1. Introduction
Table carrot (
Daucus carota subsp.
sativus) is an important root vegetable widely used in human nutrition. Every year, tens of millions of tons are produced worldwide; for example, in Russia, about 1.37 million tons of carrot were harvested in 2023 [
1]. However, a significant part of the yield may be lost during storage due to diseases. One of the most destructive diseases of stored carrot is white mold, or sclerotiniosis, caused mainly by the fungus
Sclerotinia sclerotiorum (Lib.) de Bary [
2]. Yield losses from white mold and secondary bacterial infections can reach 50–70% under unfavorable storage conditions [
3].
The main causal agent of white mold,
S. sclerotiorum, is a polyphagous pathogen that persists in soil as sclerotia for up to 5–8 years. Under favorable conditions (humidity above 80%, temperature 10–15 °C), sclerotia germinate carpogenically, forming apothecia that release ascospores dispersed by wind, infecting the aerial organs of carrot [
4]. During storage, infection occurs myceliogenically, when sclerotia or mycelium on infected leaf or root residues initiate a new outbreak, especially under conditions of mechanical damage and excessive humidity. On infected tissues, the fungus forms mycelium and new sclerotia, ensuring the survival of the pathogen and its transition to the next growing season [
5]. The pathogen has an extremely broad host range and can infect more than 400 plant species under various soil and climatic conditions, including many crops such as soybean, rapeseed, sunflower, and vegetable crops [
6]. The expansion of cultivation areas of susceptible crops (rapeseed and soybean) is associated with increased incidence of white mold on carrot [
7]. The pathogen forms specialized resting structures called sclerotia, which can persist on plant residues and in soil for several years, serving as infection sources in the field and in storage facilities, creating serious risks when these crops are rotated [
4]. In recent years, Russia has seen an increase in the area under soybean and rapeseed cultivation, accompanied by more frequent epiphytotic outbreaks and long-term persistence of sclerotia in agrocenoses. At the same time, the possibility of cross-infection of carrot by
Sclerotinia isolates from other crops remains insufficiently studied. Although
S. sclerotiorum is known to cause carrot infection in the field and during storage [
3], information on the aggressiveness of isolates from soybean and rapeseed on carrot remains limited [
8]. Therefore, assessment of the ability of
Sclerotinia isolates from rapeseed and soybean to initiate infection on carrot is an important task in phytopathological monitoring. Cross-host infection potential of
Sclerotinia species is a key challenge for both field production and long-term cold storage, where contaminated plant material from other hosts may serve as a source of primary inoculum.
In addition to
S. sclerotiorum, carrot rot can also be caused by
Sclerotinia nivalis [
9] and
S. minor [
10], but these species occur less frequently, and their economic impact is usually lower than that of
S. sclerotiorum. In Russia, the pathogen of carrot white mold has been known as
S. sclerotiorum [
11], and reports of other causal agents of the disease are absent from modern literature.
Sclerotinia nivalis, formerly known as
S. intermedia, was described relatively recently based on morphological features of the sclerotial anamorph and teleomorph obtained in culture as a pathogen of dicotyledonous herbaceous plants [
12]. The species was first reported as the causal agent of white mold on ornamental plants, weeds, and carrot (
Daucus carota) in Japan in 1997 [
9]. Further studies demonstrated its wide distribution in the Northern Hemisphere. It can be distinguished by its smaller sclerotia compared to
S. sclerotiorum, binucleate ascospores, molecular mass of major sclerotial proteins, and esterase isoenzymes in sclerotial extracts [
13].
S. nivalis is a mesophilic species, with an optimal mycelial growth temperature around 20 °C [
12].
The host range of
S. nivalis includes 84 plant species belonging to 50 genera and 19 families, including carrot (
Daucus carota) [
9], lettuce (
Lactuca sativa) [
14], stringy stonecrop (
Sedum sarmentosum) [
13], hardy kiwi (
Actinidia arguta) [
15], several weeds such as burdock (
Arctium lappa), common ragweed (
Ambrosia elatior), ribwort plantain (
Plantago lanceolata) [
9], chrysanthemum (
Chrysanthemum morifolium), bugleweed (
Ajuga reptans) [
9], japanese angelica (
Angelica acutiloba) [
9], japanese angelica tree (
Aralia elata) [
16], korean pasque flower (
Pulsatilla koreana) [
17,
18], Japanese atractylodes (
Atractylodes japonica) [
19], american ginseng (
Panax quinquefolius) [
20], and korean ginseng (
Panax ginseng) [
21]. In Russia,
S. nivalis was first detected on ornamental and medicinal plants (
Tulipa sp.,
Iris germanica,
Matricaria inodora,
Thlaspi arvense,
Phlox sp.,
Helichrysum arenarium,
Digitalis purpurea,
Sedum sp.) in botanical gardens of Yekaterinburg, Cheboksary, Kirovsk, Vladivostok, Yuzhno-Sakhalinsk, St. Petersburg, and Moscow in 2002 [
22,
23]. However, infection of carrot by this pathogen in Russia has not been previously reported. Moreover, the identification of
S. nivalis in those cases was based mainly on morphological criteria (size of sclerotia, binucleate ascospores) and molecular mass of major sclerotial proteins, which is insufficient for precise species identification. Therefore, molecular verification of the white mold pathogen on new hosts is of particular importance.
The purpose of this study was to provide the first molecular identification of S. nivalis in carrot samples affected by white mold and to compare the aggressiveness of Sclerotinia strains isolated from carrot, rapeseed, and soybean with carrot.
3. Discussion
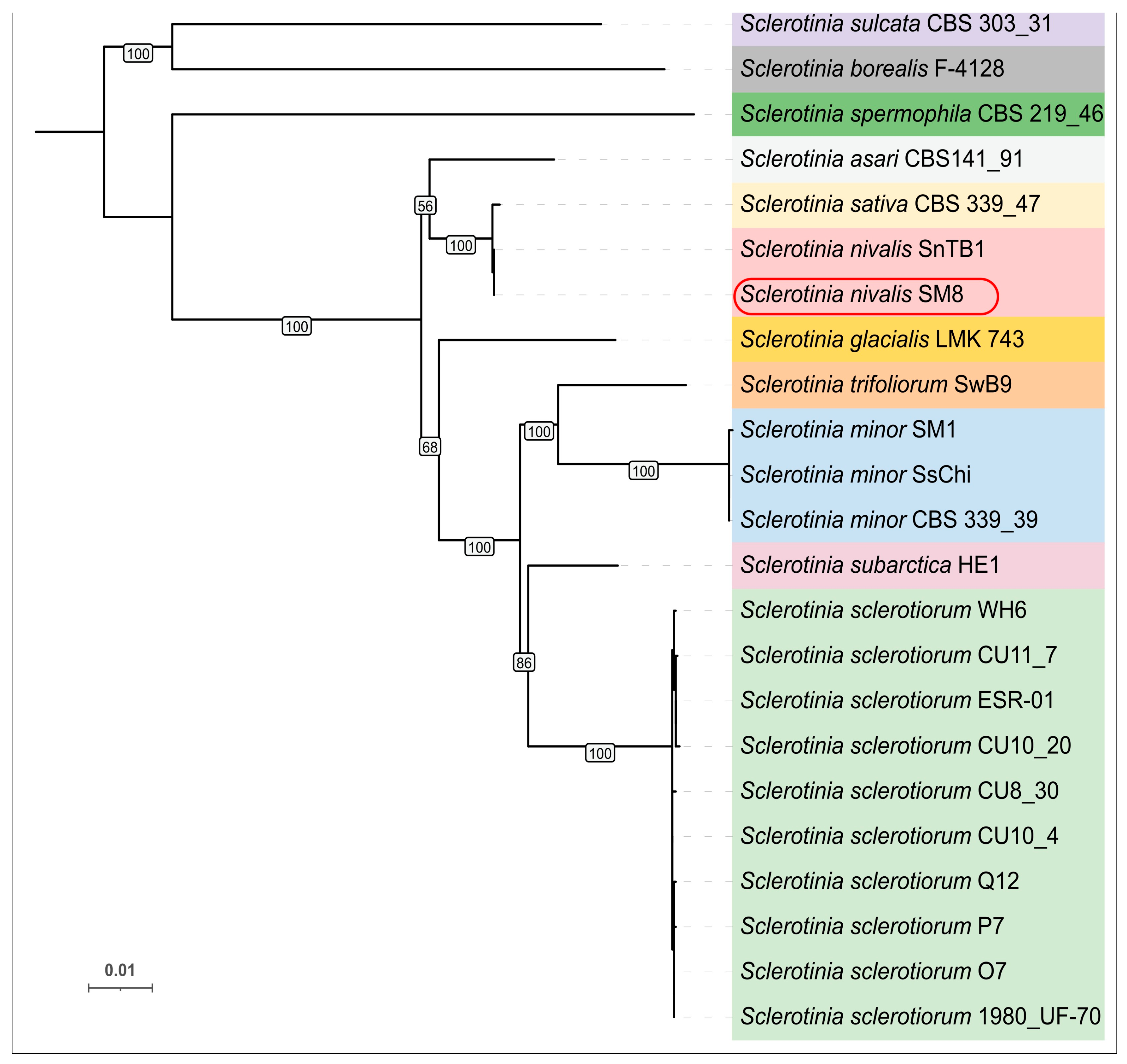
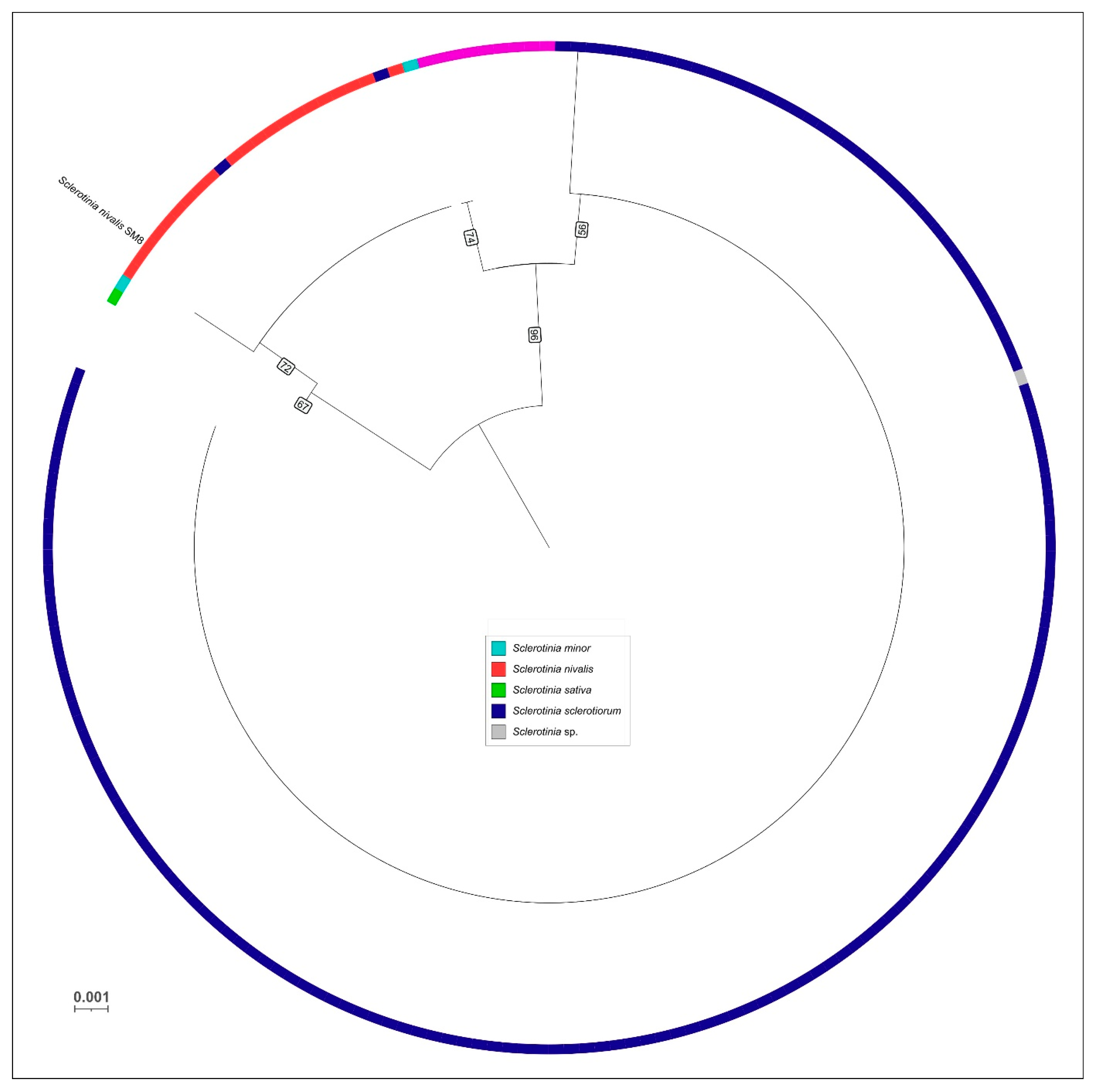
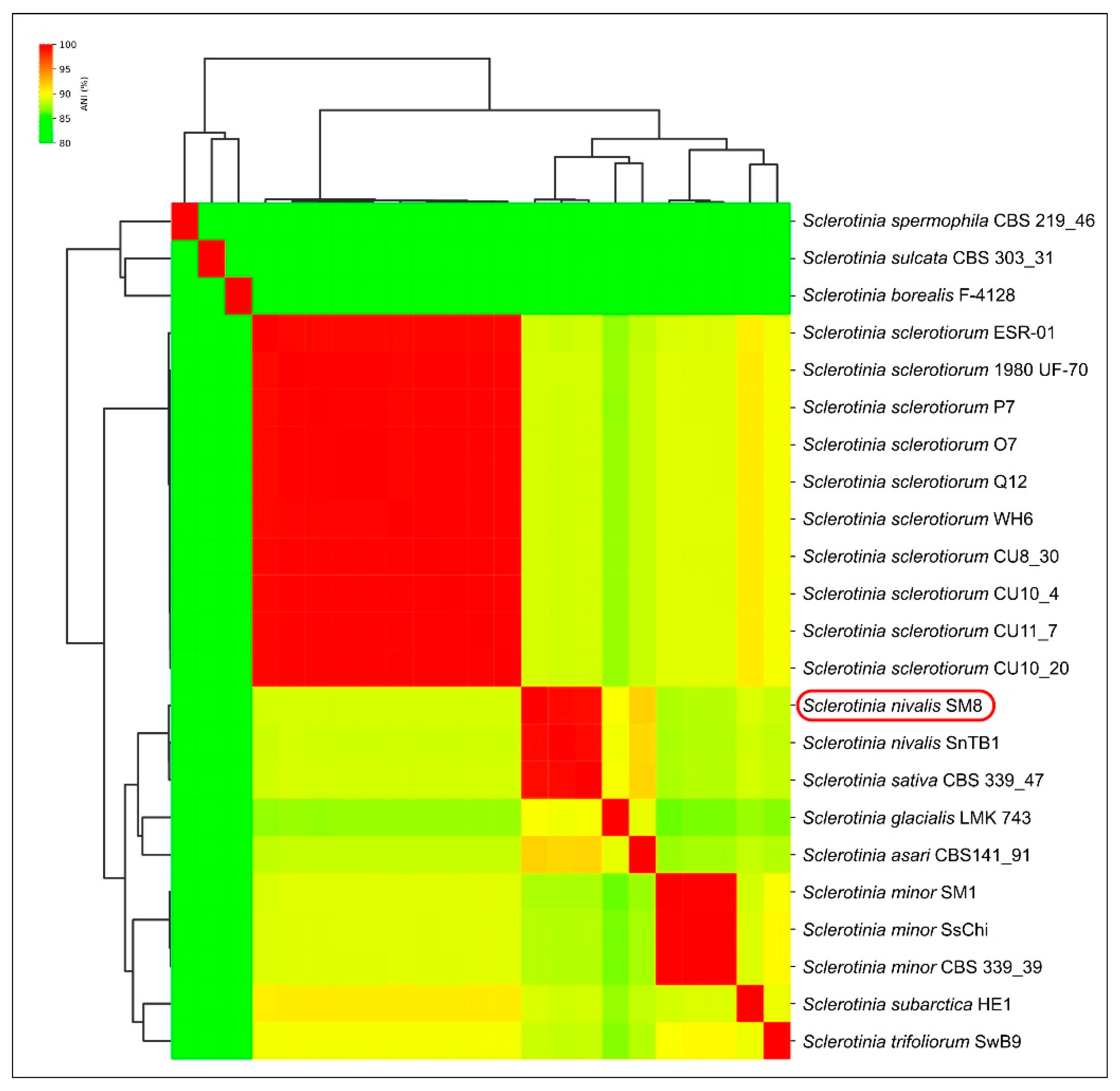
The present study provides new evidence that Sclerotinia nivalis, a species mainly associated with alpine and boreal habitats, can develop at low temperatures and pose a latent risk during carrot storage. Unlike common storage pathogens, S. nivalis demonstrates active growth at 0–5 °C, which may allow the fungus to remain undetected on asymptomatic roots while gradually producing mycelium or sclerotia over extended storage periods. This cold-tolerant behavior highlights the importance of characterizing its pathogenic and physiological traits, especially in regions where long-term storage under refrigerated conditions is essential for supply chain continuity. To the best of our knowledge, the present study reports for the first time the molecular-genetic identification of S. nivalis as a pathogen of carrot in Russia, expanding the geography of the distribution of this species worldwide. Multiple lines of evidence identify SM8 as S. nivalis. In the six-locus ML phylogeny, SM8 groups with the S. nivalis reference SnTB1 with no detectable sequence divergence. In the global ITS dataset, SM8 falls within the S. nivalis cluster, distinct from other Sclerotinia species. Whole-genome ANI between SM8 and SnTB1 is 99.37%, indicating near-identity and supporting conspecific status. Obtained data expand the documented diversity of S. nivalis by adding a new isolate from carrot.
Genomic analysis indicated a very close relatedness of
S. nivalis and
S. sativa.
Sclerotinia sativa [
25] has been treated as a separate species. However,
S. sativa CBS 339.47 shows a very small genetic distance to
S. nivalis. In the six-gene tree it is sister to
S. nivalis with negligible branch length; only a few SNPs are observed in specific loci (
ITS and
g3pdh). Genome-wide identity is similarly high: ANI between
S. sativa (CBS 339.47) and
S. nivalis (SM8/SnTB1) is ~99.3%, a level typical of intraspecific comparisons. Despite high ANI values, the species differ in sclerotial morphology and ecological adaptation to low-temperature niches, which may justify maintaining them as separate taxa. A previous analysis based on
ITS sequences has placed
S. sativa closer to
S. minor [
24], but multilocus and genomic data indicate a closer relationship to
S. nivalis. A formal synonymy is not proposed here; evaluation of original descriptions and any consistent phenotypic differences (e.g., sclerotial size, host range) is warranted. Nevertheless, if additional
S. sativa isolates exhibit < 0.5% genomic divergence from
S. nivalis, conspecific status should be considered.
ANI is widely used in bacteriology (95–96% as a common species boundary), but universal thresholds are not established for fungi [
26,
27,
28]. High ANI can occur between recently diverged or slowly evolving fungal species. The >99% ANI observed between
S. nivalis and
S. sativa suggests potential conspecificity, pending further phenotypic and population-level evidence. Notably, ANI contrasts among recognized species (e.g.,
S. sclerotiorum vs.
S. nivalis at ~88–90%) reveal clear genomic discontinuities consistent with species boundaries.
This study is based on a single
S. nivalis strain, which confirms the presence of the species but does not allow evaluation of its population diversity or geographic distribution. Therefore, the term «first report» refers specifically to the first molecular identification rather than an assessment of prevalence. Previously, this species was noted only on ornamental and wild plants in Russia [
22,
23]. Globally,
S. nivalis is known as the causal agent of white mold and snow mold in temperate and cold climates [
9,
20,
21]. On carrot, it was first described in Japan [
9], but subsequent reports of
S. nivalis infection on this crop are virtually absent. Our
S. nivalis strain SM8 shares key features with Japanese and Chinese
S. nivalis strains, including small sclerotia and optimal growth at 10–15 °C, consistent with their adaptation to cool temperate climates [
9,
13,
14].
In this regard, our study is the second in the world to prove the pathogenesis of
S. nivalis on carrot. It is likely that
S. nivalis is inferior to
S. sclerotiorum in competitiveness, manifesting only at low storage temperatures. Our data show that long-term storage of carrot at +1 °C creates conditions for its active development, whereas under these conditions
S. sclerotiorum is apparently under stress and acts only as a component of the pathocomplex [
2]. The ability of
S. nivalis to grow and form sclerotia at 0–5 °C [
12] is confirmed in our experiments. It is likely that this species was present earlier but was misidentified as
S. minor or
S. sclerotiorum due to similarity of symptoms [
13].
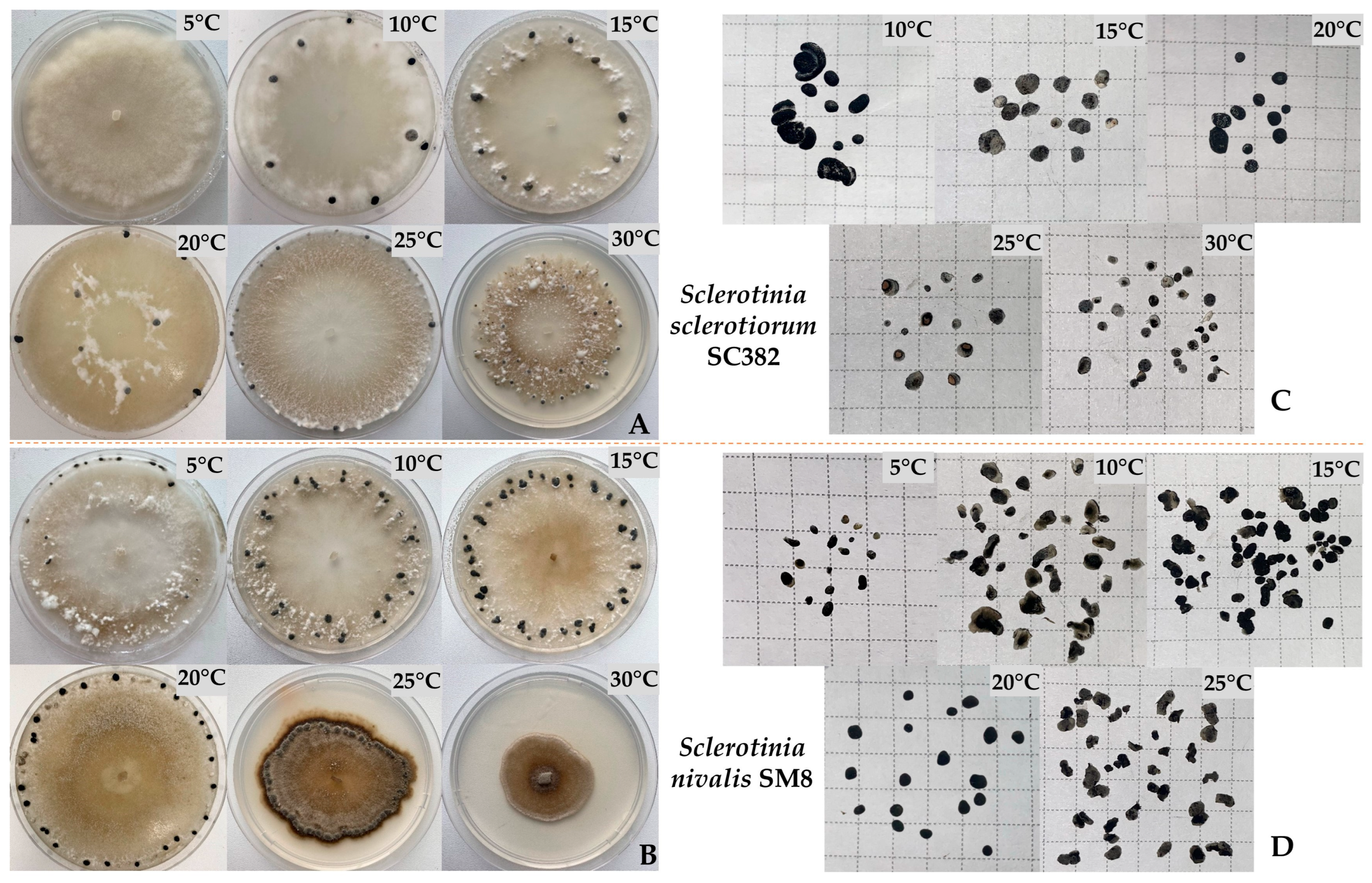
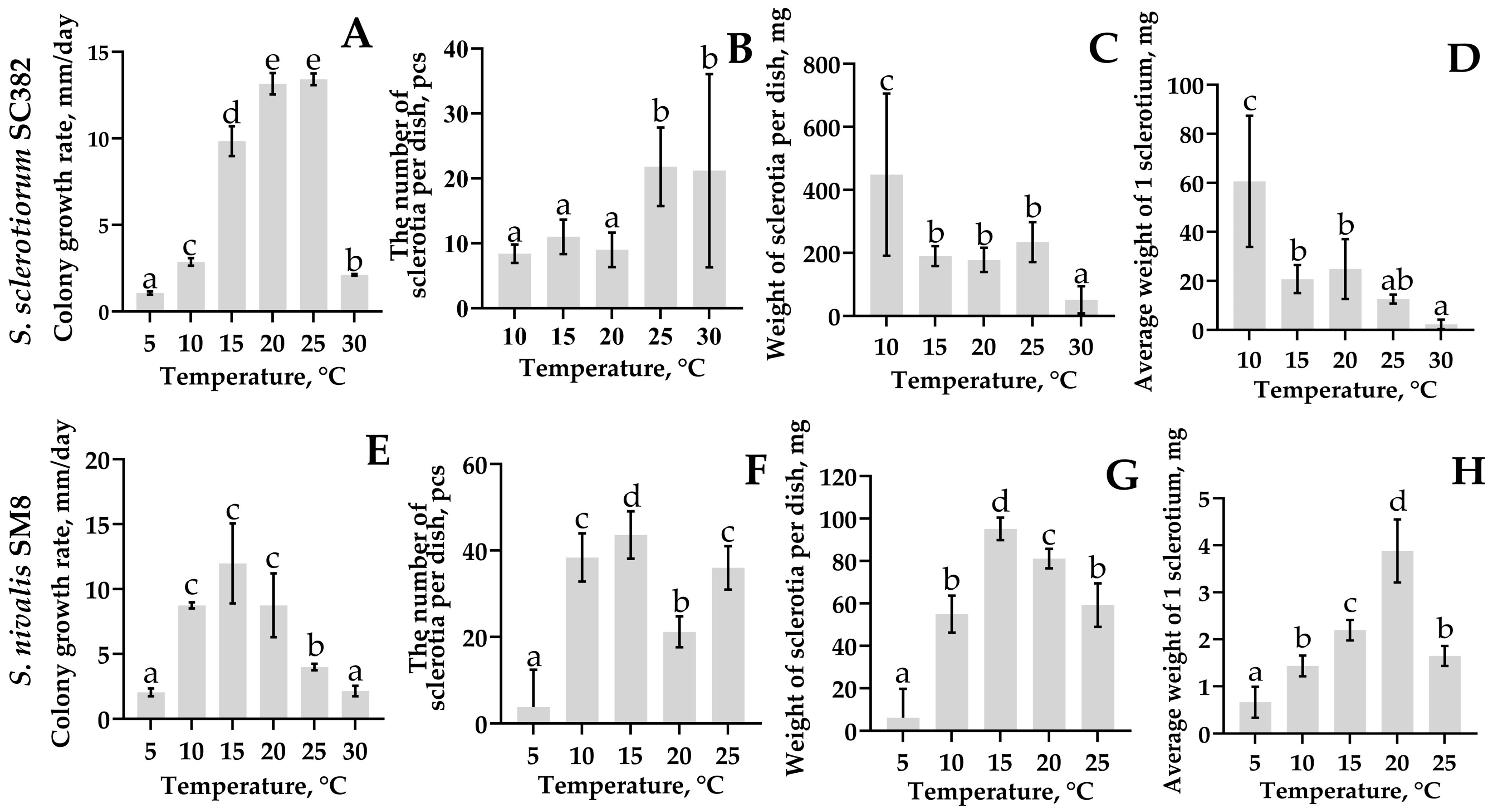
Morphologically,
S. nivalis differs from
S. sclerotiorum by slow growth and the formation of small sclerotia (2–5 mm) in larger numbers [
9,
13]. In
S. sclerotiorum, sclerotia are larger (5–15 mm) and are located mainly at the colony periphery. These differences reflect different survival strategies:
S. nivalis forms numerous small sclerotia to survive cold, whereas
S. sclerotiorum forms larger, more resource-rich structures [
12]. The optimal growth temperature for
S. nivalis was 15 °C, and for
S. sclerotiorum 20–25 °C, which is consistent with published studies and the ecological niches of the pathogen [
18,
21]. Thus,
S. nivalis is adapted to development under cool storage conditions, where competition from other phytopathogens is minimal. The ability of
S. nivalis to grow and sporulate at low temperatures highlights its potential risk for carrot storage facilities and northern agricultural regions.
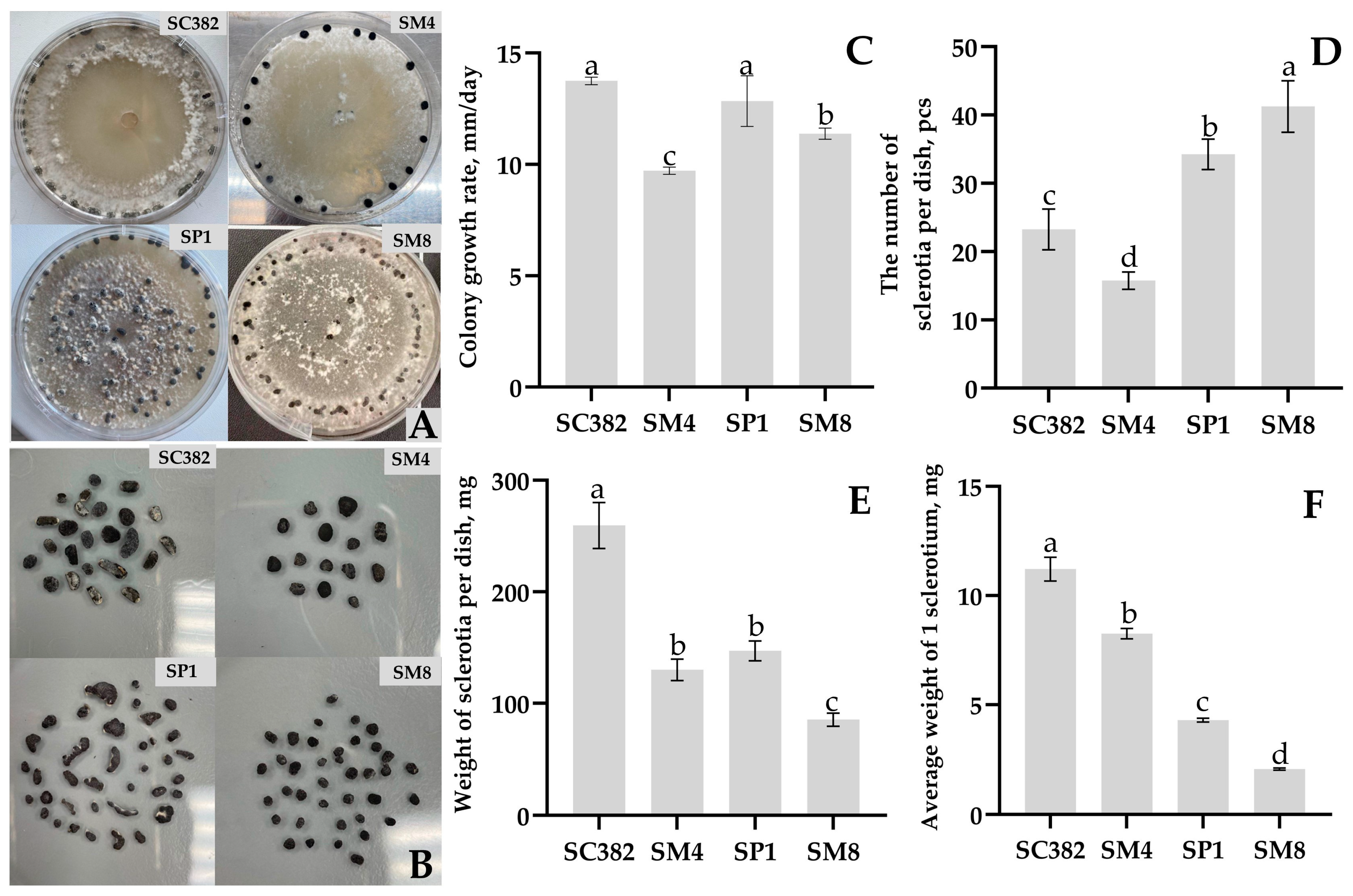
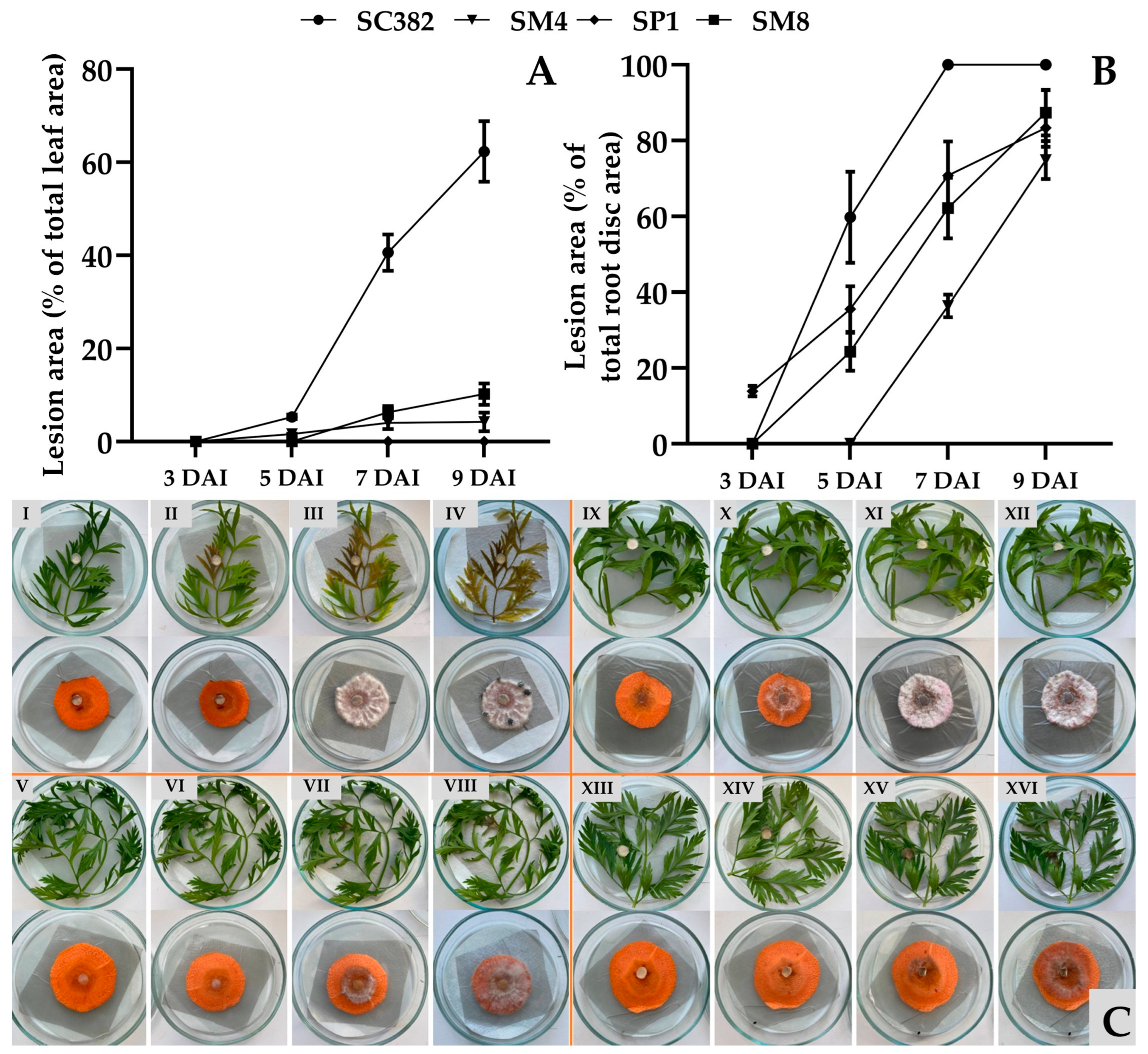
Comparison of strain aggressiveness showed that
Sclerotinia strains isolated from different hosts are capable of infecting carrot with varying degrees of aggressiveness. The most aggressive was the
S. sclerotiorum SC382 strain from soybean, which caused necrosis of up to 62% of the leaf area and complete decay of root discs within 9 days. The strain from rapeseed (
S. sclerotiorum SP1) infected only roots, indicating possible tissue specialization [
8]. The results obtained demonstrate the risk of cross-infection when rotating carrot with soybean or rapeseed, as previously noted in Canada [
7]. This underscores the need to consider sclerotiniosis when designing crop rotations and laying carrots in storage.
For the first time, the sensitivity of
S. nivalis to fungicides was evaluated. The
S. nivalis SM8 strain showed sensitivity comparable to
S. sclerotiorum to boscalid and pyraclostrobin [
29], but four times lower sensitivity to fluazinam. This may explain cases of weak fluazinam efficacy in cold storage facilities where
S. nivalis predominates and dictates the need for further studies to assess population sensitivity of
S. nivalis to fungicides. Fluazinam is a broad-spectrum fungicide whose primary mode of action is uncoupling oxidative phosphorylation in mitochondrial membranes, leading to disruption of ATP synthesis and induction of oxidative stress. Several studies demonstrated that fluazinam exposure triggers a strong glutathione-dependent detoxification response in
Sclerotinia, including upregulation of glutathione S-transferases, increased GSH/GSSG turnover, and activation of antioxidant enzymes [
30,
31]. Such responses may partly explain the higher EC
50 observed in SM8, as isolates capable of faster neutralization of reactive intermediates or enhanced antioxidant buffering can display reduced in vitro sensitivity to fluazinam. Given the variability in fluazinam EC
50 values, routine sensitivity monitoring and rotation with fungicides of different FRAC groups are recommended. Boscalid and pyraclostrobin maintained high activity against both species, making them preferable in integrated systems for protecting carrot from white mold.
4. Materials and Methods
4.1. Isolation of Sclerotinia Strains
During the inspection of vegetable storage facilities, softened carrot tissues with dark-colored sclerotia on white mycelium were collected, placed in paper bags, transported to the laboratory, and stored at 4 °C until analysis. Pure cultures were obtained following the methods of [
32,
33] with modifications. Dense, mature, dark sclerotia at least 1 mm in diameter were extracted from infected plant tissues showing typical symptoms of white mold using a sterile needle and placed in 1.5 mL Eppendorf tubes. The sclerotia were washed three times with water by pipetting to remove plant tissue and soil particles and subsequently treated with 70% ethanol for 30 s, 1% sodium hypochlorite solution for 2 min, and rinsed three times with sterile distilled water to remove residual disinfectants.
Surface-sterilized sclerotia were cut with a sterile scalpel and forceps in a laminar flow cabinet. The cut sclerotia were transferred to Petri dishes containing potato dextrose agar (PDA) (g/L: potato broth from 300 g potatoes—300.0 mL; glucose—20.0; agar—17.0; water to 1 L) supplemented with antibiotics (streptomycin sulfate and chloramphenicol, (Central Drug House (P) Ltd., Delhi, India), each at 100 mg/L) to suppress bacterial contamination. The pH values were adjusted to 5.6 by measuring using a SanXin PHS-3D-01 pH meter (SanXin Instrumentation, Shanghai, China) prior to autoclaving. Plates were incubated at 20 ± 1 °C in an KB 23 incubator (BINDER GmbH, Tuttlingen, Germany), and white mycelium growth was observed after 2–3 days, followed by sclerotia formation on days 7–9. A hyphal fragment from the colony margin not in contact with the original sclerotium was cut with a sterile needle and transferred to fresh PDA. This subculturing step was repeated three times to obtain a pure culture. Pure isolates were maintained on agar at +4 °C and in 20% glycerol at −80 °C. Strains isolated from rapeseed and soybean were purified and stored in the same way.
4.2. Pathogenicity Testing of Strains on Host Plants
Pathogenicity tests were conducted according to [
34] with some modifications. Soybean cv. Kasatka, winter rapeseed cv. Garant, and carrot cv. Shantane 2461 were grown in a glass greenhouse at 28/22 °C (14 h day/10 h night) under natural light and watered as needed. Plants were cultivated in peat-perlite substrate (Veltorf, Vologda, Russia) in plastic pots (0.5 L cell volume, AgrofloRaPak, Vologda, Russia) until 3–4 true leaves appeared. Three leaves of each plant species were used for inoculation. For inoculation, 7 mm mycelium plugs (approximately 15–20 mg of fresh biomass) were cut from 2-day PDA cultures of each isolate using a sterile cork drill, they were placed in the center of each sheet and gently pressed against the surface. Each isolate was used to inoculate the host species from which it had originally been obtained. Growth conditions remained unchanged until the end of the experiment. On day 5 after inoculation, the presence of chlorosis zones and mycelial growth on inoculated leaves was recorded. Isolates that did not cause symptoms were excluded from further work. Mock-inoculated controls (agar plugs without mycelium) remained symptom-free throughout the experiment.
After the experiment, Koch’s postulates were verified by re-isolating fungi from infected tissues on PDA with antibiotics and purifying to pure culture, as described previously. The identity of re-isolated strains was confirmed by comparing their morphological characteristics with those of the original isolates used for inoculation, as well as by sequencing the rDNA-ITS regions and comparing nucleotide sequences.
4.3. Identification of Sclerotinia Strains
Preliminary identification was carried out based on colony morphology, presence, arrangement, and size of sclerotia on PDA according to [
9]. Mycelium morphology was examined under an Axiolab 5 microscope (Carl Zeiss AG, Oberkochen, Germany).
For final identification, DNA was extracted from 7-day-old mycelium using the «Phytosorb» DNA extraction kit (Syntol LLC, Moscow, Russia) according to the manufacturer’s protocol. Reaction mixtures contained 5 µL of 5× Master-mix (5× MasDDTaqMIX-2025, Dialat LTD, Moscow, Russia), 10 µM of each primer (ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′)), 5 ng of target DNA, and PCR-grade water (Syntol LLC, Moscow, Russia) up to a total volume of 25 µL. PCR amplification of
rDNA-ITS regions was performed in a T100 thermal cycler (Bio-Rad, Hercules, CA, USA) according to [
35]. Amplicons were separated by electrophoresis in 1.5% agarose gel, stained with ethidium bromide in 0.5× TBE buffer, and visualized using a Gel Doc XR+ system (Bio-Rad, Hercules, CA, USA). PCR fragments were excised and purified using the ColGen kit (Syntol LLC, Moscow, Russia) according to the manufacturer’s instructions. Sequencing of purified PCR products was performed by the Sanger method using the BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies ThermoFisher, Waltham, MA, USA) and an automatic DNA analyzer 3730 (Thermo Fisher Scientific, Waltham, MA, USA) at Syntol LLC. The obtained sequences were compared with the GenBank database using the BLASTn algorithm. A species was considered reliably identified when sequence similarity with the type strain was ≥95% [
36].
4.4. Genome Sequencing and Assembly
The whole genome of
S. nivalis strain SM8 was sequenced and assembled into a draft genome. Genomic DNA was extracted from lyophilized mycelium by phenol–chloroform purification and sheared with a Bioruptor sonicator (Diagenode, Liège, Belgium). Samples underwent quality control by agarose gel electrophoresis (AGE). Quality control was considered passed if the sample on the electrophoregram was at the same level or higher than the 10,000 bp marker. Genomic DNA concentrations were measured on a Qubit 3.0 fluorometer (Life Technologies ThermoFisher, Waltham, MA, USA) using the dsDNA BR Assay Kit (Life Technologies ThermoFisher, Waltham, MA, USA) according to the manufacturer’s protocol. Libraries were prepared from genomic DNA using the MGIEasy FS DNA Library Prep Set according to the manufacturer’s protocol (MGI Tech Co., Shenzhen, China). A total of 260 ng of genomic DNA was used for library preparation. After fragmentation, size selection was performed using MGIEasy DNA Clean Beads—0.5× to remove long fragments and 0.36× of the initial volume to remove short fragments. Library quality control was performed by agarose gel electrophoresis (AGE). Library concentrations were measured on a Qubit 3.0 fluorometer (Life Technologies Thermo Fisher, Waltham, MA, USA) using the dsDNA HS Assay Kit (Life Technologies Thermo Fisher, Waltham, MA, USA) according to the manufacturer’s protocol. The finished library was circularized and sequenced in paired-end mode on the DNBSEQ-G99 platform using the Universal Sequencing Reaction Kit G99 SMApp-DPE150 according to the manufacturer’s protocol (MGI Tech Co., Shenzhen, China). DNB concentrations were measured on a Qubit 3.0 fluorometer (Life Technologies Thermo Fisher, USA) using the ssDNA Kit according to the manufacturer’s protocol. Primary quality control of the sequenced library was performed using FastQC v0.12.1 [
37] and MultiQC v1.18 [
38]. Subsequently, based on the obtained reports, trimming of fq.gz files and their subsequent assembly with SPAdes v4.0.0 were performed. Assembly quality control was conducted using Kraken2 v2.1.1 [
39] (taxonomic diversity, representation of contaminating groups), MetaBAT2 v2.18 [
40] (binning, detection of non-target organisms), and QUAST v5.3.0 [
41] (general assembly quality assessment). Additionally, contigs associated with bacterial contamination were removed (‘extract_kraken_reads.py’, KrakenTools software [
42]). According to the QUAST v5.3.0 report, the N50 is 31,512, the number of contigs is 19,225, of which 5071 are ≥500 bp. The maximum contig length is 255,834, and the GC content is 39.75%. Contigs were inspected and curated in Geneious Prime 2025.0.3 (Biomatters, Inc., Auckland, New Zealand). The draft genome of
S. nivalis SM8 was deposited in the NCBI GenBank database under the BioProject accession number PRJNA1344670. The size of the SM8 assembly was 47,605,970 bp and the coverage 129.4.
4.5. Phylogenetic Analysis and Average Nucleotide Identity Calculations
To determine the phylogenetic placement of strain SM8, multilocus sequence typing (MLST) using six loci (the internal transcribed spacer (
ITS) region and five protein-coding genes—β-tubulin (
tubB), histone H3 (
his3), glyceraldehyde-3-phosphate dehydrogenase (
g3pdh), heat shock protein 60 (
hsp60), and RNA polymerase II second-largest subunit (
rpb2)) was conducted. Sequences from SM8 were aligned with reference sequences of multiple
Sclerotinia species (including
S. nivalis SnTB1,
S. sativa CBS 339.47,
S. sclerotiorum,
S. minor,
S. trifoliorum, and others) using the MAFFT v7.490 [
43] applying the L-INS-i iterative refinement algorithm, and other settings were default. Phylogenetic trees were inferred under maximum likelihood in IQ-TREE 2 [
44]. The commands ‘-m TEST’ and ‘-bb 1000’ were used to automatically select the best-fit substitution model and assess branch support with 1000 bootstrap replicates. The resultant phylogeny was visualized using iTOL v7 [
45]; the tree was midpoint-rooted and annotated with species labels and bootstrap support values.
The phylogenetic tree based on ITS sequences was obtained using a wide collection of Sclerotinia isolates worldwide. Approximately ~200 ITS sequences were retrieved from GenBank, representing all major Sclerotinia species across a global distribution (including multiple isolates of S. sclerotiorum, S. minor, S. nivalis, etc.). These ITS sequences were aligned with MAFFT (L-INS-i) and manually inspected. A maximum likelihood ITS tree was generated with IQ-TREE 2 under the same parameters as above (automatic model selection, 1000 bootstrap replicates). The resultant ITS phylogeny was also visualized in iTOL, with midpoint rooting.
Average Nucleotide Identity (ANI) calculations were obtained using a panel of 23 publicly available
Sclerotinia genomes. This dataset included the genomes of
S. nivalis SnTB1,
S. sativa CBS 339.47, multiple strains of
S. sclerotiorum, and representatives of
S. minor and
S. trifoliorum, among others. ANI was computed using FastANI v1.34 [
46]. The pairwise ANI results were visualized as a clustered heatmap and dendrogram using ANIclustermap (
https://github.com/moshi4/ANIclustermap, accessed 10 September 2025).
4.6. Evaluation of Radial Growth Rate and Sclerotia Formation
An agar plug with mycelium obtained as described in
Section 4.2 was transferred to the center of a new Petri dish containing PDA, sealed with Parafilm, and incubated in an incubator at 20 ± 0.5 °C. The radial colony growth was measured daily, starting from the second day of cultivation and continuing for nine days, in two perpendicular directions using a digital caliper ADA Mechanic 150 PRO (ADA INSTRUMENTS Co LTD., Shenzhen, China). The average radial growth rate of the mycelium was calculated according to [
2] using the formula:
where V—average growth rate (mm/day); D
n—colony diameter on day n; D
0—initial diameter (7 mm); n—number of incubation days.
Sclerotia formation was assessed on the ninth day after the completion of active growth using the same Petri dishes where radial growth was measured. Starting from day 4 of cultivation, plates with cultures were transferred from a dark incubator to an incubator with alternating light conditions (12 h light/12 h dark) at the same temperature (20 ± 0.5 °C) to stimulate sclerotial morphogenesis. All sclerotia formed on the plate were removed with tweezers, counted, dried for 24 h in an incubator at 30 °C to air-dry weight, weighed on analytical balances Quintix (Sartorius AG, Göttingen, Germany), and the average mass of a single sclerotium for each strain was calculated. The experiment was performed in four replications for each strain.
4.7. Evaluation of the Effect of Cultivation Temperature on Mycelial Growth and Sclerotia Formation
An agar plug with mycelium obtained as described in
Section 4.2 was transferred to the center of a new Petri dish with PDA. Plates were sealed with Parafilm and incubated in hermetic containers to prevent medium desiccation. Cultivation was carried out at six temperature regimes: 5 °C, 10 °C, 15 °C, 20 °C, 25 °C, and 30 °C in a constant-temperature incubator (±0.5 °C) in the dark. Radial mycelial growth was measured on days 3, 7, and 10, and then every 5 days using a digital caliper. The average growth rate was calculated using the formula described in
Section 4.6. After complete sclerotia formation, their number was evaluated as described in
Section 4.6. The experiment was performed in five replications per strain.
4.8. Evaluation of Strain Virulence on Carrot Leaves and Roots
Virulence of strains was assessed according to [
34,
47] with modifications, using freshly harvested leaves and roots of carrot cv. Shantane 2461 grown as described in
Section 4.2. The roots were washed under running water to remove soil particles, sterilized with 70% ethanol, and air-dried in a sterile laminar flow cabinet for 30 min. Leaves were detached with petioles, washed under running water for 10 min, immersed in sterile water for 15 min, and similarly air-dried. Two inoculation variants were used: (I) root discs (slices 5 ± 0.5 mm thick, 30 ± 2 mm in diameter) cut from healthy surface-sterilized carrots using a sterile knife in a laminar cabinet; and (II) carrot leaves of uniform size, cut into fragments 5 ± 1 cm long. Each variant was tested in five replicates (five root discs or five leaves per strain).
Prepared root discs and leaves were placed in sterile glass Petri dishes lined with moistened filter paper (to maintain high humidity) and a layer of sterile foil. Mycelial discs obtained as described in
Section 4.2 were gently placed in the center of each root disc or on the upper surface of the leaf (at the mid-petiole region). Dishes were closed with lids, sealed in zip-lock bags, and incubated at 24 °C in the dark. High humidity in the dishes was maintained throughout the experiment by moistening the filter paper daily with sterile water.
Observations were made on days 3, 5, 7, and 9 after inoculation by measuring mycelial growth zones using the LeafDoctor application (
https://www.quantitative-plant.org/software/leaf-doctor, accessed 21 June 2025) installed on an iPhone SE 2. Each leaf and root disc was photographed separately and analyzed by adjusting the threshold slider until only symptomatic tissues appeared blue, and the percentage of affected tissue was calculated following the developer’s recommendations [
48,
49].
4.9. Evaluation of Sensitivity to Boscalid, Fluazinam, and Pyraclostrobin Using EC50
Sensitivity of the strains to fungicides was assessed according to [
29] with modifications. Effective concentrations (EC
50), at which mycelial growth was inhibited by 50% compared to the control (without fungicide), were determined. Technical-grade active ingredients (a.i. 95%) were used: boscalid (pyridinyl amide, SDHI; manufacturer Haili Guixi Chemical Co. Ltd., Yingtan, China), fluazinam (anilide; Hubei Kang Bao Tai Fine-Chemical Co. Ltd., Hubei, China), and pyraclostrobin (strobilurin, QoI; Hubei Kang Bao Tai Fine-Chemical Co. Ltd., Hubei, China). Before addition to the medium, boscalid and pyraclostrobin were dissolved in 99.8% dimethyl sulfoxide (DMSO; Component-Reaktiv LLC, Moscow, Russia), and fluazinam was dissolved in 99.8% acetone (TD HIMMED LLC, Moscow, Russia) to prepare 1000 mg a.i./mL stock solutions. Serial dilutions were prepared to obtain the required concentrations. Solvent controls (DMSO or acetone) were included at identical final concentrations as in fungicide treatments, and no inhibitory effects on mycelial growth were detected.
The prepared solutions were added with automatic pipettes to sterile glass jars containing previously autoclaved PDA medium cooled to 47 °C in a water bath, gently mixed to avoid bubbles, and poured into sterile Petri dishes (20 mL per plate) using an electric pipetting dispenser Levo Plus (DLAB Scientific Co., Ltd., Beijing, China) in a sterile laminar cabinet. The final concentrations in PDA were: for boscalid: 0 (PDA + DMSO), 0.03, 0.1, 0.3, 1.0, and 5 µg/mL; pyraclostrobin: 0 (PDA + DMSO), 0.025, 0.05, 0.1, 0.2, and 0.4 µg/mL; fluazinam: 0 (PDA + acetone), 0.0025, 0.005, 0.01, 0.05, and 0.1 µg/mL [
29,
50].
Plates were left for 30 min in the working laminar cabinet for solidification. Agar plugs (7 mm) were cut from the periphery of a 2-day-old colony of each isolate using a laboratory cork borer and placed in the center of each plate with PDA containing the respective fungicide concentration using sterile metal tweezers.
Colony diameters were measured as described in
Section 4.6 on the third day of incubation at 20 °C in the dark. Each treatment was performed in triplicate. EC
50 values were calculated by fitting a logarithmic model to the relationship between colony diameter and fungicide concentration using GraphPad Prism 9.2.0 (GraphPad Software Inc., Boston, MA, USA).
4.10. Statistical Analysis and Visualization
All statistical analyses were performed in Statistica 12.0 (TIBCO Software Inc., Palo Alto, CA, USA). Data were first tested for normality using the Shapiro–Wilk test and for homogeneity of variances using Levene’s test. When assumptions were met, differences among isolates were assessed using one-way ANOVA, followed by Duncan’s multiple range test at
p = 0.05 to separate means. For features of strains growth, pathogenicity assays and fungicides EC
50 values, each isolate had 3–5 biological replicates as indicated in
Supplementary Table S2. Graphs were visualized in GraphPad Prism 9.2.0.