Methyl Jasmonate Enhances Saponin Accumulation in Cultured Panax notoginseng Adventitious Roots
Abstract
1. Introduction
2. Results and Discussion
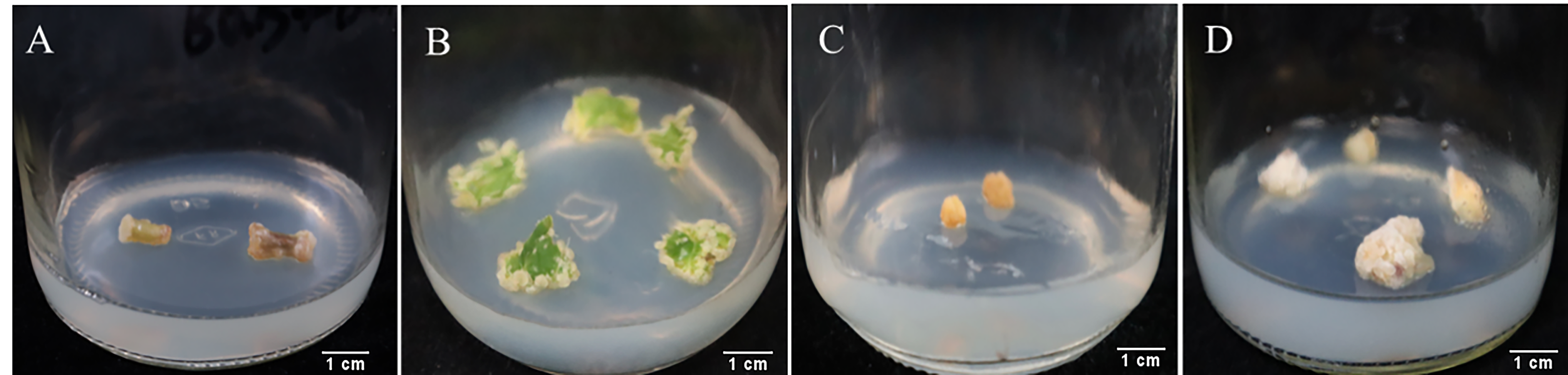
2.1. Effect of Different Explants and Plant Hormones on Callus Induction
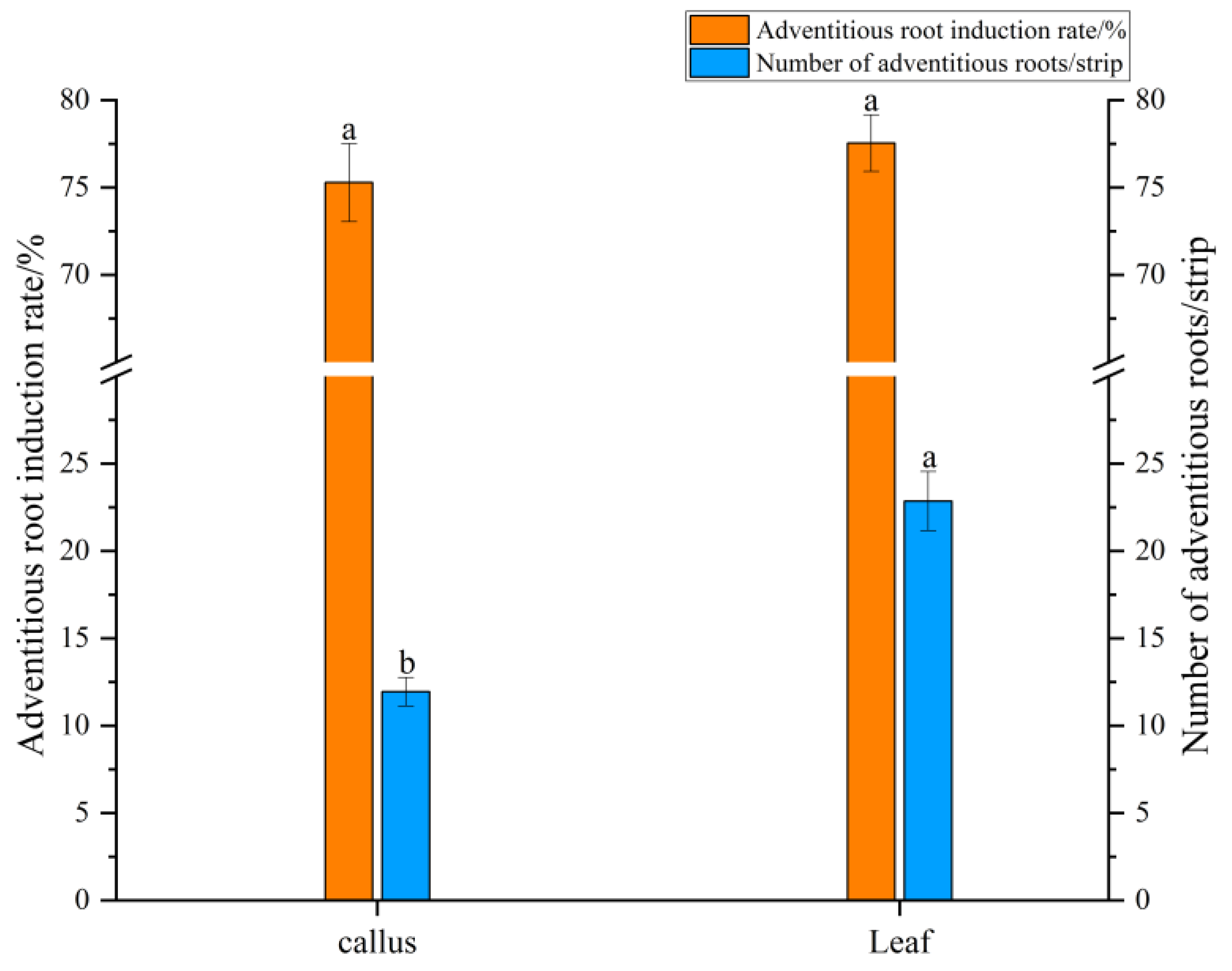
2.2. Adventitious Root Differentiation
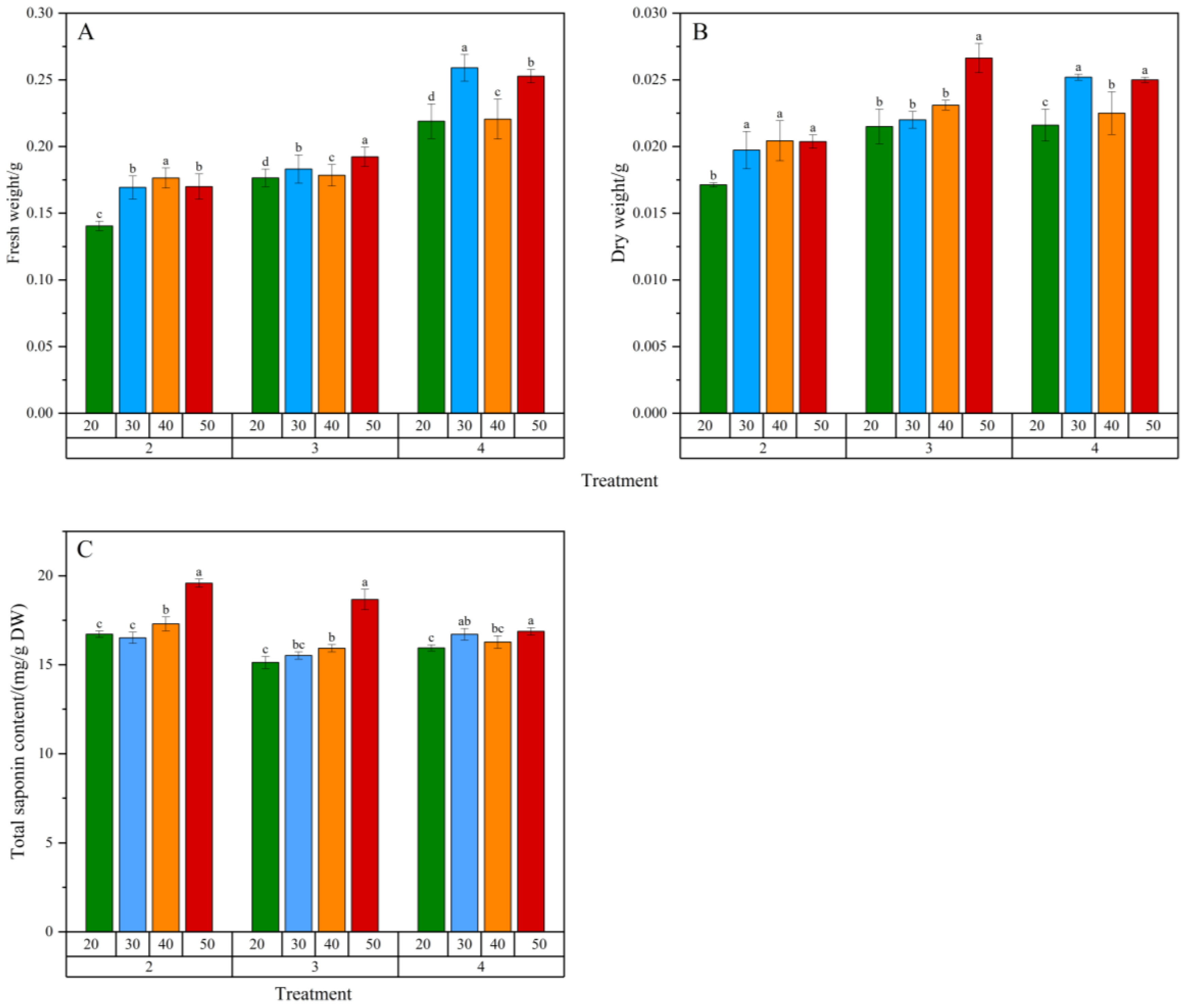
2.3. Effect of Inoculation Amount and Sucrose Concentration on Biomass and Total Saponin Content
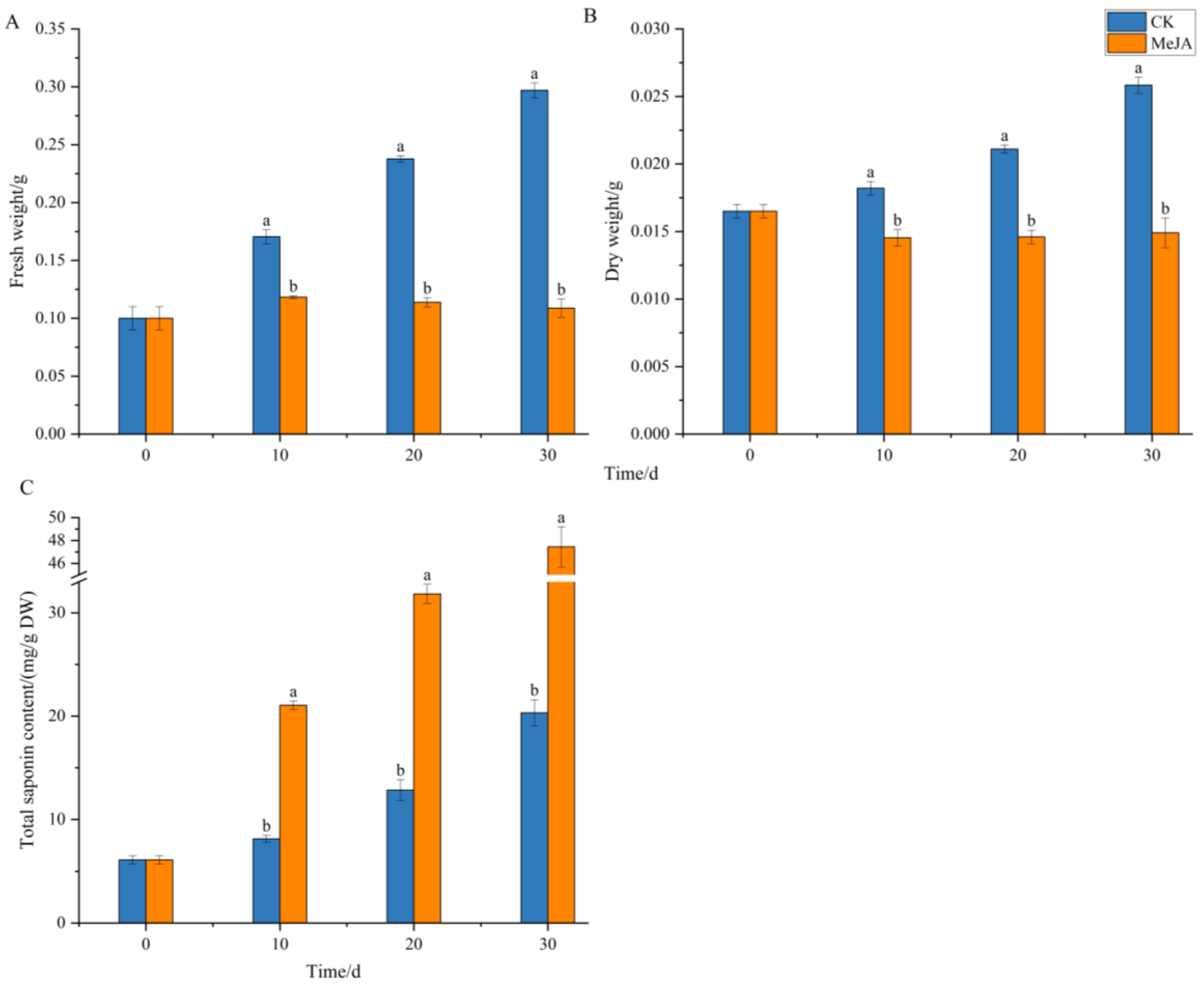
2.4. Effect of Methyl Jasmonate on Biomass and Total Saponin Content
2.5. Metabolomic Analysis of Methyl Jasmonate Induction
2.5.1. Identification of Saponin Monomers
2.5.2. Multivariate Statistical Analysis of Metabolites Induced by Methyl Jasmonate
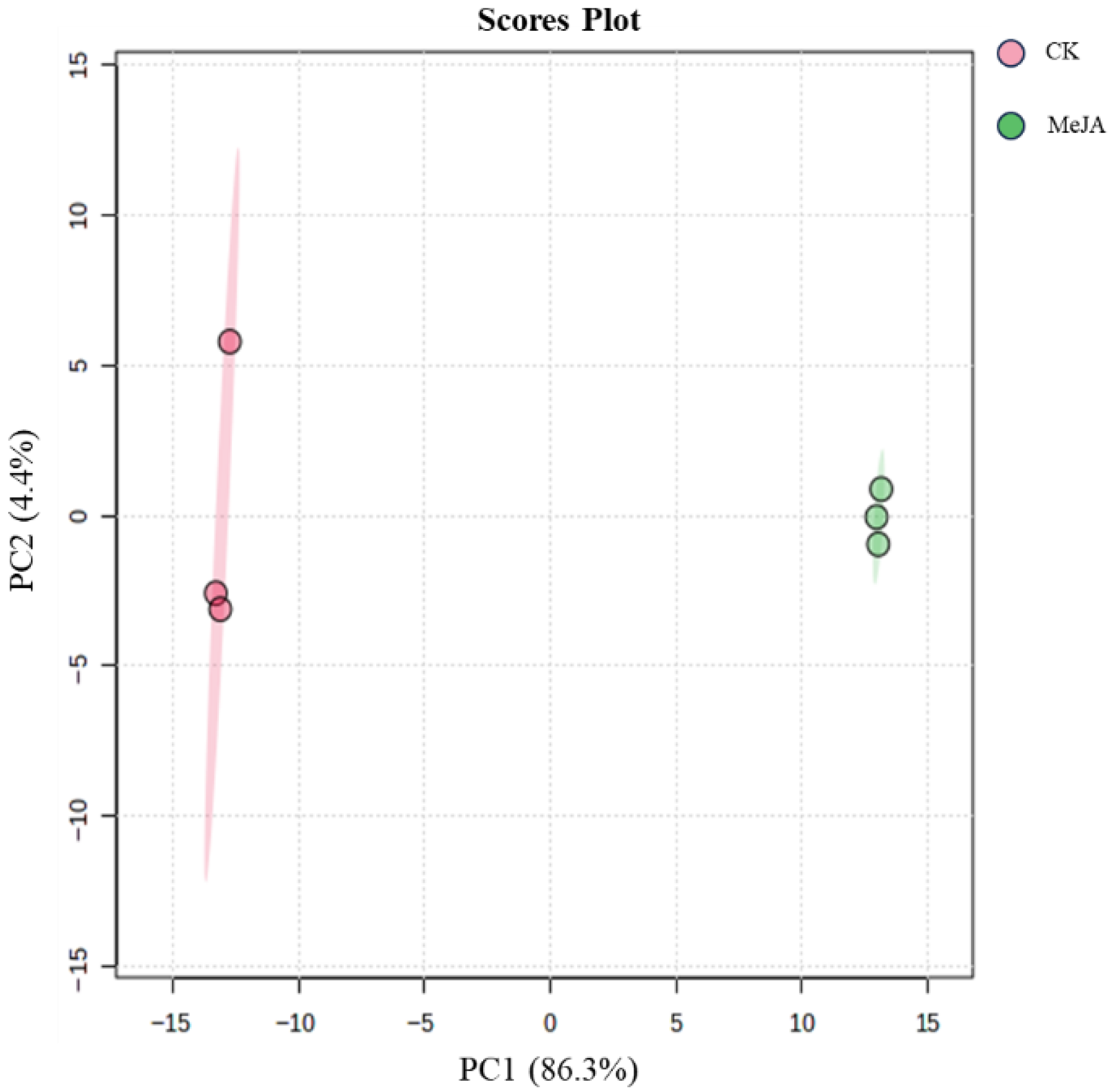
Principal Component Analysis
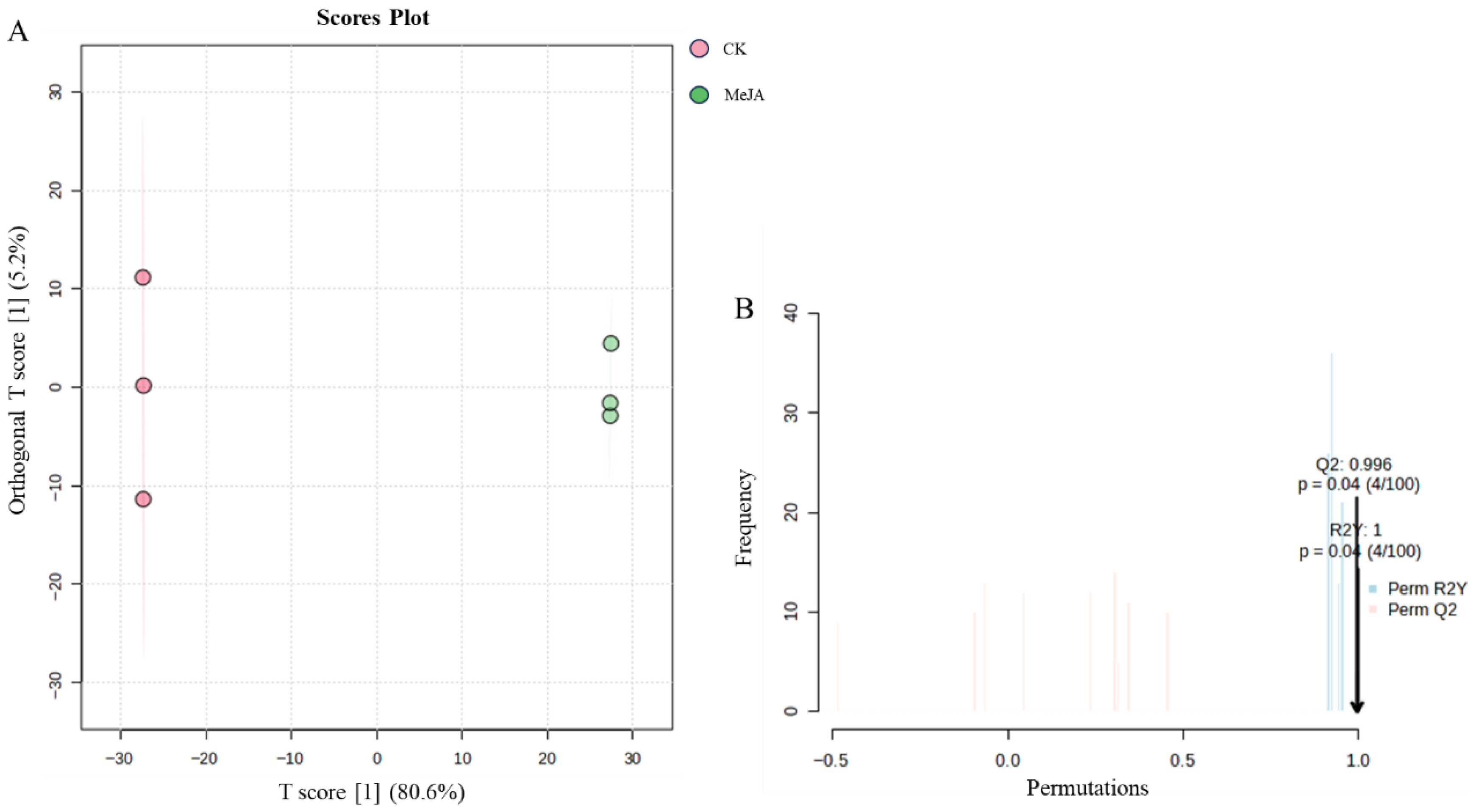
Orthogonal Partial Least Squares-Discriminant Analysis
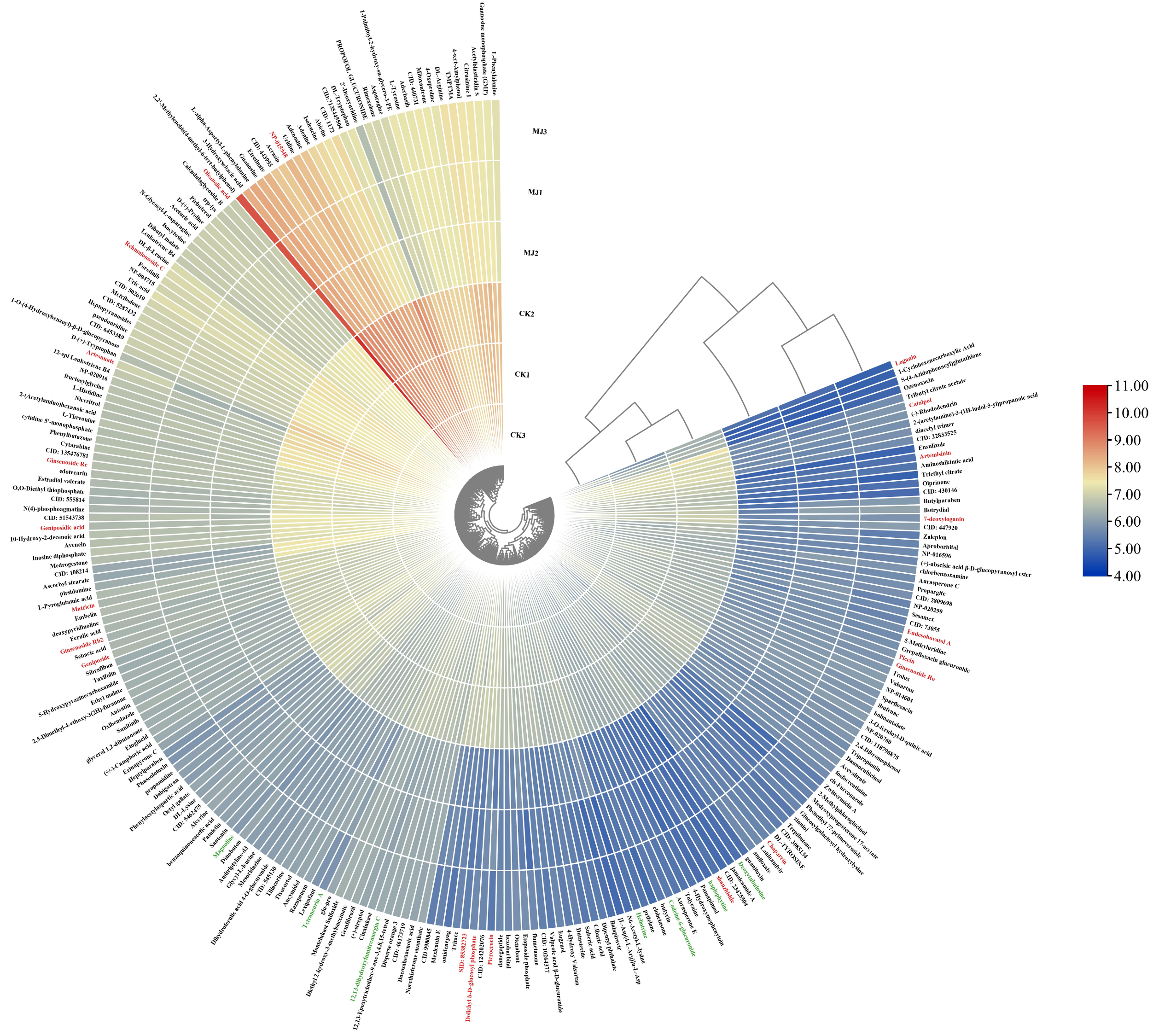
2.5.3. Screening of Differential Metabolites
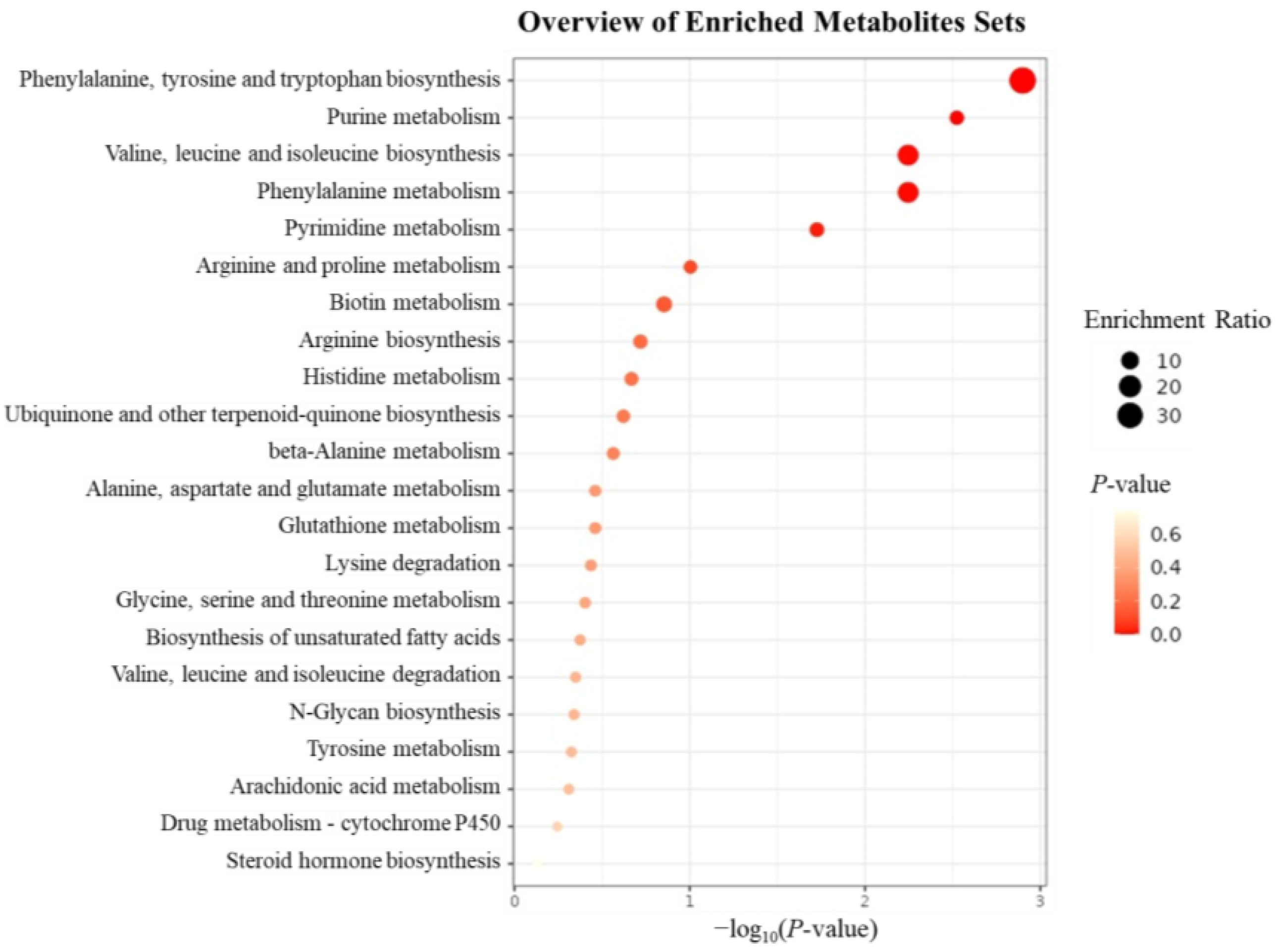
2.5.4. KEGG Pathway Enrichment
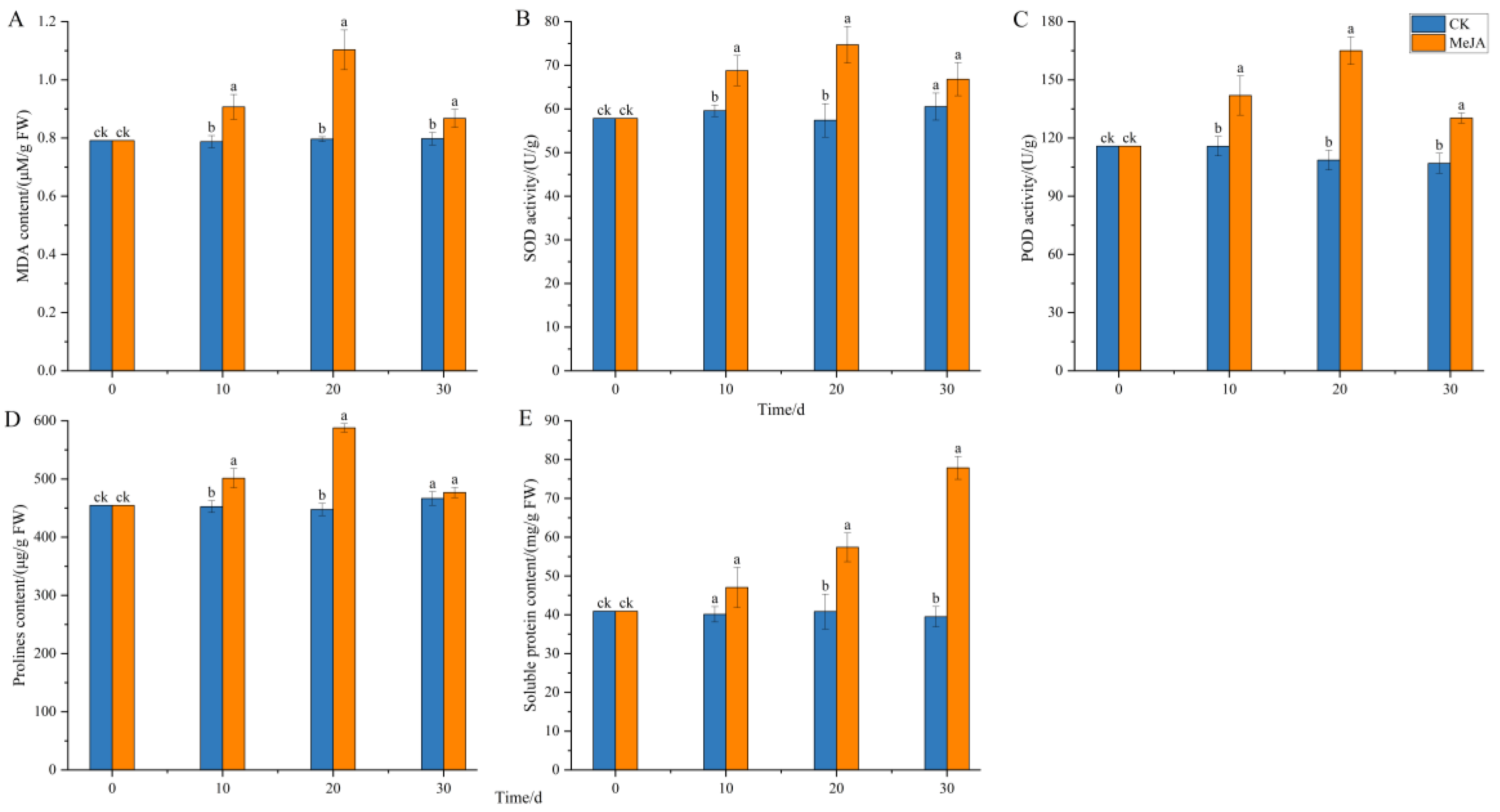
2.6. Effect of Methyl Jasmonate on Physiological Indicators of Adventitious Roots
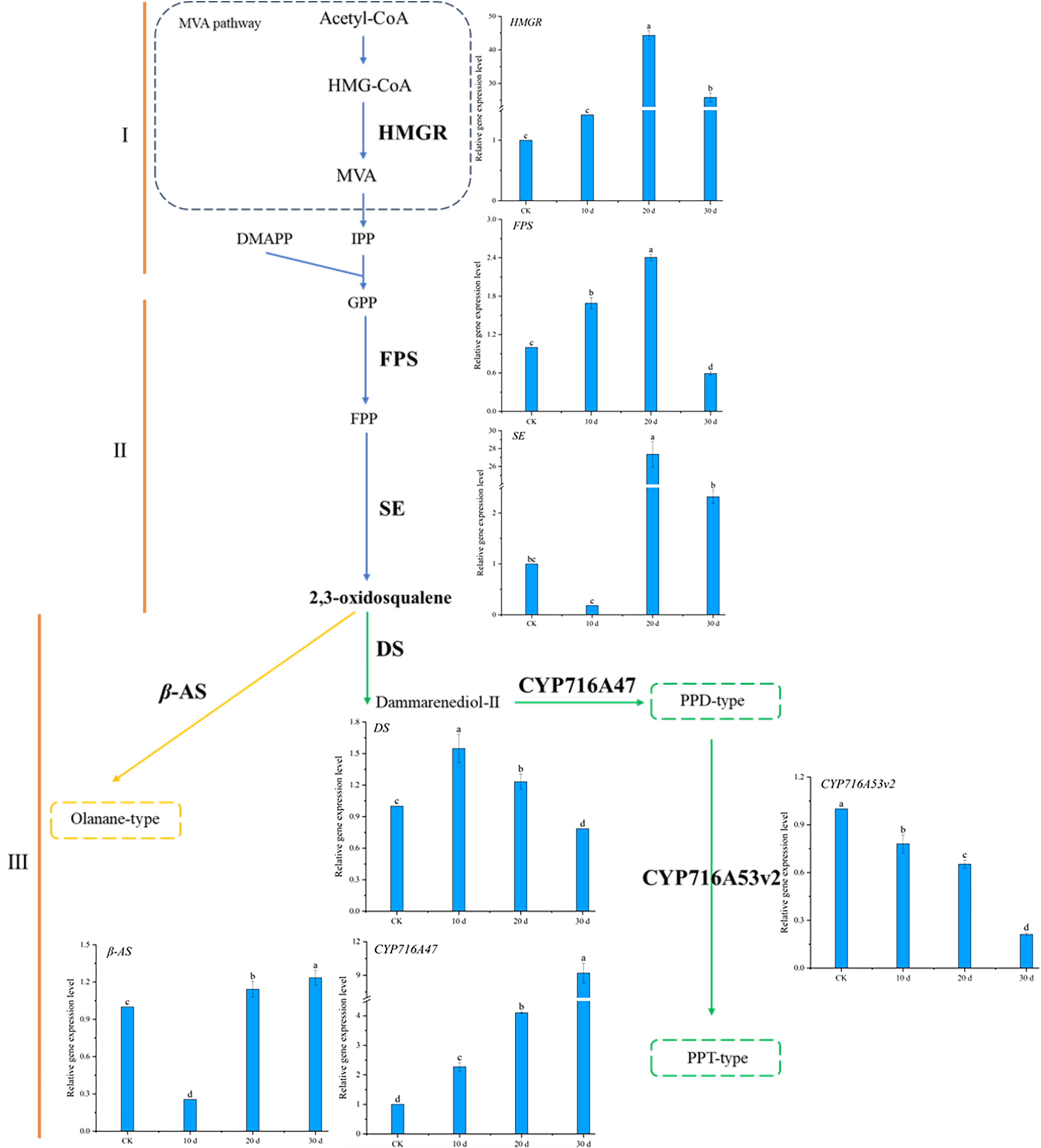
2.7. Effect of Methyl Jasmonate on the Expression of Ginsenoside Biosynthesis Genes in P. notoginseng
3. Materials and Methods
3.1. Induction and Proliferation of Callus
3.2. Adventitious Root Differentiation
3.3. Inoculation Amount and Sucrose Concentration in the Medium
3.4. Effect of Methyl Jasmonate on Adventitious Root Growth and Total Ginsenoside Content
3.5. Biomass and Total Saponin Content Measurement
- (1)
- Fresh weight: Harvested adventitious roots were filtered through a 100-mesh sieve to remove the culture medium, washed three times with deionized water, and dried with filter paper before weighing to obtain fresh weight.
- (2)
- Dry weight: The fresh weight of adventitious roots was dried in a 55 °C oven to a constant weight (approximately 2 days) and then weighed to obtain dry weight.
- (3)
- Total saponin content: Dried adventitious roots were ground into powder and extracted twice with 12 volumes of water-saturated n-butanol using ultrasonic assistance at 40 °C for 50 min [25]. After evaporating the n-butanol, the residue was dissolved in methanol, and the total saponin content was determined using the sulfuric acid-vanillin colorimetric method [26].
- (4)
- Standard curve: 3.1 mg of ginsenoside Re standard was accurately weighed and dissolved in methanol to a final volume of 10 mL, resulting in a standard solution concentration of 0.31 mg/mL. A 10 mL centrifuge tube was placed on ice, and 0.1, 0.2, 0.3, 0.4, and 0.5 mL of the standard solution were added to the tube, followed by 0.4, 0.3, 0.2, 0.1, and 0 mL of methanol to bring the total volume to 0.5 mL. A blank control was prepared using 0.5 mL of methanol. Then, 5 mL of 72% sulfuric acid and 0.5 mL of 8% vanillin in ethanol were added, mixed well, and incubated in a 60 °C water bath for 10 min. After the water bath, the reaction was immediately stopped by placing the tube in ice water for 15 min. Absorbance was measured at 544 nm. A standard curve was plotted with absorbance (y) against ginsenoside Re concentration (x), yielding the regression equation y = 0.7222x + 0.0901, with an R2 value of 0.9996.
3.6. LC-MS Conditions
3.7. Effect of Methyl Jasmonate on Physiological Indicators of Adventitious Roots
3.8. Real-Time Quantitative PCR
3.9. Data Analysis
- (1)
- Data were analyzed using SPSS Statistics 26 software. Differences between groups were determined by comparing the means of different data sets (p-value), with p < 0.05 indicating a significant difference, denoted by different lowercase letters. Additionally, the scale bars in the figures represent 1 cm.
- (2)
- The raw LC-MS data were processed using Compound Discoverer 3.0 software for untargeted metabolite analysis. ChemSpider and mzCloud databases were used to match molecular formulas, exact molecular weights, and MS1 and MS2 spectra for metabolite identification. Saponin monomers were further characterized using Thermo Xcalibur 4.1 software, with all identified notoginsenosides and ginsenosides having a mass error within 10 ppm. LC-MS data were preprocessed, and peak areas were log10-transformed for multivariate statistical analysis. Principal component analysis (PCA) was used to obtain an overview of sample distribution and identify potential outliers. Orthogonal partial least squares-discriminant analysis (OPLS-DA) was used to identify metabolites significantly contributing to clustering and discrimination. To reduce false positives, p-values were corrected using the false discovery rate (FDR). Metabolites with p < 0.05, |log2(FC)| > 1, and VIP ≥ 1 were considered differentially expressed. KEGG pathway enrichment analysis was performed.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Xia, T.; Zuo, Y.J.; Chen, Z.J.; Zhou, S.L. Development and characterization of microsatellite markers for Panax notoginseng (Araliaceae), a Chinese traditional herb. Am. J. Bot. 2011, 98, E274–E276. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.C. Exploring Panax Notoginseng; People’s Medical Publishing House: Beijing, China, 2019. [Google Scholar]
- Kim, E.J.; Kwon, K.A.; Lee, Y.E.; Kim, J.H.; Kim, S.H.; Kim, J.H. Korean Red Ginseng extract reduces hypoxia-induced epithelial-mesenchymal transition by repressing NF-κB and ERK1/2 pathways in colon cancer. J. Ginseng Res. 2018, 42, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, C.; Zhang, Y.; Wu, X. Ginseng: An Nonnegligible Natural Remedy for Healthy Aging. Aging Dis. 2017, 8, 708–720. [Google Scholar] [CrossRef]
- Chen, J.; Xue, R.; Li, L.; Xiao, L.L.; Shangguan, J.; Zhang, W.; Bai, X.; Liu, G.; Li, L. Panax notoginseng Saponins Protect Cardiac Myocytes Against Endoplasmic Reticulum Stress and Associated Apoptosis Through Mediation of Intracellular Calcium Homeostasis. Front. Pharmacol. 2019, 10, 1013. [Google Scholar] [CrossRef]
- Liao, P.; Liu, P.; Wang, Y.; Huang, C.; Lan, L.; Yang, Y.; Cui, X. Stereoscopic cultivation of Panax nowginseng: A new approach to overcome the continuous cropping obstacle. Ind. Crops Prod. 2018, 126, 38–47. [Google Scholar] [CrossRef]
- Saeed, S.; Ali, H.; Khan, T.; Kayani, W.; Khan, M.A. Impacts of methyl jasmonate and phenyl acetic acid on biomass accumulation and antioxidant potential in adventitious roots of Ajuga bracteosa Wall ex Benth., a high valued endangered medicinal plant. Physiol. Mol. Biol. Plants 2017, 23, 229–237. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, J.; Zhu, J.; He, S.; Zhang, W.; Yu, R.; Zi, J.; Song, L.; Huang, X. Effects of β-cyclodextrin and methyl jasmonate on the production of vindoline, catharanthine, and ajmalicine in Catharanthus roseus cambial meristematic cell cultures. Appl. Microbiol. Biotechnol. 2015, 99, 7035–7045. [Google Scholar] [CrossRef]
- Liu, Z.B.; Chen, J.G.; Yin, Z.P.; Shangguan, X.C.; Peng, D.Y.; Lu, T.; Lin, P. Methyl jasmonate and salicylic acid elicitation increase content and yield of chlorogenic acid and its derivatives in Gardenia jasminoides cell suspension cultures. Plant Cell Tissue Organ Cult. 2018, 134, 79–93. [Google Scholar] [CrossRef]
- Onrubia, M.; Moyano, E.; Bonfill, M.; Ma Cusido, R.; Goossens, A.; Palazon, J. Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate. J. Plant Physiol. 2013, 170, 211–219. [Google Scholar] [CrossRef]
- Xu, F.; Valappil, A.K.; Mathiyalagan, R.; Tran, T.N.A.; Ramadhania, Z.M.; Awais, M.; Yang, D.C. In Vitro Cultivation and Ginsenosides Accumulation in Panax ginseng: A Review. Plants 2023, 12, 3165. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, W.-H.; Sun, X.-Y.; Li, K.-H.; Liu, K.-J.; Wang, J.; Wang, Y.; Tan, X.; You, X.-L. A culture system for the stable and high-efficiency proliferation of adventitious roots of Panax notoginseng and ginsenoside accumulation. Ind. Crops Prod. 2020, 157, 112882. [Google Scholar] [CrossRef]
- Çelik, S.; Dervisoglu, G.; Izol, E.; Seczyk, L.; Özdemir, F.A.; Yilmaz, M.E.; Yilmaz, M.A.; Gülçin, I.; Al-Anazi, K.M.; Farah, M.A.; et al. Comprehensive phytochemical analysis of Salvia hispanica L. callus extracts using LC-MS/MS. Biomed. Chromatogr. 2024, 38, e5975. [Google Scholar] [CrossRef]
- Kotta, L.R.; Vijayalakshmi, A. Metabolic profiling of plant constituents through LC-MS. Ann. Phytomed. Int. J. 2024, 13, 469–475. [Google Scholar] [CrossRef]
- Gao, X.F.; Zhu, C.B.; Jia, W.; Gao, W.Y.; Qiu, M.F.; Zhang, Y.Y.; Xiao, P.G. Induction and characterization of adventitious roots directly from the explants of Panax notoginseng. Biotechnol. Lett. 2005, 27, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. An Introduction to Plant Tissue Culture: Advances and Perspectives. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: New York, NY, USA, 2018; pp. 3–13. [Google Scholar]
- Verstraeten, I.; Schotte, S.; Geelen, D. Hypocotyl adventitious root organogenesis differs from lateral root development. Front. Plant Sci. 2014, 5, 2014. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Sivakumar, G.; Yu, K.W.; Paek, K.Y. Production of Biomass and Ginsenosides from Adventitious Roots of Panax ginseng in Bioreactor Cultures. Eng. Life Sci. 2005, 5, 333–342. [Google Scholar] [CrossRef]
- Huang, T.-K.; McDonald, K.A. Bioreactor engineering for recombinant protein production in plant cell suspension cultures. Biochem. Eng. J. 2009, 45, 168–184. [Google Scholar] [CrossRef]
- Yang, W.Z.; Ye, M.; Qiao, X.; Liu, C.F.; Miao, W.J.; Bo, T.; Tao, H.Y.; Guo, D.A. A strategy for efficient discovery of new natural compounds by integrating orthogonal column chromatography and liquid chromatography/mass spectrometry analysis: Its application in Panax ginseng, Panax quinquefolium and Panax notoginseng to characterize 437 potential new ginsenosides. Anal. Chim. Acta 2012, 739, 56–66. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wu, X.; Liu, D.; Li, J.; Li, J.; Liu, S.; Gao, W. Jasmonic acid and methyl dihydrojasmonate enhance saponin biosynthesis as well as expression of functional genes in adventitious roots of Panax notoginseng F.H. Chen. Biotechnol. Appl. Biochem. 2017, 64, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, S.; Liang, W.; Wang, J.; Gao, W. Screening and evaluation of adventitious root lines of Panax notoginseng by morphology, gene expression, and metabolite profiles. Appl. Microbiol. Biotechnol. 2019, 103, 4405–4415. [Google Scholar] [CrossRef] [PubMed]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- GB/T 19506-2009; Product of Geographical Indication—Jilin Changbai Mountain Ginseng. Standards Press of China: Beijing, China, 2009.
- Chen, J.X.; Wang, X.F. Experimental Guide for Plant Physiology; South China University of Technology Press: Guangzhou, China, 2015. [Google Scholar]
- Adnan, M.; Morton, G.; Hadi, S. Analysis of rpoS and bolA gene expression under various stress-induced environments in planktonic and biofilm phase using 2−ΔΔCT method. Mol. Cell. Biochem. 2011, 357, 275–282. [Google Scholar] [CrossRef]

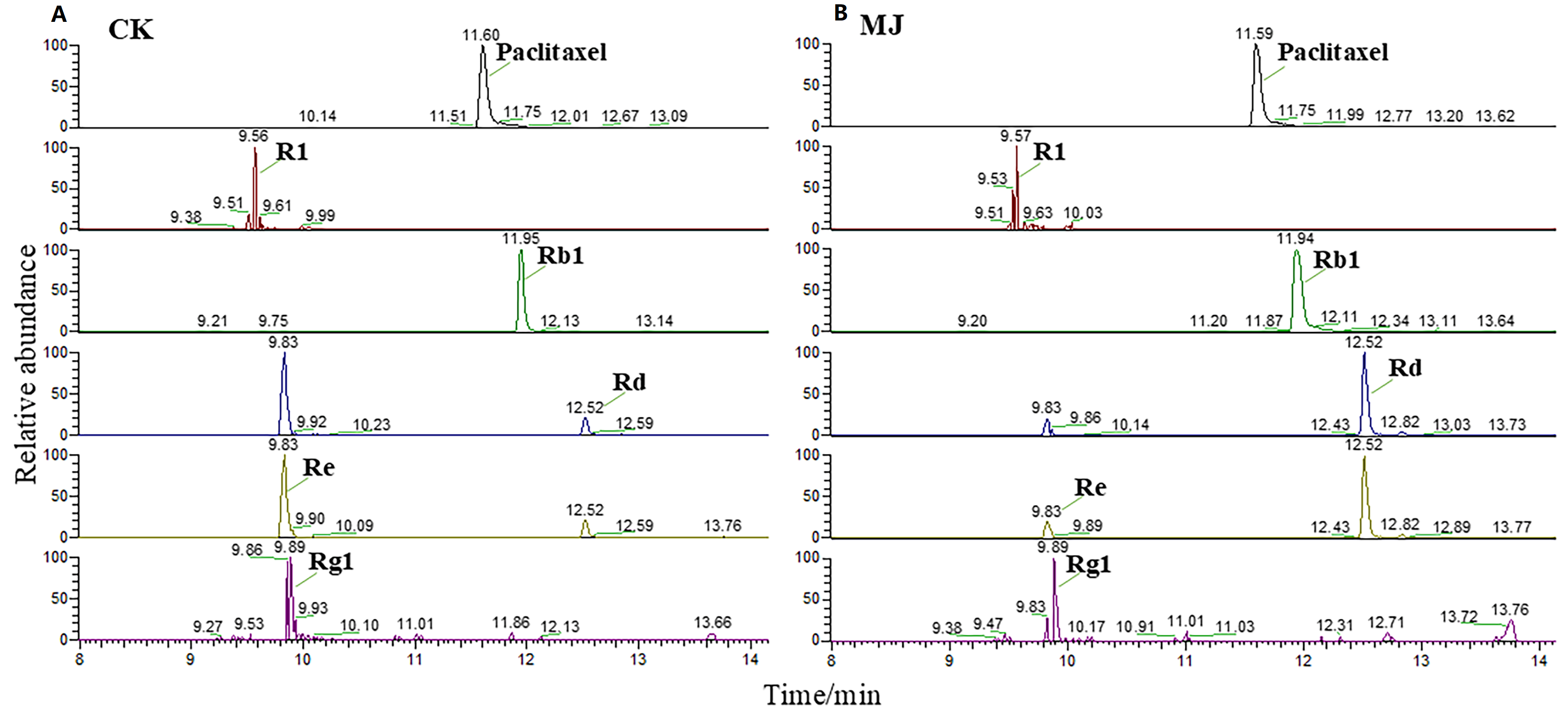
| Type | Name | RT /min | Theoretical Molecular Weight [M-H]− | Measured Molecular Weight | Mass Error/ppm | MS2 |
|---|---|---|---|---|---|---|
| Notoginsenoside R1 | 9.57 | 931.5271 | 931.5219 | −5.582 | 637.4309, 619.4176, 475.3781 | |
| Notoginsenoside R2 | 11.11 | 767.4743 | 767.4709 | −4.430 | 637.4300, 475.3779, | |
| Notoginsenoside Fa | 11.75 | 1239.6379 | 1239.6330 | −3.953 | 1239.6363, 1107.5931, 1077.5827, 945.5407, 783.4886, 459.3831 | |
| Notoginsenoside Fc | 12.06 | 1209.6273 | 1209.6239 | −2.811 | 1209.6193, 1077.8514, 915.5327, 783.4890, 621.4357 | |
| PPD | Ginsenoside Rb1 | 11.94 | 1107.5956 | 1107.5911 | −4.063 | 945.5422, 783.4874, 621.4358, 459.3807 |
| PPD | Ginsenoside Rd | 12.52 | 945.5428 | 945.5388 | −4.230 | 945.5399, 783.4881, 621.4354, 459.3832 |
| PPT | Ginsenoside Re | 9.83 | 945.5428 | 945.5364 | −6.769 | 945.5369, 799.4810, 783.4882, 637.4309, 475.3779 |
| PPT | Ginsenoside Rg1 | 9.89 | 799.4849 | 799.4823 | −3.252 | 637.4296, 475.3780 |
| PPT | Ginsenoside Rg2 | 11.42 | 783.49 | 783.4860 | −5.105 | 783.4895, 637.4303, 619.4180, 475.3782 |
| PPT | Ginsenoside Ro | 11.91 | 955.4908 | 955.4884 | −2.512 | 955.4882, 793.4371, 569.3833 |
| PPD | Ginsenoside Rc | 12.2 | 1077.585 | 1077.5819 | −2.877 | 1077.5860, 945.5431, 783.4896, 765.4788, 621.4369, 459.3830 |
| PPD | Ginsenoside Rg3 | 13.44 | 783.49 | 783.4860 | −5.105 | 783.4851, 621.4358, 459.3837 |
| PPD | Ginsenoside Rb2 | 12.24 | 1077.585 | 1077.5845 | −0.464 | 1077.5843, 945.5335, 783.4890, 621.4356, 459.3837 |
| PPD | Ginsenoside Rb3 | 12.35 | 1077.585 | 1077.5817 | −3.062 | 945.5474, 785.4865, 623.4396, 459.3848 |
| Gene | Accession Number | Forward Primer | Reverse Primer |
|---|---|---|---|
| GADPH | KF815711 | GATTCGGCATTGTTGAGG | CAGTGGGAACTCGGAAGG |
| HMGR | KJ804166 | CCTGATAGCTGGGACATTC | CCGCAACTACTGCGTTAA |
| FPS | KJ804175 | TGGGAAGATTGGCACAGA | TCGGCAAATACATCCTGAA |
| SE | KJ804171 | TTTTGGATATGCCCTTTAC | CTTTCTCCCTCATTCGTT |
| DS | KJ804174 | ATGTGGAAGCTGAAGGTTGCT | TTAAATTTTGAGCTGCTGGTGC |
| β-AS | KJ804177 | AGGTAGGAGATGACGAGGTA | GCTGGGAACACTGTATCAA |
| CYP716A47 | OR514680.1 | ATGTCGTGTCGGGTGTTT | TTGGGACGCTTGCTTATT |
| CYP716A53v2 | MZ277754.1 | TTTCTGCGGTGCCTCGG | CTTGTGGATTGCTTCGGGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Li, P.; Li, W. Methyl Jasmonate Enhances Saponin Accumulation in Cultured Panax notoginseng Adventitious Roots. Plants 2025, 14, 3462. https://doi.org/10.3390/plants14223462
Liu K, Li P, Li W. Methyl Jasmonate Enhances Saponin Accumulation in Cultured Panax notoginseng Adventitious Roots. Plants. 2025; 14(22):3462. https://doi.org/10.3390/plants14223462
Chicago/Turabian StyleLiu, Kaiyang, Ping Li, and Wenlan Li. 2025. "Methyl Jasmonate Enhances Saponin Accumulation in Cultured Panax notoginseng Adventitious Roots" Plants 14, no. 22: 3462. https://doi.org/10.3390/plants14223462
APA StyleLiu, K., Li, P., & Li, W. (2025). Methyl Jasmonate Enhances Saponin Accumulation in Cultured Panax notoginseng Adventitious Roots. Plants, 14(22), 3462. https://doi.org/10.3390/plants14223462






