Comparative Evaluation of the Digestive Enzyme Inhibition, Protein, and Starch Components of Ten Macrotyloma uniflorum (Lam.) Verdc. Accessions
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collection
2.2. Methanol Extraction for In Vitro Assays
2.3. Propagation of M. uniflorum Seeds
2.4. In Vitro Enzyme Inhibition Assays
2.4.1. Reagents
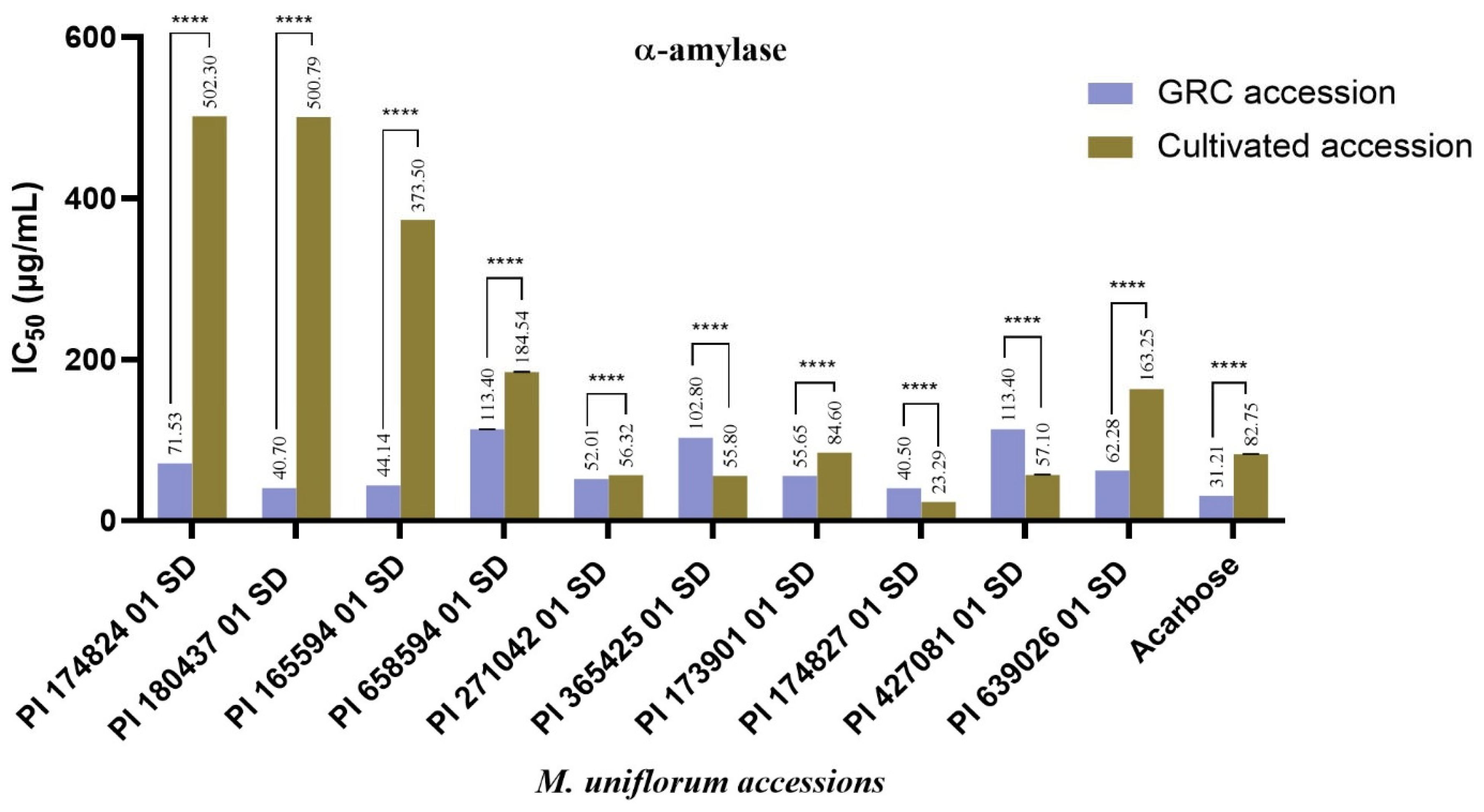
2.4.2. In Vitro α-Amylase Inhibition
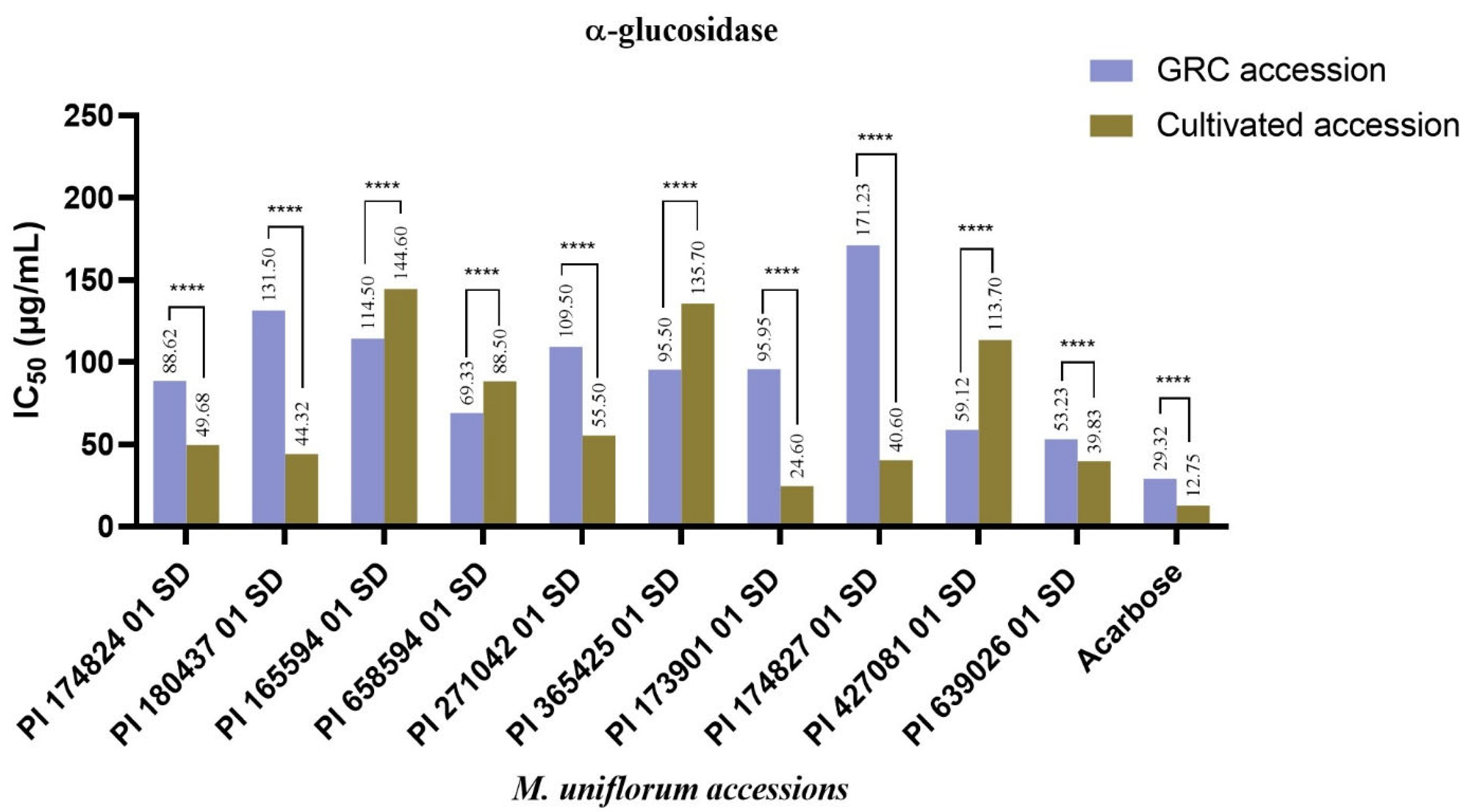
2.4.3. α-Glucosidase Inhibition
2.5. Total Protein Content
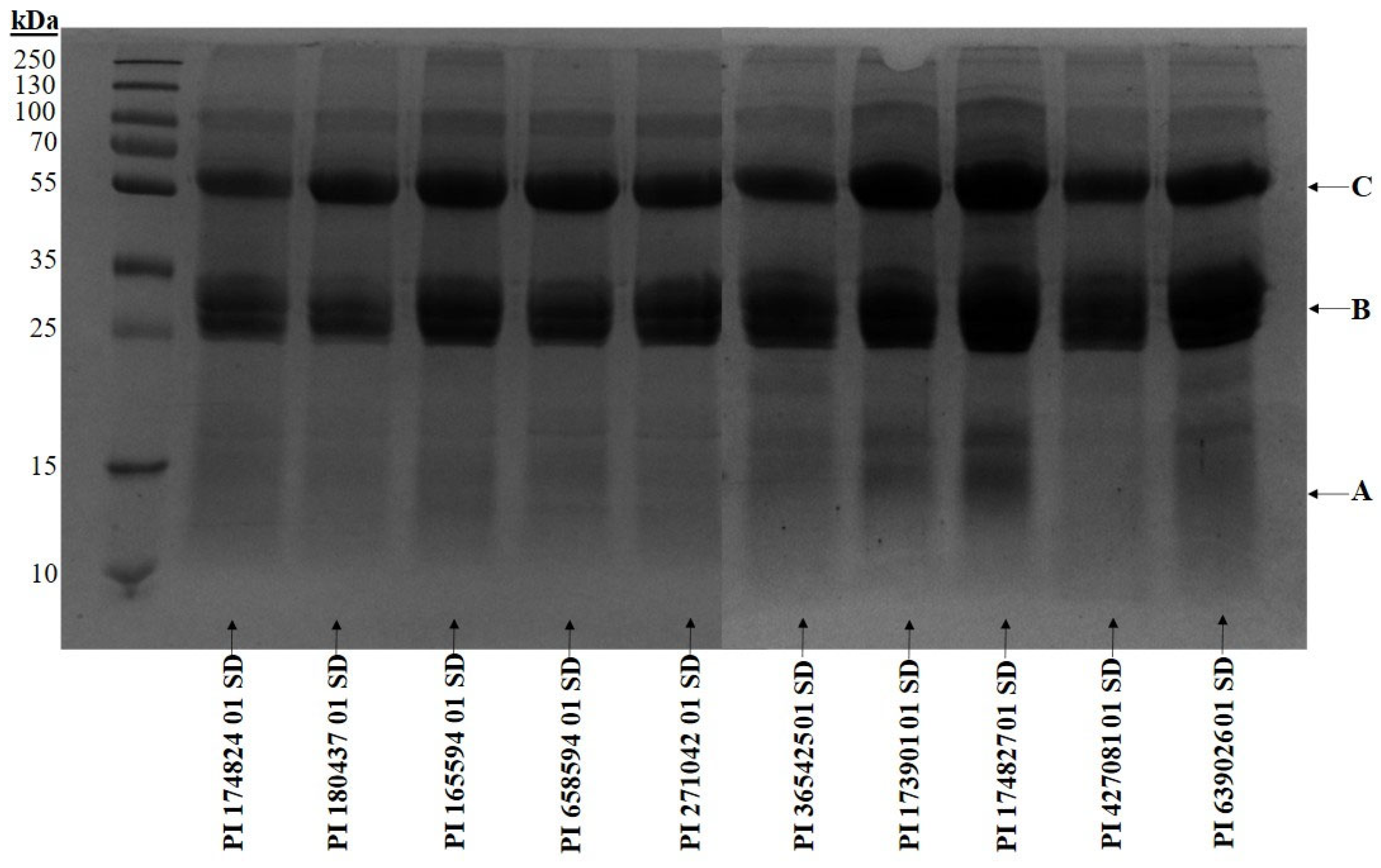
2.6. Electrophoretic Separation of Proteins
2.7. Total Starch Content
2.8. Amylose Content
2.9. Resistant Starch Content
2.10. Data and Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philanim, W.S.; Kumar, A.; Bhupenchandra, I.; Ngangkham, U.; Touthang, L.; Singh, B.K.; Jaiswal, S.; Sangma, R.C.; Singh, N.R.; Chandora, K. Horse Gram (Macrotyloma uniflorum (Lam.) Verdc.). In Potential Pulses: Genetic and Genomic Resources; CABI GB: Wallingford, UK, 2024; pp. 199–216. [Google Scholar]
- Oli, P.; Joshi, K.; Punetha, S. Traditional uses, phytochemistry, pharmacology, and nutraceutical potential of horse gram (Macrotyloma uniflorum): A systematic review. J. Food Sci. 2024, 89, 8102–8127. [Google Scholar] [CrossRef]
- Ain, S.; Ain, Q. Macrotyloma uniflorum (Lam.) Verdc. In Medicinal and Aromatic Plants of India; Springer: Berlin/Heidelberg, Germany, 2024; Volume 3, pp. 199–209. [Google Scholar]
- Singh, N.; Maurya, V.; Sharma, A.; Kumar, R. Exploring Salt Stress Tolerance in Horsegram (Macrotyloma uniflorum): Insights from Growth, Physiology and Biochemical Approaches. J. Crop Health 2024, 77, 35. [Google Scholar] [CrossRef]
- Ingle, K.P.; Al-Khayri, J.M.; Chakraborty, P.; Narkhede, G.W.; Suprasanna, P. Bioactive compounds of horse gram (Macrotyloma uniflorum lam. [Verdc.]). In Bioactive Compounds in Underutilized Vegetables and Legumes; Springer: Cham, Switzerland, 2020; pp. 1–3. [Google Scholar]
- Vashishth, R.; Semwal, A.D.; Murugan, M.P.; Khan, M.A.; Goel, C. Influence of processing on bioactive compounds, Type-II diabetes related enzyme regulation potential and antiurolithiatic potential of underutilized legume Macrotyloma uniflorum. J. Food Sci. Technol. 2022, 59, 3220–3230. [Google Scholar] [CrossRef] [PubMed]
- Kashid, R.R.; Talekar, S.M.; Sonajirao, K. Horse gram (Macrotyloma uniflorum): Nutraceutical pulse crop: A review. J. Food Sci. Technol. 2021, 52, 2489–2499. [Google Scholar]
- Stachelek, R.K. An Ethnobotanical Study of Herbal Emmenagogues Used by Ayurvedic Doctors in Sri Lanka. Master’s Thesis, California State University, Fullerton, CA, USA, 1998. [Google Scholar]
- Arya, P.; Pandey, S.; Verma, V. Kidney stones formation and use of medicinal plants as antiurolithiatic agents. Univers. J. Pharm. Res. 2017, 2, 2456–8058. [Google Scholar] [CrossRef]
- Vashishth, R.; Semwal, A.; Naika, M.; Sharma, G.; Kumar, R. Influence of cooking methods on antinutritional factors, oligosaccharides and protein quality of underutilized legume Macrotyloma uniflorum. Food Res. Int. 2021, 143, 110299. [Google Scholar] [CrossRef]
- Venketeish, K.N.; Govindarajan, N.; Pandiselvam, R.; Khaneghah, A.M. Unlocking the potential of underutilized legumes: Nutritional benefits, anti-nutritional challenges, and mitigation strategies. J. Food Meas. Charact. 2024, 18, 9588–9602. [Google Scholar] [CrossRef]
- Rlds, R.; Erhss, E. Medicinal and nutritional values of Macrotyloma uniflorum (Lam.) verdc (kulattha): A conceptual study. Glob. J. Pharm. Pharm. Sci. 2017, 1, 44–53. [Google Scholar] [CrossRef]
- Gautam, M.; Katoch, S.; Chahota, R.K. Comprehensive nutritional profiling and activity directed identification of lead antioxidant, antilithiatic agent from Macrotyloma uniflorum (Lam.) Verdc. Food Res. Int. 2020, 137, 109600. [Google Scholar] [CrossRef]
- Hafiz, M.S.Y. The Effect of Pulse Consumption on Carbohydrate Digestion, Markers of Glycaemia and Satiety. Ph.D. Thesis, University of Leeds, Leeds, UK, 2022. [Google Scholar]
- Ogbole, O.O.; Noleto-Dias, C.; Kamdem, R.S.; Akinleye, T.E.; Nkumah, A.; Ward, J.L.; Beale, M.H. γ-Glutamyl-β-phenylethylamine, a novel α-glucosidase and α-amylase inhibitory compound from Termitomyces robustus, an edible Nigerian mushroom. Nat. Prod. Res. 2021, 36, 4675–4685. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, J.F.; Khan, A.; Fettke, J. Gradual Analytics of Starch-Interacting Proteins Revealed the Involvement of Starch-Phosphorylating Enzymes during Synthesis of Storage Starch in Potato (Solanum tuberosum L.) Tubers. Molecules 2023, 28, 6219. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Laignelet, B. An improved colorimetric procedure for determining apparent and total amylose in cereal and other starches. J. Cereal Sci. 1983, 1, 9–20. [Google Scholar] [CrossRef]
- Konik-Rose, C.; Thistleton, J.; Chanvrier, H.; Tan, I.; Halley, P.; Gidley, M.; Kosar-Hashemi, B.; Wang, H.; Larroque, O.; Ikea, J.; et al. Effects of starch synthase IIa gene dosage on grain, protein and starch in endosperm of wheat. Theor. Appl. Genet. 2007, 115, 1053–1065. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Food-derived non-phenolic α-amylase and α-glucosidase inhibitors for controlling starch digestion rate and guiding diabetes-friendly recipes. LWT 2022, 153, 112455. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Mishra, A. α-Glucosidase inhibitors for diabetes/blood sugar regulation. In Natural Products as Enzyme Inhibitors: An Industrial Perspective; Springer: Singapore, 2022; pp. 269–283. [Google Scholar]
- McClements, D.J. Food hydrocolloids: Application as functional ingredients to control lipid digestion and bioavailability. Food Hydrocoll. 2021, 111, 106404. [Google Scholar] [CrossRef]
- Tsukamoto, C.; Kikuchi, A.; Harada, K.; Kitamura, K.; Okubo, K. Genetic and chemical polymorphisms of saponins in soybean seed. Phytochemistry 1993, 34, 1351–1356. [Google Scholar] [CrossRef]
- Azam, M.; Zhang, S.; Abdelghany, A.M.; Shaibu, A.S.; Feng, Y.; Li, Y.; Tian, Y.; Hong, H.; Li, B.; Sun, J. Seed isoflavone profiling of 1168 soybean accessions from major growing ecoregions in China. Food Res. Int. 2020, 130, 108957. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of environmental conditions on secondary metabolites in medicinal plants: A Literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Adekola, O.S.; Akinleye, T.E.; Nkumah, A.O.; Oyatomi, O.A.; Ogbole, O.O.; Odeku, O.A.; Abberton, M.T. Inhibitory activities of ten accessions of underutilised West Africa legume Macrotyloma geocarpum (Harms) Maréchal & Baudet, growing in Nigeria against metabolic enzymes α-amylase and α-glucosidase. Discov. Food 2024, 4, 71. [Google Scholar] [CrossRef]
- Shvachko, N.A.; Khlestkina, E.K. Molecular genetic bases of seed resistance to oxidative stress during storage. Vavilov J. Genet. Breed. 2020, 24, 451–458. [Google Scholar] [CrossRef]
- Gupta, L.H.; Badole, S.L.; Bodhankar, S.L.; Sabharwal, S.G. Antidiabetic potential of α-amylase inhibitor from the seeds of Macrotyloma uniflorum in streptozotocin-nicotinamide-induced diabetic mice. Pharm. Biol. 2010, 49, 182–189. [Google Scholar] [CrossRef]
- Copeland, L. Cereal starches (excluding rice and maize). In Starch in Food; Elsevier: Amsterdam, The Netherlands, 2024; pp. 259–274. [Google Scholar]
- Oyeyinka, S.A.; Kayitesi, E.; Diarra, S.S.; Adedeji, A.A.; Amonsou, E.O.; Singh, S. Bambara Groundnut Starch. In Food and Potential Industrial Applications of Bambara Groundnut; Springer: Cham, Switzerland, 2021; pp. 85–118. [Google Scholar]
- Wang, Y.; Ou, X.; Al-Maqtari, Q.A.; He, H.J.; Othman, N. Evaluation of amylose content: Structural and functional properties, analytical techniques, and future prospects. Food Chem.: X 2024, 24, 101830. [Google Scholar]
- Koekuyt, H.A.; Marangoni, A.G. The solid state and nanostructure of starch: Effects on starch functionality. Crit. Rev. Food Sci. Nutr. 2024, 65, 4488–4509. [Google Scholar] [CrossRef]
- Bhagyawant, S.S.; Narvekar, D.T.; Gupta, N.; Bhadkaria, A.; Gautam, A.K.; Srivastava, N. Chickpea (Cicer arietinum L.) Lectin Exhibit Inhibition of ACE-I, α-amylase and α-glucosidase Activity. Protein Pept. Lett. 2019, 26, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, V.A.; Tank, J.G. Biomolecules regulating defense mechanism in plants. Proc. Natl. Acad. Sci. India Sect. B: Biol. Sci. 2023, 93, 17–25. [Google Scholar] [CrossRef]
- Lannoo, N.; Van Damme, E.J. Lectin domains at the frontiers of plant defense. Front. Plant Sci. 2014, 5, 397. [Google Scholar] [CrossRef] [PubMed]
- Jeyachandran, S.; Vibhute, P. Lectins: Versatile proteins in plant life with diverse applications in immunomodulation, antibacterial defense, and disease-fighting. J. Carbohydr. Chem. 2024, 43, 243–288. [Google Scholar] [CrossRef]
- Mishra, A.; Behura, A.; Mawatwal, S.; Kumar, A.; Naik, L.; Mohanty, S.S.; Manna, D.; Dokania, P.; Mishra, A.; Patra, S.K.; et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol. 2019, 134, 110827. [Google Scholar] [CrossRef]
| Accession | Total Protein (%w/w d.w) | Total Starch (%w/w d.w.) | Resistant Starch (%w/w d.w.) | Amylose Content (%w/w d.w.) |
|---|---|---|---|---|
| PI 174824 01 SD | 24.13 ± 0.98 a | 27.48 ± 1.89 f | 6.99 ± 0.00 ab | 36.07 ± 0.68 e |
| PI 180437 01 SD | 27.08 ± 0.66 a | 38.83 ± 5.56 d | 5.89 ± 0.00 d | 27.05 ± 0.51 f |
| PI 165594 01 SD | 14.90 ± 0.25 c | 38.17 ± 2.81 d | 7.04 ± 0.00 a | 34.57 ± 0.28 e |
| PI 658594 01 SD | 26.64 ± 0.53 a | 51.23 ± 2.99 b | 7.09 ± 0.00 a | 38.28 ± 0.58 d |
| PI 271042 01 SD | 13.81 ± 0.46 c | 33.60 ± 4.94 e | 6.03 ± 0.00 d | 35.12 ± 0.30 e |
| PI 365425 01 SD | 13.85 ± 0.50 c | 34.01 ± 6.92 e | 6.65 ± 0.00 b | 42.76 ± 1.55 c |
| PI 173901 01 SD | 18.69 ± 0.73 b | 42.84 ± 5.55 c | 6.31 ± 0.01 c | 38.68 ± 0.55 d |
| PI 174827 01 SD | 19.19 ± 0.28 b | 40.05 ± 5.09 cd | 6.97 ± 0.00 ab | 48.13 ± 0.26 a |
| PI 427081 01 SD | 14.67 ± 0.31 c | 42.08 ± 4.02 c | 6.49 ± 0.01 c | 46.24 ± 0.74 b |
| PI 639026 01 SD | 21.19 ± 0.80 b | 54.70 ± 4.51 a | 6.79 ± 0.01 b | 40.49 ± 0.93 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogunniyi, Q.A.; Molokwu, A.F.; Nkumah, A.O.; Adegoke, A.A.; Oyatomi, O.A.; Ogbole, O.O.; Odeku, O.A.; Fettke, J.; Abberton, M.T. Comparative Evaluation of the Digestive Enzyme Inhibition, Protein, and Starch Components of Ten Macrotyloma uniflorum (Lam.) Verdc. Accessions. Plants 2025, 14, 3483. https://doi.org/10.3390/plants14223483
Ogunniyi QA, Molokwu AF, Nkumah AO, Adegoke AA, Oyatomi OA, Ogbole OO, Odeku OA, Fettke J, Abberton MT. Comparative Evaluation of the Digestive Enzyme Inhibition, Protein, and Starch Components of Ten Macrotyloma uniflorum (Lam.) Verdc. Accessions. Plants. 2025; 14(22):3483. https://doi.org/10.3390/plants14223483
Chicago/Turabian StyleOgunniyi, Queeneth A., Ada F. Molokwu, Abraham O. Nkumah, Abdullahi A. Adegoke, Olaniyi A. Oyatomi, Omonike O. Ogbole, Oluwatoyin A. Odeku, Joerg Fettke, and Michael T. Abberton. 2025. "Comparative Evaluation of the Digestive Enzyme Inhibition, Protein, and Starch Components of Ten Macrotyloma uniflorum (Lam.) Verdc. Accessions" Plants 14, no. 22: 3483. https://doi.org/10.3390/plants14223483
APA StyleOgunniyi, Q. A., Molokwu, A. F., Nkumah, A. O., Adegoke, A. A., Oyatomi, O. A., Ogbole, O. O., Odeku, O. A., Fettke, J., & Abberton, M. T. (2025). Comparative Evaluation of the Digestive Enzyme Inhibition, Protein, and Starch Components of Ten Macrotyloma uniflorum (Lam.) Verdc. Accessions. Plants, 14(22), 3483. https://doi.org/10.3390/plants14223483





