1. Introduction
The conservation and restoration of forest ecosystems constitute a critical objective of sustainable development [
1,
2]. In recent years, this goal has progressively shifted from forest area expansion to biodiversity enhancement [
3,
4]. Understory vegetation plays a vital role in forest ecosystems. Although its biomass is typically far smaller than that of the canopy layer, its rapid biomass turnover rate exerts significant impacts on ecosystem nutrient cycling, litter decomposition, and microclimate regulation [
5]. Furthermore, understory vegetation often represents the primary component of forest biodiversity [
6], particularly in plantations, where it contributes substantially to nutrient cycling and energy flow [
7]. Consequently, investigating the variations in plantation biodiversity and their underlying drivers has emerged as a focal research topic in recent years [
8,
9].
In the 1970s, rapid economic growth and infrastructure development in south China led to the extensive degradation of zonal vegetation—primarily evergreen broad-leaved forests [
10]. To restore vegetation and improve soil quality, large-scale afforestation programs were initiated, establishing monoculture plantations (e.g.,
Pinus massoniana,
Cunninghamia lanceolata) and coniferous—broadleaf mixed plantations [
11]. However, most plantations in this region have now reached maturity and face multiple ecological challenges, including impeded natural succession [
12], soil degradation [
13], and unbalanced stand structure [
14], collectively driving vegetation diversity decline and compromised ecological functionality. Consequently, there is an urgent need to quantitatively assess understory vegetation diversity and biomass in south China’s plantations, identify key drivers of understory development, and formulate adaptive management strategies to address these issues.
Forest structure and understory light environment are critical determinants of understory vegetation diversity and biomass [
15]. Regulating stand density, species composition, and spatial configuration can optimize understory growth conditions, thereby enhancing both biodiversity and biomass accumulation [
16]. Empirical studies demonstrate that understory species richness increases with stand density until reaching a threshold, beyond which intensified competition for resources (e.g., light, water, nutrients) suppresses understory vegetation development and reduces diversity [
17]. Canopy structural heterogeneity further modulates understory light regimes, influencing the photomorphogenic responses and light-foraging strategies of understory plants [
18]. Additionally, tree distribution patterns govern light penetration through canopy gaps, directly affecting the photosynthetic efficiency and growth dynamics of understory communities. Helbach et al. [
18] demonstrated that appropriate canopy gap size critically regulates understory plant communities by enhancing light availability, thereby driving species diversity and biomass dynamics.
South China’s subtropical monsoon climate, characterized by high temperatures and abundant rainfall, drives the widespread distribution of acidic lateritic red soils and latosolic red soils (pH < 5.5) with severe nutrient leaching [
19]. The interplay between these edaphic properties and vegetation dynamics critically governs the sustainability of plantation ecosystems [
20]. Studies confirm that stand type significantly modulates soil nutrient profiles, including total nitrogen (TN), TP, and soil organic carbon (SOC) [
21]. For instance, introducing broadleaf species into
Cunninghamia lanceolata plantations alters the Acidobacteria subpopulation composition and upregulates N-cycling gene abundance, thereby enhancing soil fertility [
22]. Furthermore, interspecific variations in litter decomposition rates and nutrient release patterns create divergent soil nutrient availabilities, ultimately shaping understory vegetation composition and productivity [
23]. Collectively, stand type mediates understory diversity and biomass through synergistic modifications of soil physicochemical properties and canopy structure.
Despite this progress, the mechanisms underlying these interactions in subtropical plantations remain poorly resolved. Critical knowledge gaps persist regarding (1) the interactive effects of multiple drivers (e.g., light × nutrient × stand structure) and (2) their quantitative contributions to understory biomass allocation. For example, while broadleaf admixtures in conifer monocultures may elevate soil P availability via diversified litter inputs [
24], whether this facilitation significantly enhances understory growth compared to conifer monocultures remains unverified. Moreover, no comparative studies have addressed the understory characteristics between exotic species (e.g.,
Pinus caribaea) and native counterparts (e.g.,
Pinus massoniana), leaving their ecological equivalence in plantation management unresolved.
Building upon this foundation, we conducted a comparative study across four dominant plantation types in south China: Pinus massoniana (native), Pinus caribaea (exotic), Cunninghamia lanceolata monoculture, and Cunninghamia lanceolata–broadleaf mixed forests. Integrating stand structural inventories, understory light environment monitoring, and soil nutrient quantification, we addressed three pivotal questions: (1) Do exotic Pinus caribaea plantations exhibit significantly divergent understory vegetation diversity and biomass allocation patterns compared to native Pinus massoniana stands under comparable site conditions? (2) Does introducing broadleaf species into Cunninghamia lanceolata monocultures enhance understory species richness and biomass productivity through improved light heterogeneity and soil P availability? (3) What synergistic interactions exist among stand structure, light regimes, and soil properties in driving understory vegetation dynamics? To resolve these questions, we employed SEM to disentangle direct and indirect drivers of understory community assembly. Our findings elucidate species-specific adaptation strategies across plantation types, particularly highlighting how exotic conifers trade off understory biodiversity for faster biomass accumulation. This work establishes a quantitative framework for optimizing stand structure–soil–light interactions, informing precision forest management strategies to enhance ecological functions (e.g., carbon sequestration, nutrient cycling) in subtropical plantations while supporting carbon neutrality goals.
3. Discussion
Understory vegetation, as a crucial component of forest ecosystems, plays an indispensable role in plantations due to its rapid nutrient accumulation and biological turnover rates [
5]. However, influenced by species diversity, stand structure, and understory light conditions, vegetation in different stands demonstrates significant spatial heterogeneity in a niche occupation, which is manifested through distinct community composition, vertical stratification, and distribution patterns [
25]. As dominant plantation types in south China,
Pinus massoniana and
Pinus caribaea stands exhibit marked differences in understory characteristics.
Pinus massoniana forests generally support higher understory diversity, where naturally regenerated species like
Castanopsis fissa and
Psychotria asiatica form stratified shrub communities resembling early successional features of tropical secondary forests. This phenomenon likely stems from complex interdependencies with local soil, climate, and biotic factors, with long-term co-evolution enabling
Pinus massoniana stands to sustain richer native understory communities [
26]. In contrast,
Pinus caribaea stands with greater canopy openness, favoring light-demanding species such as
Melicope pteleifolia,
Ilex asprella, and
Wendlandia uvariifolia that dominate ecological niches while suppressing other vegetation [
27]. This intensified light availability concurrently contributes to greater shrub biomass accumulation in
Pinus caribaea stands compared to
Pinus massoniana plantations.
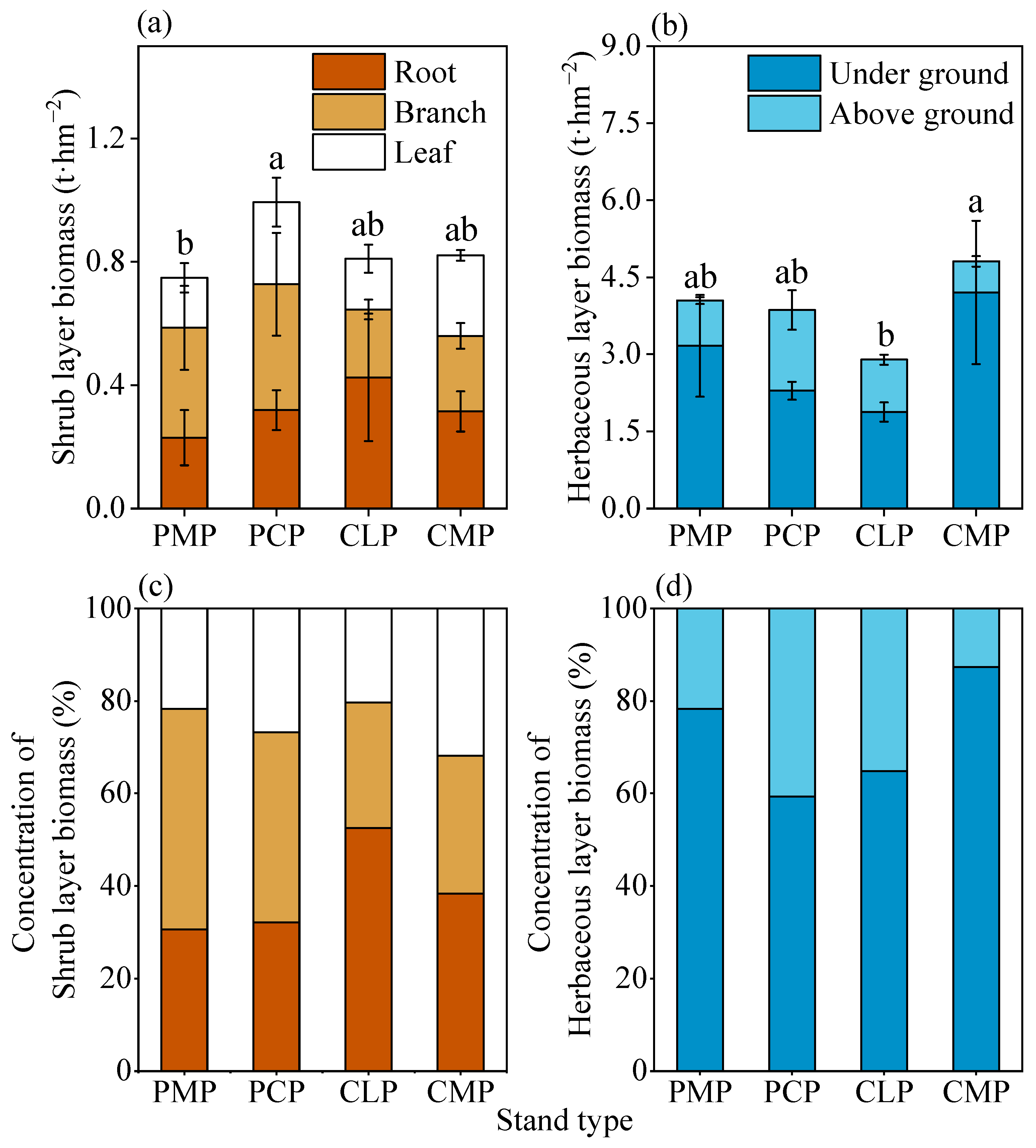
In comparison, the Chinese fir–broadleaf mixed forest’s high density (2016 trees·hm
−2) and random distribution pattern (angular scale 0.52) likely suppress shrub growth through root competition and light interception, while its elevated species mingling degree (0.63) enhances stand habitat heterogeneity, thereby promoting herbaceous layer biomass accumulation. The introduction of broadleaf species into pure stands may accelerate nutrient cycling through diversified litter composition and quality differences [
28], consequently improving herb layer diversity. While understory light resources provide essential energy for plant growth, they may simultaneously inhibit shade-tolerant species [
29], potentially explaining the lower herb diversity in Chinese fir pure stands compared to mixed forests. The mixed forest’s reduced light intensity appears to drive herbaceous plants toward photo adaptive strategies, such as increased leaf area and thicker laminar structures, resulting in 24.5–66.1% greater herb biomass than pure Chinese fir stands. These physiological adaptations likely correlate with species-specific functional traits (e.g., specific leaf area, chlorophyll concentration), though experimental validation remains necessary [
30]. Furthermore, beyond the live canopy (LAI), other factors likely influenced the understory light environment. We observed that
Cunninghamia lanceolata forest monocultures often had a dense, persistent layer of undecomposed
Cunninghamia lanceolata needles, which could create a physical barrier and further reduce light penetration to the herbaceous layer [
31]. In contrast, the mixed stands had a more heterogeneous litter layer composed of broadleaf and conifer litter, which likely decomposed faster and formed a less continuous barrier. The retention of dead lower branches, particularly in the dense
Cunninghamia lanceolata stands, may have contributed to the interception of light before it reached the forest floor, a factor not captured by our hemispherical photography which primarily assesses the live canopy.
Soil physicochemical properties significantly influence understory vegetation’s resource allocation strategies, particularly determining the distribution patterns of shallow-rooted herbs and shrubs [
31]. P and K play crucial roles in plant energy metabolism (ATP synthesis) and water regulation (stomatal control). Although P deficiency commonly occurs in south China’s lateritic red soils [
32], the Chinese fir–broadleaf mixed forest exhibits superior TP (0.83 g·kg
−1) and TK (33.65 g·kg
−1) concentrations, potentially explaining its exceptional herbaceous biomass (4.05 t·hm
−2). This nutrient enrichment likely stems from diversified litter inputs—broadleaf species like
Cinnamomum camphora contribute a low C/N ratio litter that accelerates microbial mineralization [
33], while reduced bulk density (1.26 g·cm
−3) facilitates root proliferation and nutrient uptake efficiency. Notably, Caribbean pine stands demonstrate superior carbon sequestration capacity with SOM content 1.64-fold higher than Masson pine forests, coupled with elevated moisture levels mitigating seasonal drought stress. However, concerning evidence suggests Caribbean pine litter contains elevated resin acids and other allelopathic compounds that may inhibit P mineralization through microbial suppression [
34], potentially causing long-term P depletion that compromises understory productivity, which particularly affects shallow-rooted herbs dependent on labile nutrient pools [
35].
In biomass allocation, shrubs in Chinese fir pure stands and fir–broadleaf mixed forests preferentially allocate greater proportions to root systems (52.48% and 38.31% respectively), while
Pinus massoniana and
Pinus caribaea stands exhibit predominant biomass investment in aboveground branches (47.61% and 41.09%). This divergence likely relates to soil resource heterogeneity—Chinese fir stands with the highest soil bulk density (1.41 g·cm
−3) may mechanically force shrubs to develop deeper root systems for water acquisition [
36], whereas elevated organic matter content in
Pinus massoniana and
Pinus caribaea stands could reduce root construction costs, possibly reducing belowground investment while promoting aerial photic competition [
37].
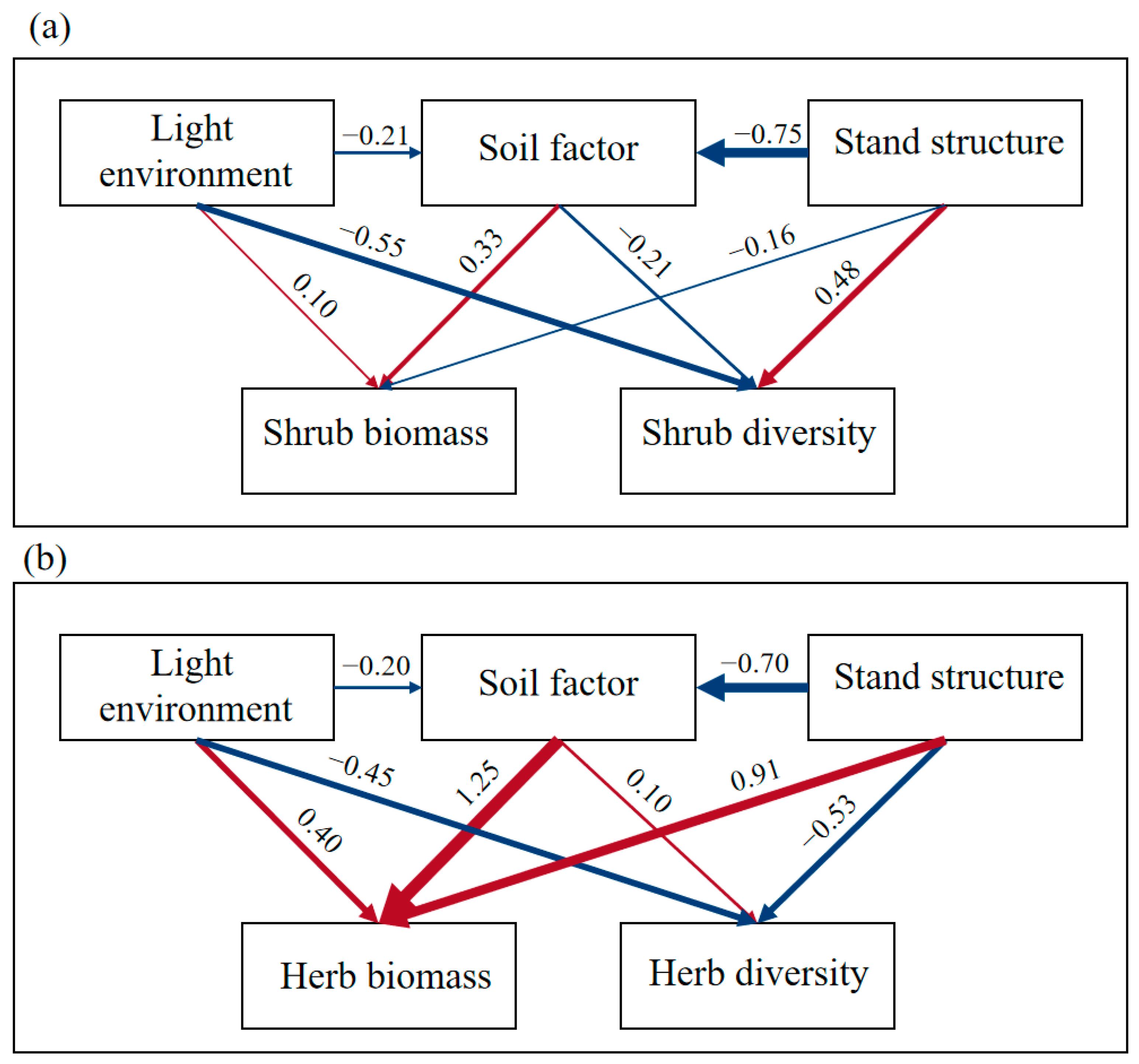
SEM analysis revealed that stand structure exerted significantly stronger direct positive effects on shrub diversity (path coefficient = 0.48) than the negative impacts from light environment and soil factors, confirming the critical role of canopy management practices (e.g., thinning and pruning) in regulating understory vegetation [
6]. The herbaceous layer biomass showed a primary dependence on soil factors (path coefficient = 1.25), particularly TP and pH regulation, suggesting that P fertilizer application or soil amendments could effectively enhance herb productivity in plantation management. Notably, contrasting responses emerged between vegetation layers—the understory light environment positively influenced herb diversity (path coefficient = 0.40) while negatively affecting shrubs (path coefficient = −0.55), reflecting niche differentiation in photic adaptation across plant life forms [
38].
It is important to note that the quantitative strengths of the drivers identified (e.g., the primacy of soil P for herb biomass) may vary across different geographical locations and soil types in south China’s extensive plantation landscapes. Therefore, our findings should be interpreted as a mechanistic case study that reveals key interactive processes. The management suggestions below are proposed as testable hypotheses for local application, which should be validated through broader regional studies before large-scale implementation. (1) Structural optimization of Chinese fir monocultures: Implement the gradual introduction of shade-tolerant broadleaf species (e.g., Cinnamomum burmannii, Schima superba) to establish coniferous–broadleaf mosaic structures. (2) Nutrient management in pine-dominated stands: Address P limitations through slow-release P fertilizers during tending phases or enhanced nutrient cycling via litter retention. This study provides scientific basis for ecological restoration and the sustainable management of subtropical plantations, though long-term monitoring remains essential to decipher dynamic responses of understory vegetation to climate change and anthropogenic disturbances. Future research should prioritize investigating soil microbiome–vegetation interactions across stand types and climate change impacts on understory community assembly.
5. Conclusions
This study systematically investigated the understory vegetation diversity and biomass characteristics across four subtropical plantations in south China, elucidating their driving mechanisms. Key findings include the following:
(1) Native vs. exotic species contrast: Pinus massoniana stands exhibited higher shrub diversity (dominated by Castanopsis fissa) due to their larger diameter at DBH, broader crown widths, and random spatial patterns. In contrast, Pinus caribaea stands demonstrated greater shrub biomass (dominated by Melicope pteleifolia and Wendlandia uvariifolia), which was attributed to their superior soil organic matter and moisture content. (2) Monoculture vs. mixed plantation comparison: The Chinese fir–broadleaf mixed forest displayed 24.50–66.06% higher herbaceous biomass and enhanced diversity, which was driven by their high mingling degree, random distribution patterns, and enriched phosphorus–potassium soil conditions. (3) Differentiated drivers: Stand structure parameters (D, density, mingling degree) directly regulated shrub development through spatial configuration and light redistribution (path coefficient = 0.48), while herbaceous layers were predominantly controlled by soil–light interactions, particularly P availability and pH modulation.
These findings reveal the adaptive strategies of understory vegetation to heterogeneous environments in subtropical plantations, highlighting critical constraints from inappropriate stand structures and P-deficient acidic soils. Our structural equation models revealed that stand structure is a key driver of understory dynamics. This finding suggests that manipulating stand density through thinning could be a potential management tool to improve light availability and heterogeneity. However, the optimal intensity and timing of thinning remain unknown. Therefore, we recommend that future research prioritizes long-term thinning experiments that monitor the response of understory vegetation and soil biota to a gradient of stocking densities. Such manipulative studies are essential to move from correlative understanding to predictive, prescriptive management. This work advances our understanding of understory ecological processes in subtropical plantations, offering actionable insights for sustainable forest management and carbon-neutral initiatives.