ± 0.06 cde | 0.27 ± 0.01 bc |
| Iron−Based Nanomaterials | Treatment | Net Photosynthetic Rate (μmol m−2 s−1) | Intercellular CO2 Concentration (μmol mol−1) | Transpiration Rate (mmol m−2 s−1) | Stomatal Conductance (mmol m−2 s−1) |
|---|---|---|---|---|---|
| S−nZVI | T0 | 6.78 ± 0.16 f | 147.43 ± 5.50 e | 1.28 ± 0.14 e | 50.16 ± 0.34 e |
| T1 | 7.58 ± 0.07 f | 282.65 ± 14.38 c | 3.33 ± 0.23 bc | 53.90 ± 0.17 e | |
| T2 | 12.64 ± 0.06 e | 340.10 ± 9.98 ab | 3.86 ± 0.37 b | 80.24 ± 0.19 d | |
| T3 | 16.98 ± 0.11 cd | 382.03 ± 6.11 a | 3 ± 0.26 bc | 90.04 ± 0.22 cd | |
| T4 | 20.73 ± 0.21 a | 380.71 ± 16.75 a | 5.97 ± 0.15 a | 156.45 ± 0.09 a | |
| T5 | 17.80 ± 0.31 bc | 345.18 ± 13.47 ab | 6.35 ± 0.19 a | 97.60 ± 0.11 c | |
| nZVI | T0 | 6.78 ± 0.16 f | 147.42 ± 5.49 e | 1.28 ± 0.14 e | 50.16 ± 0.34 e |
| T1 | 7.22 ± 0.26 f | 154.18 ± 14.30 e | 1.63 ± 0.094 de | 52.79 ± 2.47 e | |
| T2 | 11.34 ± 0.45 e | 227.22 ± 8.94 d | 3.16 ± 0.14 bc | 77.97 ± 4.05 d | |
| T3 | 15.42 ± 0.80 d | 268.85 ± 5.14 cd | 3.41 ± 0.12 bc | 89.99 ± 2.25 cd | |
| T4 | 17.31 ± 0.77 bc | 312.88 ± 6.43 bc | 3.68 ± 0.231 b | 132.93 ± 7.63 b | |
| T5 | 16.94 ± 0.50 cd | 308.07 ± 5.39 bc | 2.55 ± 0.29 cd | 44.47 ± 3.34 e |
| Iron−Based Nanomaterials | Treatment | Minimal Fluorescence F0 | Maximum Fluorescence Fm | Fv/Fm PSII Maximum Photoelectrochemical Quantum Yield | Actual Photochemical Efficiency of Photosystem II |
|---|---|---|---|---|---|
| S−nZVI | T0 | 0.20 ± 0.01 cd | 0.49 ± 0.01 h | 0.59 ± 0.01 f | 0.54 ± 0.01 h |
| T1 | 0.21 ± 0.01 bc | 0.58 ± 0.01 ef | 0.61 ± 0.02 ef | 0.64 ± 0.01 f | |
| T2 | 0.25 ± 0.02 a | 0.71 ± 0.02 b | 0.64 ± 0.01 ef | 0.69 ± 0.02 de | |
| T3 | 0.19 ± 0.01 cd | 0.68 ± 0.01 bc | 0.69 ± 0.01 bcd | 0.68 ± 0.02 de | |
| T4 | 0.18 ± 0.034 d | 0.76 ± 0.01 a | 0.75 ± 0.01 a | 0.9 ± 0.01 a | |
| T5 | 0.15 ± 0.01 e | 0.59 ± 0.02 ef | 0.74 ± 0.04 ab | 0.74 ± 0.03 bc | |
| nZVI | T0 | 0.20 ± 0.01 cd | 0.49 ± 0.01 h | 0.59 ± 0.01 f | 0.54 ± 0.01 h |
| T1 | 0.21 ± 0.01 bc | 0.52 ± 0.01 gh | 0.61 ± 0.01 ef | 0.59 ± 0.01 g | |
| T2 | 0.23 ± 0.01 ab | 0.61 ± 0.01 de | 0.64 ± 0.03 def | 0.63 ± 0.01 fg | |
| T3 | 0.19 ± 0.02 cd | 0.65 ± 0.01 cd | 0.66 ± 0.02 cde | 0.67 ± 0.01 ef | |
| T4 | 0.14 ± 0.01 e | 0.69 ± 0.01 b | 0.69 ± 0.01 bcd | 0.78 ± 0.02 b | |
| T5 | 0.13 ± 0.02 e | 0.56 ± 0.03 fg | 0.71 ± 0.02 abc | 0.72 ± 0.04 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Yu, J.; Wei, Y.; Munir, F.; Haider, F.U.; Cai, L. Foliar Application of Iron Nanoparticles Improves Chinese Cabbage Growth. Plants 2025, 14, 3509. https://doi.org/10.3390/plants14223509
He M, Yu J, Wei Y, Munir F, Haider FU, Cai L. Foliar Application of Iron Nanoparticles Improves Chinese Cabbage Growth. Plants. 2025; 14(22):3509. https://doi.org/10.3390/plants14223509
Chicago/Turabian StyleHe, Miaomiao, Jialu Yu, Yuzhen Wei, Fahad Munir, Fasih Ullah Haider, and Liqun Cai. 2025. "Foliar Application of Iron Nanoparticles Improves Chinese Cabbage Growth" Plants 14, no. 22: 3509. https://doi.org/10.3390/plants14223509
APA StyleHe, M., Yu, J., Wei, Y., Munir, F., Haider, F. U., & Cai, L. (2025). Foliar Application of Iron Nanoparticles Improves Chinese Cabbage Growth. Plants, 14(22), 3509. https://doi.org/10.3390/plants14223509







