Phytoremediation Potential of Heavy Metals Using Biochar and Accumulator Plants: A Sustainable Approach Towards Cleaner Environments
Abstract
1. Introduction
2. Results
2.1. Physicochemical Characterization and Heavy Metal Content in Coconut Fiber Biochar
2.2. Coconut Fiber Biochar FTIR Analysis
2.3. Thermogravimetric Analysis (TGA) of the Coconut Fiber Biochar
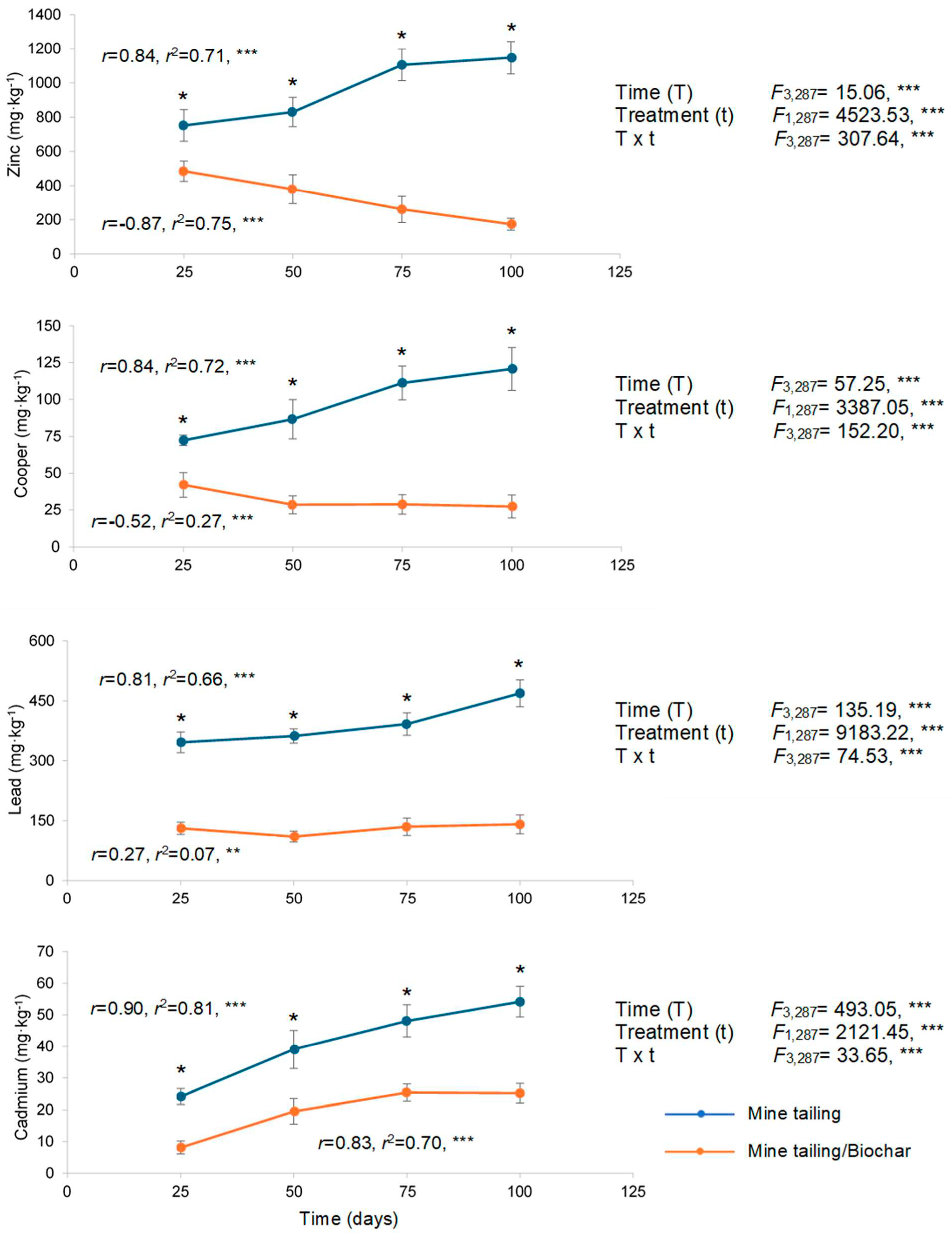
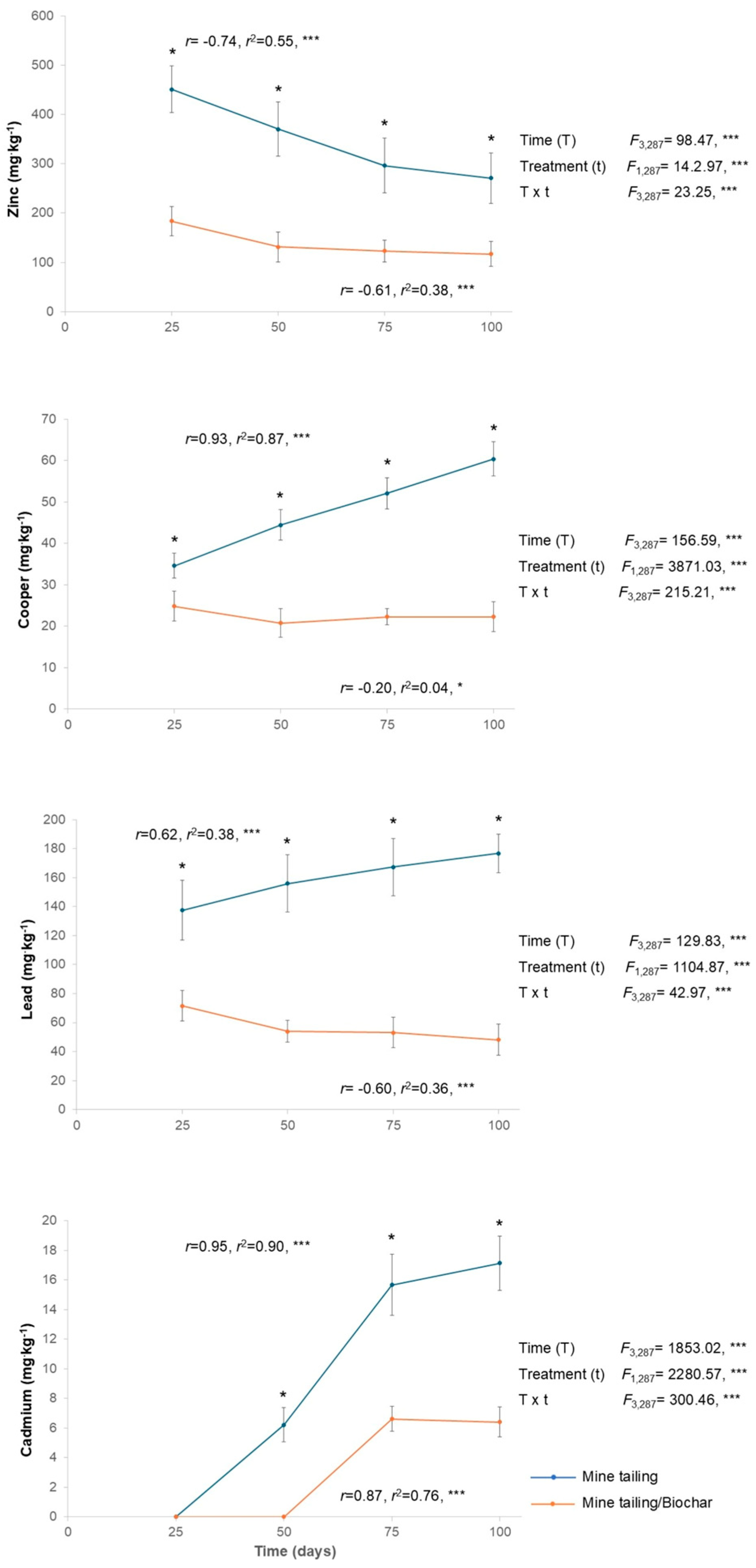
2.4. Heavy Metal Bioaccumulation in Roots and Leaves of S. procumbens Growing on Tailing/Tailing-Biochar Substrates
2.4.1. Roots
2.4.2. Leaves
2.5. Bioconcentration and Translocation Factors of S. procumbens Grown in Tailing and Tailing/Biochar Substrates
2.6. Effect of Tailing and Tailing/Biochar Substrates on S. procumbens Biomass
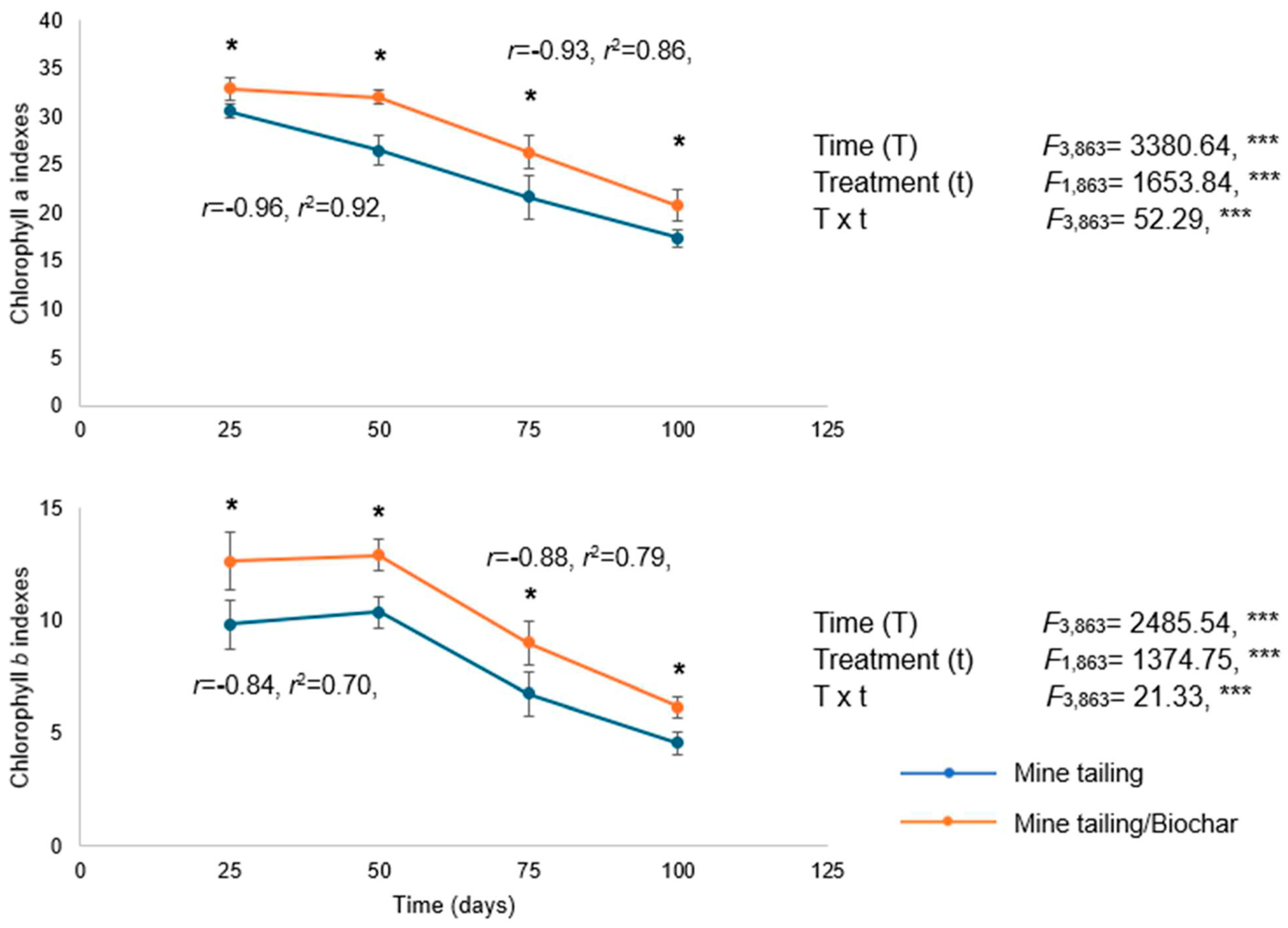
2.7. Effect of Tailing and Tailing/Biochar Substrates on Chlorophyll a and b Content of S. procumbens Individuals
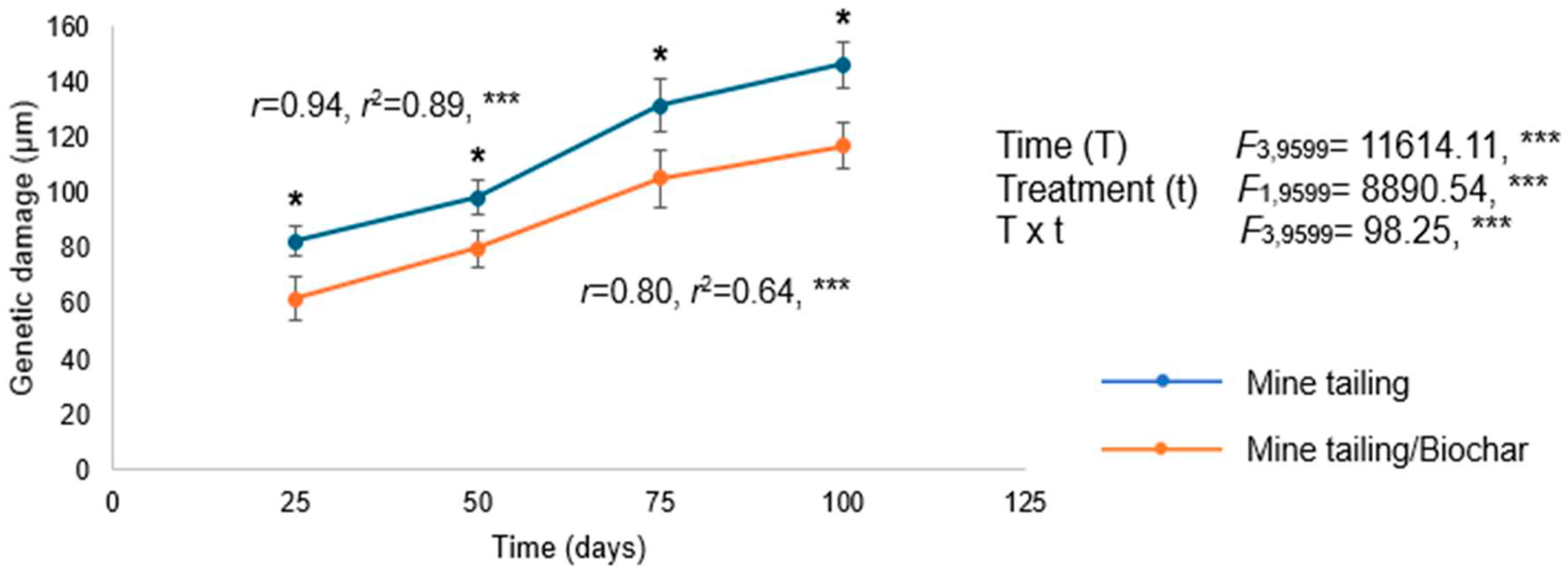
2.8. Effect of Tailing and Tailing/Biochar Substrates on Genetic Damage (Single-Strand DNA Breaks) of S. procumbens Individuals
3. Discussion
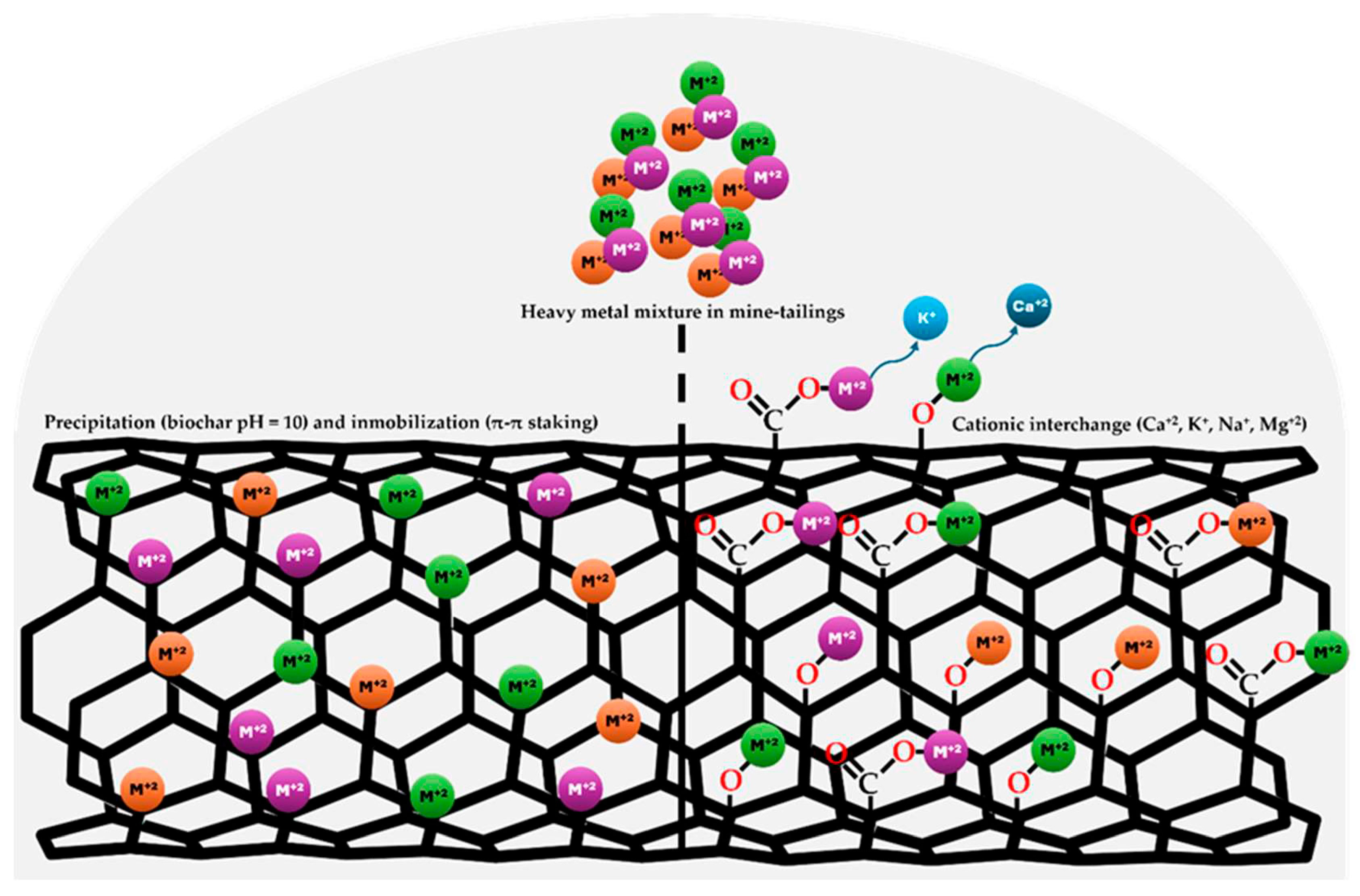
3.1. Physicochemical Characterization of Coconut Biochar and Its Influence on Heavy Metal Immobilization in Soil
3.2. Influence of Coconut Fiber Biochar Incorporated in Tailing Substrate on HM Bioaccumulation, Bioconcentration and Translocation Factors in S. procumbens Individuals
3.3. Influence of Coconut Fiber Biochar Incorporated in Tailing Substrate on Biomass Traits in S. procumbens Individuals
3.4. Influence of Coconut Fiber Biochar Incorporated in Tailing Substrate on Chlorophyll a and b Content in S. procumbens Individuals
3.5. Influence of Coconut Fiber Biochar Incorporated in Tailing Substrate on Genotoxic Damage (DNA Single Strand Breaks) in S. procumbens Individuals
4. Materials and Methods
4.1. Study Site
4.2. Study Species
4.3. Seed Germination and Seedling Establishment
4.4. Coconut Fiber Biochar Production and Physico-Chemical Characterization
4.5. Biomass Determination
4.6. Heavy Metal Concentrations
4.7. Bioconcentration Factor (BCF) and Translocation Factor (TF)
4.8. Single Cell Gel Electrophoresis or Comet Assay for Leaf Tissue
4.9. Chlorophyll a and b Content
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Stefani, P.; Rogelio, C.; Amílcar, S.; del Carmen, A. Fitorremediación de un residuo de mina asistida con enmiendas y bacterias promotoras de crecimiento. Rev. Latinoam. Biotecnol. Ambient. Algal. 2015, 6, 3. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.; Li, J.; Baek, K.; Hou, D.; Ding, S.; Poon, C. Recycling dredged sediment into fill materials, partition blocks, and paving blocks: Technical and economic assessment. J. Clean. Prod. 2018, 199, 69–76. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Y.; He, Z.; Stoffella, P. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 339–353. [Google Scholar] [CrossRef]
- Ogundiran, M.; Mekwunyei, N.; Adejumo, S. Compost and biochar assisted phytoremediation potentials of Moringa oleifera for remediation of lead contaminated soil. J. Environ. Chem. Eng. 2018, 6, 2206–2213. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Mendez, A.; Gasco, G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.; Berruti, F.; Kozinski, J. Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste Biomass Valorization 2016, 7, 201–235. [Google Scholar] [CrossRef]
- Wu, W.; Li, J.; Lan, T.; Müller, K.; Niazi, N.; Chen, X.; Xu, S.; Zheng, L.; Chu, Y.; Li, J.; et al. Unraveling sorption of lead in aqueous solutions by chemically modified biochar derived from coconut fiber: A microscopic and spectroscopic investigation. Sci. Total Environ. 2017, 576, 766–774. [Google Scholar] [CrossRef]
- Luna, D.; González, A.; Gordon, M.; Martín, N. Obtención de carbón activado a partir de la cáscara de coco. ContactoS 2007, 64, 39–48. [Google Scholar]
- Dhar, S.; Sakib, T.; Hilary, L. Effects of pyrolysis temperature on production and physicochemical characterization of biochar derived from coconut fiber biomass through slow pyrolysis process. Biomass Convers. Biorefinery 2022, 12, 2631–2647. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Guo, X.; Liu, H.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2020, 102, 884–899. [Google Scholar] [CrossRef] [PubMed]
- Field, J.; Keske, C.; Birch, G.; DeFoort, M.; Cotrufo, M. Distributed biochar and bioenergy coproduction: A regionally specific case study of environmental benefits and economic impacts. Gcb Bioenergy 2013, 5, 177–191. [Google Scholar] [CrossRef]
- Patra, J.; Panda, S.; Dhal, N. Biochar as a low-cost adsorbent for heavy metal removal: A review. Int. J. Res. Biosci. 2017, 6, 1–7. [Google Scholar]
- Rasheed, A.; Hassan, M.; Aamer, M.; Bian, J.; Xu, Z.; He, X.; Wu, Z. Iron toxicity, tolerance and quantitative trait loci mapping in rice; a review. Appl. Ecol. Environ. Res. 2020, 18, 7483–7498. [Google Scholar] [CrossRef]
- Raza, A.; Habib, M.; Charagh, S.; Kakavand, S. Genetic engineering of plants to tolerate toxic metals and metalloids. In Handbook of bioremediation; Academic Press: Cambridge, MA, USA, 2021; pp. 411–436. [Google Scholar] [CrossRef]
- Cho-Ruk, K.; Kurukote, J.; Supprung, P.; Vetayasuporn, S. Perennial plants in the phytoremediation of lead-contaminated soils. Biotechnology 2006, 5, 1–4. [Google Scholar] [CrossRef]
- González, R.; González-Chávez, M. Metal accumulation in wild plants surrounding mining wastes. Environ. Pollut. 2006, 144, 84–92. [Google Scholar] [CrossRef]
- Rasti, S.; Rajabzadeh, M.; Park, J. Effective phytoremediation of soil contamination through native iranian hyperaccumulator plant species. Int. J. Environ. Sci. Technol. 2025, 22, 16991–17012. [Google Scholar] [CrossRef]
- Huang, R.; Xing, C.; Yang, Y.; Yu, W.; Zeng, L.; Li, Y.; Tan, Z.; Li, Z. Phytoremediation and environmental effects of three Amaranthaceae plants in contaminated soil under intercropping systems. Sci. Total Environ. 2024, 914, 169900. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Y.; Norbu, N.; Wang, Z. Remediation of biochar on heavy metal polluted soils. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 042113. [Google Scholar] [CrossRef]
- Ramírez-Zamora, J.; Mussali-Galante, P.; Rodríguez, A.; Castrejón-Godínez, M.; Valencia-Cuevas, L.; Tovar-Sánchez, E. Assisted phytostabilization of mine-tailings with Prosopis laevigata (Fabaceae) and biochar. Plants 2022, 11, 3441. [Google Scholar] [CrossRef]
- Rosas-Ramírez, M.; Tovar-Sánchez, E.; Rodríguez-Solís, A.; Flores-Trujillo, K.; Castrejón-Godínez, M.L.; Mussali-Galante, P. Assisted phytoremediation between biochar and Crotalaria pumila to phytostabilize heavy metals in mine tailings. Plants 2024, 13, 2516. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Zhang, J.; Zheng, L.; Chen, D.; Wu, Z.; Shaheen, S.; Rinklebe, J.; Ok, Y.; Wang, H.; et al. Coconut-fiber biochar reduced the bioavailability of lead but increased it translocation rate in rice plants: Elucidation of immobilization mechanisms and significance of iron plaque barrier on roots using spectroscopic techniques. J. Hazard. Mater. 2020, 389, 122177. [Google Scholar] [CrossRef]
- Tan, X.; Zhu, S.; Wang, R.; Chen, Y.; Show, P.; Zhang, F.; Ho, S. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T. Characteristics of activated carbon prepared from pistachio-nut shell by zinc chloride activation under nitrogen and vacuum conditions. J. Colloid Interface Sci. 2005, 290, 505–513. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the Removal of Contaminants from Soil and Water: A Review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.; Lim, J.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.; Ok, Y. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Ajien, A.; Idris, J.; Md Sofwan, N.; Husen, R.; Seli, H. Coconut shell and husk biochar: A review of production and activation technology, economic, financial aspect and application. Waste Manag. Res. 2023, 41, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Ighalo, J.; Conradie, J.; Ohoro, C.; Amaku, J.; Oyedotun, K.; Maxakato, N.; Akpomie, K.; Okeke, E.; Olisah, C.; Malloum, A. Biochar from Coconut Residues: An Overview of Production, Properties, and Applications. Ind. Crops Prod. 2023, 204, 117300. [Google Scholar] [CrossRef]
- Vasić, V.; Kukić, D.; Šćiban, M.; Đurišić-Mladenović, N.; Velić, N.; Pajin, B.; Crespo, J.; Farre, M.; Šereš, Z. Lignocellulose-Based Biosorbents for the Removal of Contaminants of Emerging Concern (CECs) from Water: A Review. Water 2023, 15, 1853. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total Environ. 2020, 713, 136433. [Google Scholar] [CrossRef]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar applications and modern techniques for characterization. Clean Technol. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]
- Yi, Q.; Qi, F.; Cheng, G.; Zhang, Y.; Xiao, B.; Hu, Z.; Liu, S.; Cai, H.; Xu, S. Thermogravimetric analysis of co-combustion of biomass and biochar. J. Therm. Anal. Calorim. 2013, 112, 1475–1479. [Google Scholar] [CrossRef]
- Pflieger, C.; Eckhard, T.; Schmitz, G.; Angenent, V.; Göckeler, M.; Senneca, O.; Schmid, R.; Cerniello, F.; Muhler, M. Thermicity of the decomposition of oxygen functional groups on cellulose-derived chars. ACS Omega 2022, 7, 48606–48614. [Google Scholar] [CrossRef]
- Alkharabsheh, H.; Seleiman, M.; Battaglia, M.; Shami, A.; Jalal, R.; Alhammad, B.; Almutairi, K.; Al-Saif, A. Biochar and Its Broad Impacts in Soil Quality and Fertility, Nutrient Leaching and Crop Productivity: A Review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application Research of Biochar for the Remediation of Soil Heavy Metals Contamination: A Review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wang, Y.; Wei, Y. Advancements in Biochar for Soil Remediation of Heavy Metals and/or Organic Pollutants. Materials 2025, 18, 1524. [Google Scholar] [CrossRef] [PubMed]
- Sukartono, W.H.; Nugroho, W.H.; Kusuma, Z. Simple biochar production generated from cattle dung and coconut shell. J. Basic Appl. Sci. Res. 2011, 1, 680–1685. [Google Scholar]
- Devens, K.U.; Neto, S.P.; Oliveira, D.L.D.A.; Gonçalves, M.S. Characterization of biochar from green coconut shell and orange peel wastes. Rev. Virtual Quim. 2018, 10, 288–294. [Google Scholar] [CrossRef]
- Guarnieri, S.F.; do Nascimento, E.C.; Costa Junior, R.F.; de Faria, J.L.B.; de Almeida Lobo, F. Coconut fiber biochar alters physical and chemical properties in sandy soils. Acta Sci. Agron. 2021, 43, e51801. [Google Scholar] [CrossRef]
- de Lima, K.C.; de Souza, D.M.; Kappler, G.K.K.; Modolo, R.C.E.; Mendes Moraes, C.A. In natura and carbonized coconut fiber characterization from pyrolysis process. Mix Sustentável 2023, 8, 27–38. [Google Scholar] [CrossRef]
- Ma, L.; Xu, R.; Jiang, J. Adsorption and desorption of Cu (II) and Pb (II) in paddy soils cultivated for various years in the subtropical China. J. Environ. Sci. 2010, 22, 689–695. [Google Scholar] [CrossRef]
- Uchimiya, M.; Lima, I.; Klasson, K.; Wartelle, L. Contaminant immobilization and nutrient release by biochar soil amendment: Roles of natural organic matter. Chemosphere 2010, 80, 935–940. [Google Scholar] [CrossRef]
- Shen, X.; Huang, D.; Ren, X.; Zhu, H.; Wang, S.; Xu, C.; He, Y.; Luo, Z.; Zhu, Q. Phytoavailability of Cd and Pb in crop straw biochar-amended soil is related to the heavy metal content of both biochar and soil. J. Environ. Manag. 2016, 168, 245–251. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, L.; Li, Z.; Zhou, X.; Cheng, S.; Zhang, L.; Zhang, Q. Effects of various pyrolysis conditions and feedstock compositions on the physicochemical characteristics of cow manure-derived biochar. J. Clean. Prod. 2021, 311, 127458. [Google Scholar] [CrossRef]
- Wood, R.; Mašek, O.; Erastova, V. Developing a Molecular-Level Understanding of Biochar Materials Using Public Characterization Data. Cell Rep. Phys. Sci. 2024, 5, 1. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Wu, W.; Li, J.; Niazi, N.; Müller, K.; Chu, Y.; Zhang, L.; Yuan, G.; Lu, K.; Song, Z.; Wang, H. Influence of pyrolysis temperature on lead immobilization by chemically modified coconut fiber-derived biochars in aqueous environments. Environ. Sci. Pollut. Res. 2016, 23, 22890–22896. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Gautam, S. Pyrolysis of coconut husk biomass: Analysis of its biochar properties. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 761–767. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; Da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Romero Millán, L.; Sierra Vargas, F.; Nzihou, A. Characterization of steam gasification biochars from lignocellulosic agrowaste towards soil applications. Waste Biomass Valorization 2021, 12, 4141–4155. [Google Scholar] [CrossRef]
- Mohammad, I.; Abakr, Y.; Kabir, F.; Yusuf, S.; Alshareef, I.; Chin, S. Pyrolysis of Napier grass in a fixed bed reactor: Effect of operating conditions on product yields and characteristics. BioResources 2015, 10, 6457–6478. [Google Scholar] [CrossRef]
- Lee, J.; Kidder, M.; Evans, B.; Paik, S.; Buchanan Iii, A.; Garten, C.; Brown, R. Characterization of biochars produced from cornstovers for soil amendment. Environ. Sci. Technol. 2010, 44, 7970–7974. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal–a review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Noronha, F.; Manikandan, S.; Nair, V. Role of coconut shell biochar and earthworm (Eudrilus euginea) in bioremediation and palak spinach (Spinacia oleracea L.) growth in cadmium-contaminated soil. J. Environ. Manag. 2022, 302, 114057. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; de Jesus Santos, J.C.; Ganassali Junior, L.F.; Fontes, P.T.N.; Araújo, J.d.S.; Gonzaga, T.A.S. Copper uptake, physiological response, and phytoremediation potential of Brassica juncea under biochar application. Int. J. Phytorem. 2022, 24, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Aborisade, M.; Geng, H.; Oba, B.; Kumar, A.; Ndudi, E.; Battamo, A.; Liu, J.; Chen, D.; Okimiji, O.; Ojekunle, O.; et al. Remediation of soil polluted with Pb and Cd and alleviation of oxidative stress in Brassica rapa plant using nanoscale zerovalent iron supported with coconut-husk biochar. J. Plant Physiol. 2023, 287, 154023. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.; El-Sherbini, M.; Selim, E. Effects of biochar on soil properties, heavy metal availability and uptake, and growth of summer squash grown in metal-contaminated soil. Sci. Hortic. 2022, 301, 111097. [Google Scholar] [CrossRef]
- Eissa, M.A. Effect of cow manure biochar on heavy metals uptake and translocation by zucchini (Cucurbita pepo L). Arab. J. Geosci. 2019, 12, 48. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.; Tang, L.; Dong, H.; Huang, B.; et al. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.; Lee, S.; Al-Wabel, M.; Tsang, D.; Ok, Y. Biochar-induced changes in soil properties affected immobilization/mobilization of metals/metalloids in contaminated soils. J. Soils Sediments 2017, 17, 717–730. [Google Scholar] [CrossRef]
- Granados, D.; López, G. Manejo de la palma de coco (Cocos nucifera L.) en México. Revista Chapingo. Ser. Cienc. For. Ambiente 2002, 8, 39. [Google Scholar]
- Trujillo-Sánchez, A.F.; Arias-Maya, L.S. El coco, recurso renovable para el diseño de materiales verdes. Entre Cienc. Ing. 2024, 17, 45–58. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Biomass Bioenergy 2013, 57, 196–204. [Google Scholar] [CrossRef]
- Fiaz, K.; Danish, S.; Younis, U.; Malik, S.; Raza Shah, M.; Niaz, S. Drought impact on Pb/Cd toxicity remediated by biochar in Brassica campestris. Soil Sci. Plant Nutr. 2014, 14, 845–854. [Google Scholar] [CrossRef]
- Shi, G.; Xia, S.; Liu, C.; Zhang, Z. Cadmium accumulation and growth response to cadmium stress of eighteen plant species. Environ. Sci. Pollut. Res. 2016, 23, 23071–23080. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.; Macri, C.; Miard, F.; Hattab, N.; Motelica, M.; Morabito, D.; Bourgerie, S. Effect of biochar amendments on As and Pb mobility and phytoavailability in contaminated mine technosols phytoremediated by Salix. J. Geochem. Explor. 2017, 182, 149–156. [Google Scholar] [CrossRef]
- Irfan, M.; Muhammad, M.; Muhammad, J.; Khadim, M.; Dost, M.; Ishaq, A.; Ishaq, A.; Waqas, A.; Shah, F.; Shah, S.; et al. Heavy metals immobilization and improvement in maize (Zea mays L.) growth amended with biochar and compost. Sci. Rep. 2021, 11, 18416. [Google Scholar] [CrossRef]
- Liu, H.; Xu, F.; Xie, Y.; Wang, C.; Zhang, A.; Li, L.; Xu, H. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci. Total Environ. 2018, 645, 702–709. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Zheng, L.; Chen, D.; Wu, Z.; Xie, Y.; Wu, W.; Niazi, N.; Ok, Y.; Rinklebe, J. Sorption of lead in soil amended with coconut fiber biochar: Geochemical and spectroscopic investigations. Geoderma 2019, 350, 52–60. [Google Scholar] [CrossRef]
- Abid, M.; Danish, S.; Zafar-ul-Hye, M.; Shaaban, M.; Iqbal, M.; Rehim, A.; Qayyum, M.; Naqqash, M. Biochar increased photosynthetic and accessory pigments in tomato (Solanum lycopersicum L.) plants by reducing cadmium concentration under various irrigation waters. Environ. Sci. Pollut. Res. 2017, 24, 22111–22118. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ahmad, W.; Hussain, F.; Ahamd, W.; Numan, M.; Shah, M.; Ahmad, I. Phytostabalization of the heavy metals in the soil with biochar applications, the impact on chlorophyll, carotene, soil fertility and tomato crop yield. J. Clean. Prod. 2020, 255, 120318. [Google Scholar] [CrossRef]
- Lyu, S.; Du, G.; Liu, Z.; Zhao, L.; Lyu, D. Effects of biochar on photosystem function and activities of protective enzymes in Pyrus ussuriensis Maxim. under drought stress. Acta Physiol. Plant. 2016, 38, 220. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Shakya, K.; Chettri, M.; Sawidis, T. Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch. Environ. Contam. Toxicol. 2008, 54, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Li, S.; Zeng, L.; Su, Z. Wheat growth, photosynthesis and physiological characteristics under different soil Zn levels. J. Integr. Agric. 2022, 21, 1927–1940. [Google Scholar] [CrossRef]
- Anwar, T.; Shehzadi, A.; Qureshi, H.; Shah, M.; Danish, S.; Salmen, S.; Ansari, M. Alleviation of cadmium and drought stress in wheat by improving growth and chlorophyll contents amended with GA3 enriched deashed biochar. Sci. Rep. 2023, 13, 18503. [Google Scholar] [CrossRef]
- Whiteside, J.; Box, C.; McMillan, T.; Allinson, S. Cadmium and copper inhibit both DNA repair activities of polynucleotide kinase. DNA Repair. 2010, 9, 83–89. [Google Scholar] [CrossRef]
- Ventura, L.; Giovannini, A.; Savio, M.; Donà, M.; Macovei, A.; Buttafava, A.; Carbonera, D.; Balestrazzi, A. Single cell gel electrophoresis (Comet) assay with plants: Research on DNA repair and ecogenotoxicity testing. Chemosphere 2013, 92, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Shen, F.; Guo, H.; Wang, Z.; Yang, G.; Wang, L.; Zhang, Y.; Zeng, Y.; Deng, S. Phytotoxicity assessment on corn stover biochar, derived from fast pyrolysis, based on seed germination, early growth, and potential plant cell damage. Environ. Sci. Pollut. Res. 2015, 22, 9534–9543. [Google Scholar] [CrossRef]
- Zulfiqar, B.; Raza, M.; Saleem, M.; Aslam, M.; Iqbal, R.; Muhammad, F.; Amin, J.; Ibrahim, M.; Khan, I. Biochar enhances wheat crop productivity by mitigating the effects of drought: Insights into physiological and antioxidant defense mechanisms. PLoS ONE 2022, 17, e0267819. [Google Scholar] [CrossRef]
- SEMARNAT. Evaluación de Tecnologías de Remediación Para Suelos Contaminados Con Metales, Etapa II; Secretaría del Medio Ambiente y Recursos Naturales, Instituto Nacional de Ecología, SEMARNAT-INE: México City, México, 2005; p. 36.
- Santoyo-Martínez, M.; Mussali-Galante, P.; Hernández-Plata, I.; Valencia-Cuevas, L.; Rodríguez-Solís, A.; Castrejón-Godínez, M.L.; Tovar-Sánchez, E. Phytoremediation Potential of Crotalaria pumila (Fabaceae) in Soils Polluted with Heavy Metals: Evidence from Field and Controlled Experiments. Plants 2024, 13, 1947. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Núñez, L.F.; Mussali-Galante, P.; Castrejón-Godínez, M.L.; Rodríguez-Solís, A.; Castañeda-Espinoza, J.D.; Tovar-Sánchez, E. In situ phytoremediation of mine tailings with high concentrations of cadmium and lead using Dodonaea viscosa (Sapindaceae). Plants 2024, 14, 69. [Google Scholar] [CrossRef]
- Comisión Nacional para el Conocimiento y Uso de La Biodiversidad (CONABIO). (2009). Malezas de México. Sanvitalia procumbens Lam. Available online: http://www.conabio.gob.mx/malezasdemexico/asteraceae/sanvitalia-procumbens/fichas/ficha.htm (accessed on September 2024).
- Rzedowski, C.; Rzedowski, J. Flora Fanerogámica del Valle de México, 2nd ed.; Instituto de Ecología, A.C. y Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Pátzcuaro, Mexico, 2001; 1406p.
- Gold, K.; León, P.; Way, M. Manual de Recolección de Semillas de Plantas Silvestres; Instituto de Investigaciones Agropecuarias, Centro Regional de Investigación Intihuasi: La Serena, Chile, 2004; pp. 10–62. [Google Scholar]
- NOM-021-SEMARNAT-2000; Establece Las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudios, Muestreos y Análisis. Norma Oficial Mexicana. Diario Oficial de la Federación del: Mexico City, Mexico, 2000.
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Covarrubias, S.; Cabriales, J. Contaminación ambiental por metales pesados en México: Problemática y estrategias de fitorremediación. Rev. Int. Contam. Ambient. 2017, 33, 7–21. [Google Scholar] [CrossRef]
- Tice, R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Sasaki, Y. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Statsoft, Inc. Statistica for Windows; Statsoft, Inc.: Tulsa, OK, USA, 2007. [Google Scholar]

| Concentration (mg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|
| Metal | Time (Days) | BCF (Root) | BCF (Leaf) | TF | TF | ||
| Tailing | Tailing/Biochar | Tailing | Tailing/Biochar | Tailing | Tailing/Biochar | ||
| Zinc | |||||||
| 25 | 1.76 | 1.13 | 1.05 | 0.42 | 0.60 | 0.37 | |
| 50 | 1.94 | 0.98 | 0.86 | 0.30 | 0.44 | 0.34 | |
| 75 | 2.58 | 0.61 | 0.69 | 0.28 | 0.26 | 0.47 | |
| 100 | 2.68 | 0.41 | 0.63 | 0.27 | 0.23 | 0.67 | |
| mean ± SD | 2.24 ± 0.46 | 0.76 ± 0.32 | 0.81 ± 0.18 | 0.32 ± 0.07 | 0.39 ± 0.16 | 0.50 ± 0.20 | |
| t-student | 32.195 *** | 24.938 *** | 4.407 *** | ||||
| Copper | |||||||
| 25 | 9.01 | 5.23 | 4.31 | 3.09 | 0.47 | 0.59 | |
| 50 | 10.79 | 3.53 | 5.53 | 2.58 | 0.51 | 0.73 | |
| 75 | 13.85 | 3.57 | 6.49 | 2.77 | 0.46 | 0.77 | |
| 100 | 15.09 | 3.39 | 7.52 | 2.76 | 0.50 | 0.81 | |
| mean ± SD | 13.17 ± 2.76 | 3.93 ± 0.86 | 5.96 ± 1.36 | 2.80 ± 0.20 | 0.49 ± 0.07 | 0.76 ± 0.21 | |
| t-student | 32.656 *** | 28.167 *** | 12.486 *** | ||||
| Lead | |||||||
| 25 | 49.70 | 18.81 | 36.22 | 11.40 | 0.39 | 0.54 | |
| 50 | 51.97 | 15.82 | 15.92 | 5.97 | 0.43 | 0.48 | |
| 75 | 55.72 | 19.34 | 21.29 | 7.61 | 0.42 | 0.39 | |
| 100 | 67.27 | 20.23 | 22.55 | 6.89 | 0.37 | 0.34 | |
| mean ± SD | 56.17 ± 7.81 | 18.54 ± 3.16 | 23.99 ± 8.64 | 7.97 ± 2.38 | 0.40 ± 0.05 | 0.45 ± 0.12 | |
| t-student | 53.747 *** | 19.893 *** | 3.495 *** | ||||
| Cadmium | |||||||
| 25 | 2.88 | 0.97 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 50 | 4.66 | 2.33 | 0.74 | 0.00 | 0.15 | 0.00 | |
| 75 | 5.76 | 3.09 | 1.87 | 0.79 | 0.32 | 0.26 | |
| 100 | 6.47 | 3.01 | 2.04 | 0.76 | 0.31 | 0.25 | |
| mean ± SD | 4.94 ± 1.46 | 2.33 ± 0.91 | 1.16 ± 0.86 | 0.38 ± 0.39 | 0.20 ± 0.15 | 0.12 ± 0.14 | |
| t-student | 18.061 *** | 9.837 *** | 4.484 *** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Ramírez, M.; Tovar-Sánchez, E.; Rodríguez, A.; Castrejón-Godínez, M.L.; Saldarriaga-Noreña, H.A.; Bretón-Deval, L.; Mussali-Galante, P. Phytoremediation Potential of Heavy Metals Using Biochar and Accumulator Plants: A Sustainable Approach Towards Cleaner Environments. Plants 2025, 14, 3470. https://doi.org/10.3390/plants14223470
Rosas-Ramírez M, Tovar-Sánchez E, Rodríguez A, Castrejón-Godínez ML, Saldarriaga-Noreña HA, Bretón-Deval L, Mussali-Galante P. Phytoremediation Potential of Heavy Metals Using Biochar and Accumulator Plants: A Sustainable Approach Towards Cleaner Environments. Plants. 2025; 14(22):3470. https://doi.org/10.3390/plants14223470
Chicago/Turabian StyleRosas-Ramírez, Marcos, Efraín Tovar-Sánchez, Alexis Rodríguez, María Luisa Castrejón-Godínez, Hugo Albeiro Saldarriaga-Noreña, Luz Bretón-Deval, and Patricia Mussali-Galante. 2025. "Phytoremediation Potential of Heavy Metals Using Biochar and Accumulator Plants: A Sustainable Approach Towards Cleaner Environments" Plants 14, no. 22: 3470. https://doi.org/10.3390/plants14223470
APA StyleRosas-Ramírez, M., Tovar-Sánchez, E., Rodríguez, A., Castrejón-Godínez, M. L., Saldarriaga-Noreña, H. A., Bretón-Deval, L., & Mussali-Galante, P. (2025). Phytoremediation Potential of Heavy Metals Using Biochar and Accumulator Plants: A Sustainable Approach Towards Cleaner Environments. Plants, 14(22), 3470. https://doi.org/10.3390/plants14223470










