Durum Wheat Kernel: Influence of the Genotype and Environment on the Mineral Profile of Grains and Ashes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Site
2.3. Field Experiment
2.4. Quality Evaluation
2.5. Statistical Analyses
3. Results
3.1. Climatic Conditions
3.2. Genotype and Environmental (Site) Effects on Durum Wheat Technological Quality
3.3. Genotype and Environmental (Local) Effects on Durum Wheat Grain and Ash Mineral Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, X.; Ling, H.Q. Current advances in genome sequencing of common wheat and its ancestral species. Crop. J. 2018, 6, 15–21. [Google Scholar] [CrossRef]
- Almeida, A.S.; Maçãs, B.; Coutinho, J.; Costa, R.; Pinheiro, N.; Gomes, C.; Coco, J.; Costa, A.; Bagulho, A.S.; Jézequel, S. Understanding and reducing yield gap under Mediterranean climate. Searching for adapted wheat varieties. WL. Ciheam 2016, 37, 2114–3129. Available online: https://www.ciheam.org/uploads/attachments/255/011_Almeida_WL_37.pdf (accessed on 1 July 2025).
- Filip, E.; Woronko, K.; Stępień, E.; Czarniecka, N. An overview of factors affecting the functional quality of common wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2023, 24, 7524. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Wang, Y.; Sun, H. Impact of temperature stresses on wheat quality: A focus on starch and protein composition. Foods 2025, 14, 2178. [Google Scholar] [CrossRef]
- Ru, C.; Liu, Y.; Wang, W.; Hu, X. Moderate intermittent water deficit enhances dry matter remobilization, nitrogen uptake, and water and nitrogen use efficiency in winter wheat. Agric. Commun. 2025, 3, 100098. [Google Scholar] [CrossRef]
- Bagulho, A.S.; Moreira, J.; Costa, R.; Pinheiro, N.; Gomes, C.; Almeida, A.S.; Costa, A.; Coutinho, J.; Maçãs, B. Trigo duro—Influência da variedade e adubação fracionada na produção e qualidade. Vida Rural 2021, 1864, 52–58. [Google Scholar]
- Bagulho, A.S.; Moreira, J.; Costa, R.; Pinheiro, N.; Gomes, C.; Almeida, A.S.; Costa, A.; Coutinho, J.; Dôres, J.; Costa, N.; et al. Qualidade do trigo-duro—Dependência de fatores genéticos, ambientais e fertilização. Vida Rural 2021, 1869, 64–71. [Google Scholar]
- Brier, N.; Gomand, S.V.; Donner, E.; Paterson, D.; Delcour, J.A.; Lombi, E.; Smolders, E. Distribution of minerals in wheat grains (Triticum aestivum L.) and in roller milling fractions affected by pearling. J. Agric. Food Chem. 2015, 63, 1276–1285. [Google Scholar] [CrossRef]
- Cubadda, F.; Aureli, F.; Raggi, A.; Carcea, M. Effect of milling, pasta making and cooking on minerals in durum wheat. J. Cereal Sci. 2009, 49, 92–97. [Google Scholar] [CrossRef]
- Peleg, Z.; Cakmak, I.; Ozturk, L.; Yazici, A.; Jun, Y.; Budak, H.; Korol, A.B.; Fahima, T.; Saranga, Y. Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat × wild emmer wheat RIL population. Theor. Appl. Genet. 2009, 119, 353–369. [Google Scholar] [CrossRef]
- Lyons, G.; Monasterio, I.O.; Stangoulis, J.; Graham, R. Selenium concentration in wheat grain: Is there sufficient genotypic variation to use in breeding? Plant Soil 2005, 269, 369–380. [Google Scholar] [CrossRef]
- Hamnér, K.; Weih, M.; Eriksson, J.; Kirchmann, H. Influence of nitrogen supply on macro- and micronutrient accumulation during growth of winter wheat. Field Crops Res. 2017, 213, 118–129. [Google Scholar] [CrossRef]
- Reznick, J.P.; Barth, G.; Kaschuk, G.; Pauletti, V. Nitrogen and cultivars as field strategies to improve the nutritional status of wheat grain and flour. J. Cereal Sci. 2021, 102, 103290. [Google Scholar] [CrossRef]
- Wysocka, K.; Cacak-Pietrzak, G.; Sosulski, T. Mineral concentration in spring wheat grain under organic, integrated, and conventional farming systems and their alterations during processing. Plants 2025, 14, 1003. [Google Scholar] [CrossRef]
- Islam, M.Z.; Nur-Alam, M.; Rahman, M.M.; Islam, M.Z.; Rahman, A. Nutritional values of wheat and the roles and functions of its compositions in health. Plant Sci. 2024, Preprints. [Google Scholar] [CrossRef]
- Meenu, M.; Kaur, S.; Yadav, M.; Sharma, A.; Tiwari, V.; Ali, U.; Giri, L.; Badwal, A.K.; Garg, M. Impact of inherent chemical composition of wheat and various processing technologies on whole wheat flour and its final products. Cereal Res. Commun. 2025, 53, 409–424. [Google Scholar] [CrossRef]
- Grant, C.; Cubadda, F.; Carcea, M.; Pogna, N.; Gazza, L. Vitamins, minerals, and nutritional value of durum wheat. In Durum Wheat: Chemistry and Technology, 2nd ed.; Sissons, M., Abécassis, J., Marchylo, B., Carcea, M., Eds.; AACC International Press: St. Paul, MN, USA, 2012; pp. 125–137. [Google Scholar]
- Emam, A.; Kamal, N.; Gorafi, Y.; Tahir, I.; Balla, M.; Tsujimoto, H.; Takayoshi, I. Enriched grain minerals in Aegilops tauschii-derived common wheat population under heat-stress environments. Sci. Rep. 2025, 15, 5624. [Google Scholar] [CrossRef] [PubMed]
- Arif, L.; Hamza, M.; Iqbal, E.; Kaleem, Z. Role of micronutrients (vitamins & minerals). Int. J. Multidiscip. Sci. Arts 2024, 3, 333–337. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Biofortification and bioavailability of Zn, Fe and Se in wheat: Present status and future prospects. Theor. Appl. Genet. 2021, 134, 1–35. [Google Scholar] [CrossRef]
- Rodrigo, S.; Lidon, F.; Reboredo, F.H.; Silva, M.M.; Simões, M.M.; Costa, A.R. Wheat plant response to zinc enrichment: Results from a big plot assay. Emir. J. Food Agric. 2023, 35, 946–955. [Google Scholar] [CrossRef]
- Ram, H.; Naeem, A.; Rashid, A.; Kaur, C.; Ashraf, M.Y.; Malik, S.S.; Aslam, M.; Mavi, G.S.; Tutus, Y.; Yazici, M.A.; et al. Agronomic biofortification of genetically biofortified wheat genotypes with zinc, selenium, iodine, and iron under field conditions. Front. Plant Sci. 2024, 15, 1455901. [Google Scholar] [CrossRef]
- INFOSOLO. Available online: https://projects.iniav.pt/infosolo/sig/websig (accessed on 3 July 2025).
- NP EN 15948: 2020; Cereals—Determination of Moisture and Protein—Method Using Near-Infrared-Spectroscopy in Whole Kernels. CEN (European Committee for Standardization): Brussels, Belgium, 2020; 12p.
- ISO 2171: 2023; Cereals, Pulses and By-Products—Determination of Ash Yield by Incineration. International Organization for Standardization (ISO): Geneva, Switzerland, 2023; 6p.
- Gallardo, H.; Queralt, I.; Tapias, J.; Guerra, M.; Carvalho, M.L.; Marguí, E. Possibilities of low-power X-ray fluorescence spectrometry methods for rapid multielemental analysis and imaging of vegetal foodstuffs. J. Food Compos. Anal. 2016, 50, 1–9. [Google Scholar] [CrossRef]
- Bannayan, M.; Sanjani, S. Weather conditions associated with irrigated crops in an arid and semi-arid environment. Agric. For. Meteorol. 2011, 151, 1589–1598. [Google Scholar] [CrossRef]
- Başçiftçi, Z.B.; Olgun, M.; Erdoğan, S. Evaluation of climate drought yield relationships on wheat (T. aestivum L.) by Krigging method in Turkey. Selcuk. J. Agri. Food Sci. 2012, 26, 57–65. Available online: https://dergipark.org.tr/en/download/article-file/3110566 (accessed on 20 August 2025).
- Kheiri, M.; Soufizadeh, S.; Ghaffari, A.; AghaAlikhani, M.; Eskandari, A. Association between temperature and precipitation with dryland wheat yield in northwest of Iran. Clim. Change 2017, 141, 703–717. [Google Scholar] [CrossRef]
- Campiglia, E.; Mancinelli, R.; De Stefanis, E.; Pucciarmati, S.; Radicetti, E. The long-term effects of conventional and organic cropping systems, tillage managements and weather conditions on yield and grain quality of durum wheat (Triticum durum Desf.) in the Mediterranean environment of Central Italy. Field Crops Res. 2015, 176, 34–44. [Google Scholar] [CrossRef]
- Michel, S.; Loeschenberger, F.; Ametz, C.; Pachler, B.; Sparry, E.; Buerstmayr, H. Combining grain yield, protein content and protein quality by multi-trait genomic selection in bread wheat. Theor. Appl. Genet. 2019, 132, 2767–2780. [Google Scholar] [CrossRef] [PubMed]
- Chaurand, M.; Lempereur, I.; Roulland, T.M.; Autran, J.C.; Abecassis, J. Genetic and agronomic effects on semolina milling value of durum wheat. Crop. Sci. 1999, 39, 790–795. [Google Scholar] [CrossRef]
- Miravalles, M.T.; Bermúdez, C.; Aprile, E.; Seghezzo, M.L.; Molfese, E.; Möckel, F. Variaciones en los niveles de cenizas del grano de trigo para fideos en el Sur Bonaerense. In Proceedings of the VII Congreso Nacional de Trigo, Santa Rosa, Argentina, 2 July 2008; EdUNLPump: La Pampa, Argentina, 2008; Volume C7, pp. 1–5. [Google Scholar]
- Adams, C.; Kongraksawech, T.; Ross, A.; Long, D.; Neely, C.; Marshall, J.; Graebner, R.; Reardon, C.; Liang, X. As grain mineral densities have declined over time, have densities converged across wheat classes?—Insights from the US Pacific Northwest and worldwide benchmarks. Crop. Sci. 2024, 65, 1–20. [Google Scholar] [CrossRef]
- Von Grebmer, K.; Bernstein, J.; Resnick, D.; Wiemers, M.; Reiner, L.; Bachmeier, M.; Hanano, A.; Towey, O.; Chéilleachair, R.N.; Foley, C.; et al. Global Hunger Index: Food Systems Transformation and Local Governance; Welthungerhilfe and Concern Worldwide: Bonn, Germany; Dublin, Ireland, 2022. [Google Scholar]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- Neal, B.; Wu, Y.; Feng, X.; Zhang, R.; Zhang, Y.; Shi, J.; Zhang, J.; Tian, M.; Huang, L.; Li, Z.; et al. Effect of salt substitution on cardiovascular events and death. N. Engl. J. Med. 2021, 385, 1067–1077. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, J.; Kumar, S.; Singh, P. Metabolism of macro-elements (calcium, magnesium, sodium, potassium, chloride and phosphorus) and associated disorders. In Clinical Applications of Biomolecules in Disease Diagnosis, 1st ed.; Singh, R.L., Singh, P., Pathak, N., Eds.; Springer: Singapore, 2024; pp. 177–203. [Google Scholar] [CrossRef]
- Hocking, P.J. Dry-matter production, mineral nutrient concentrations, and nutrient distribution and redistribution in irrigated spring wheat. J. Plant Nutr. 1994, 17, 1289–1308. [Google Scholar] [CrossRef]
- El Houssni, I.; Zahidi, A.; Khedid, K.; Hassikou, R. Nutrient and anti-nutrient composition of durum, soft and red wheat landraces: Implications for nutrition and mineral bioavailability. J. Agric. Food Res. 2024, 15, 101078. [Google Scholar] [CrossRef]
- Fan, M.S.; Zhao, F.J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 2008, 22, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Cazetta, J.O.; Carlin, S.; Telles, B. Drought-induced alterations in the uptake of nitrogen, phosphorus and potassium, and the relation with drought tolerance in sugar cane. Ciênc. Agrotec. 2017, 41, 117–127. [Google Scholar] [CrossRef]
- Raza, S.; Saleem, F.M.; Shah, M.G.; Jamil, M.; Khan, H.I. Potassium applied under drought improves physiological and nutrient uptake performances of wheat (Triticum aestivum L.). J. Soil Sci. Plant Nutr. 2013, 13, 175–185. [Google Scholar] [CrossRef]
- Hussain, A.; Larsson, H.; Kuktaite, R.; Johansson, E. Mineral composition of organically grown wheat genotypes: Contribution to daily minerals intake. Int. J. Environ. Res. Public Health 2010, 7, 3442–3456. [Google Scholar] [CrossRef]
- Broadley, M.R.; Alcock, J.; Alford, J.; Cartwright, P.; Foot, I.; Fairweather-Tait, S.J.; Hart, D.; Hurst, R.; Knott, P.; McGrath, S.P.; et al. Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 2010, 332, 5–18. [Google Scholar] [CrossRef]
- Malavolta, E. Manual de Calagem e Adubação Das Principais Culturas, 1st ed.; Ceres: São Paulo, Brazil, 1987; pp. 1–40. [Google Scholar]
- Sankara-Rao, D.S.; Deosthale, Y.G. Mineral and trace element composition of wheat and wheat flours of different extraction rates. J. Plant Foods 1981, 3, 251–257. [Google Scholar] [CrossRef]
- Benguella, R.; Meziani, S.; Zohra, C.F.; Barek, S.; Aissaoui, M.; Rahmoun, M.N.; Demmouche, A. Comparison of the nutritional and antioxidant values of the peripheral layers in two species of wheat (soft and hard) grown in Algeria. Chil. J. Agric. Anim. Sci. 2022, 38, 15–25. [Google Scholar] [CrossRef]
- Ficco, D.B.; Riefolo, C.; Nicastro, G.; Simone, V.; Di Gesù, A.M.; Beleggia, R.; Platani, C.; Cattivelli, L.; De Vita, P. Phytate and mineral elements concentration in a collection of Italian durum wheat cultivars. Field Crops Res. 2009, 111, 235–242. [Google Scholar] [CrossRef]
- Racz, C.J.; Haluschak, P.W. Effects of phosphorus concentration on Cu, Zn, Fe and Mn utilization by wheat. Can. J. Soil Sci. 1974, 54, 357–367. [Google Scholar] [CrossRef]
- Mladenov, N.; Przulj, N.; Hristov, N.; Djuric, V.; Milovanovic, M. Cultivar-by environment interactions for wheat quality traits in semiarid conditions. Cereal Chem. 2001, 78, 363–367. [Google Scholar] [CrossRef]
- Taghouti, M.; Gaboun, F.; Nsarellah, N.; Rhrib, R.; El-Haila, M.; Kamar, M.; Abbad-Andaloussi, F.; Udupa, S.M. Genotype x Environment interaction for quality traits in durum wheat cultivars adapted to different environments. Afr. J. Biotechnol. 2010, 9, 3054–3062. Available online: https://www.ajol.info/index.php/ajb/article/view/80550 (accessed on 20 August 2025).
- Surma, M.; Adamski, T.; Banaszak, Z.; Kaczmarek, Z.; Kuczynska, A.; Majcher, M.; Lugowska, B.; Obuchowski, W.; Salmanowicz, B.P.; Krystkowiak, K. Effect of genotype, environment and their interaction on quality parameters of wheat breeding lines of diverse grain hardness. Plant Prod. Sci. 2012, 15, 192–203. [Google Scholar] [CrossRef]
- Alkhatib, K.; Paulsen, G.M. Photosynthesis and productivity during high-temperature stress of wheat genotypes from major world regions. Crop. Sci. 1990, 30, 1127–1132. [Google Scholar] [CrossRef]
- Aftab, T. Omics Analysis of Plants Under Abiotic Stress, 1st ed.; Apple Academic Press: Palm Bay, FL, USA, 2023; ISBN 978-1-00-328276-1. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, B.; Singh, B.N.; Hidangmayum, A.; Jatav, H.S.; Chandra, K.; Singhal, R.K.; Sathyanarayana, E.; Patra, A.; Mohapatra, K.K. Physiological mechanisms and adaptation strategies of plants under nutrient deficiency and toxicity conditions. In Plant Perspectives to Global Climate Changes, 1st ed.; Aftab, T., Roychoudhury, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 173–194. [Google Scholar] [CrossRef]
- Menezes, E.W.; (Universidade de São Paulo, São Paulo, Brasil); Purgatto, E.; (Universidade de São Paulo, São Paulo, Brasil). Determinação de Cinzas em Alimentos. Personal communication, 2016. [Google Scholar]
- Bilge, G.; Sezer, B.; Efe Eseller, K.; Berberoglu, H.; Koksel, H.; Hakki-Boyaci, I. Ash analysis of flour sample by using laser-induced breakdown spectroscopy. Spectrochim. Acta B At. Spectrosc. 2016, 124, 74–78. [Google Scholar] [CrossRef]
- Johansen, J.M.; Jakobsen, J.G.; Frandsen, F.J.; Glarborg, P. Release of K, Cl, and S during Pyrolysis and Combustion of High-Chlorine Biomass. Energy Fuels 2011, 25, 4961–4971. [Google Scholar] [CrossRef]
- Terzioglu, P.; Yucel, S.; Rababah, T.M.; Özçimen, D. Characterization of wheat hull and wheat hull ash as a potential source of SiO2. BioResources 2013, 8, 4406–4420. [Google Scholar] [CrossRef]
- Colas, A. Dosage des cendres et matières minérales. In Guide Pratique D’Analyses Dans Les Industries Des Céréales, 2nd ed.; Godon, B., Loisel, W., Eds.; Lavoisier Tec & Doc.: Paris, France, 1997; pp. 295–313. ISBN 978-2-7430-0123-0. [Google Scholar]
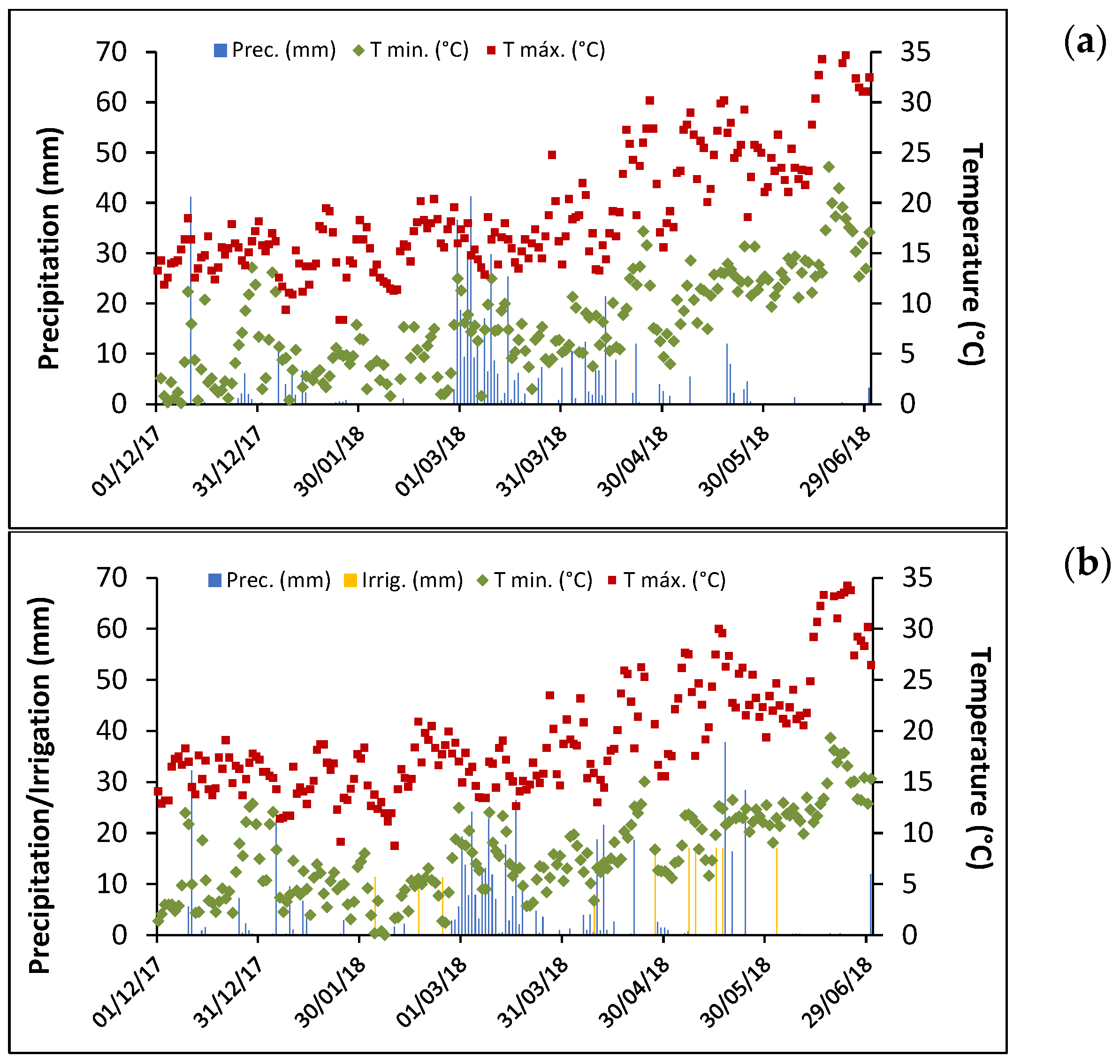
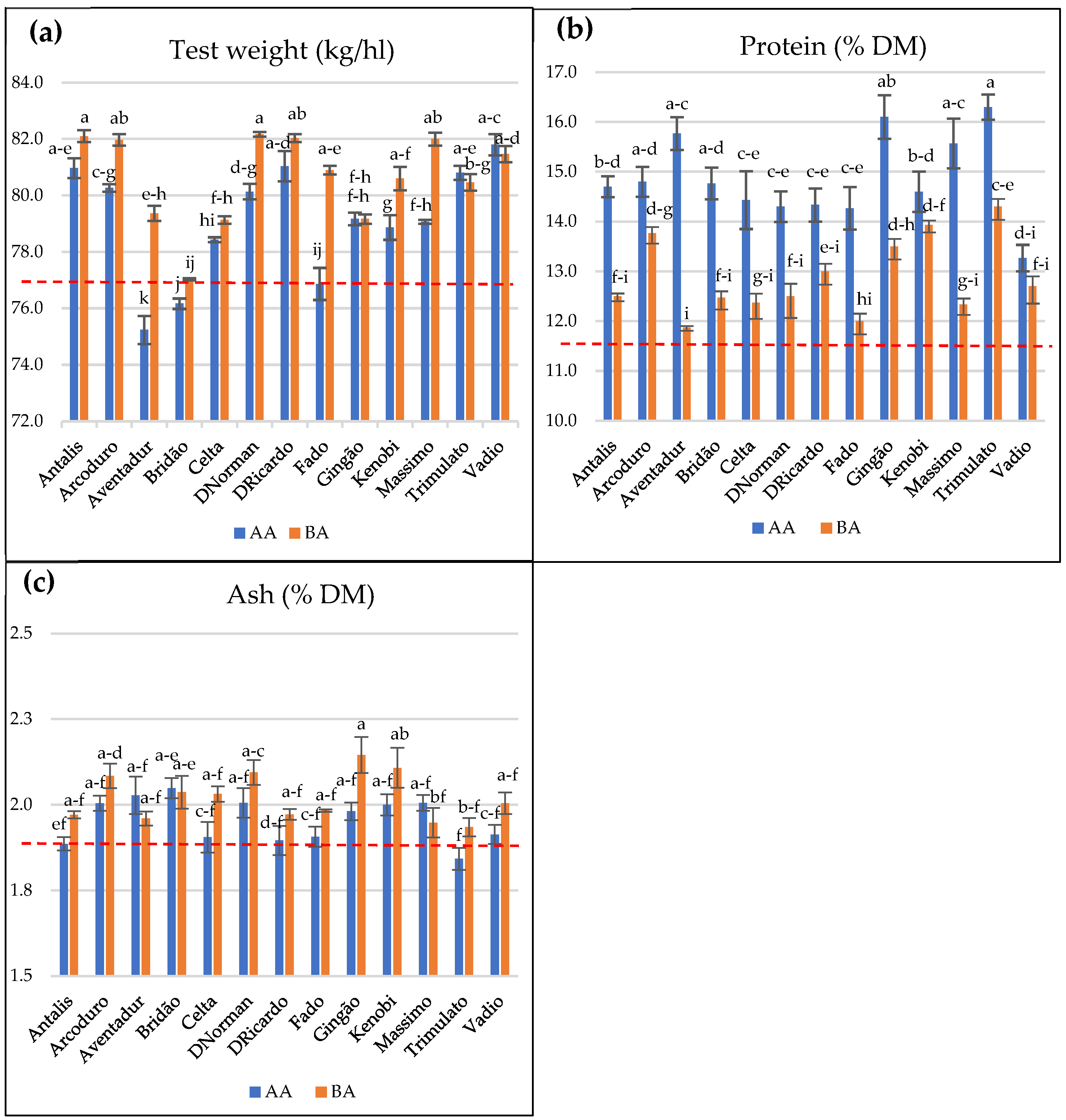
| Source of Variation | Test Weight (R2 = 0.955) | Protein Content (R2 = 0.909) | Ash Content (R2 = 0.685) | ||||
|---|---|---|---|---|---|---|---|
| df | MS | F | MS | F | MS | F | |
| Model | 25 | 11.22 | 43.82 *** | 5.02 | 20.79 *** | 0.016 | 4.53 *** |
| Genotype (G) | 12 | 16.41 | 64.09 *** | 2.60 | 10.77 *** | 0.020 | 5.52 *** |
| Environment (E) | 1 | 44.33 | 173.13 *** | 77.80 | 322.44 *** | 0.083 | 23.24 *** |
| G × E | 12 | 3.27 | 12.78 *** | 1. 37 | 5.67 *** | 0.007 | 1.98 * |
| Error | 52 | 0.26 | 0.24 | 0.004 | |||
| Macro Concent. | GR Samples | GRash Samples | ||
|---|---|---|---|---|
| AA (g/kg) | BA (g/kg) | AA (g/kg) | BA (g/kg) | |
| K | 8.02 ± 0.16 a | 4.94 ± 0.08 b | 195.9 ± 3.6 a | 110.1 ± 2.2 b |
| P | 1.69 ± 0.04 b | 2.06 ± 0.04 a | 52.9 ± 1.1 a | 52.6 ± 1.1 a |
| Ca | 0.533 ± 0.015 a | 0.408 ± 0.009 b | 14.3 ± 0.5 a | 8.2 ± 0.3 b |
| S | 0.532 ± 0.013 b | 0.597 ± 0.006 a | - | - |
| Cl | 0.162 ± 0.010 b | 0.243 ± 0.006 a | - | - |
| Total (%) | 99.1 | 98.6 | 98.3 | 97.9 |
| Micro Concent. | AA (mg/kg) | BA (mg/kg) | AA (g/kg) | BA (g/kg) |
| Fe | 44.0 ± 1.2 a | 42.1 ± 0.9 a | 1.300 ± 0.035 a | 0.789 ± 0.015 b |
| Mn | 35.9 ± 1.1 a | 37.1 ± 0.9 a | 1.120 ± 0.038 a | 0.762 ± 0.019 b |
| Zn | 21.3 ± 0.6 b | 30.1 ± 0.5 a | 0.727 ± 0.019 a | 0.650 ± 0.014 b |
| Cu | 3.49 ± 0.24 b | 7.91 ± 0.18 a | 0.153 ± 0.005 a | 0.124 ± 0.002 b |
| Si | - | - | 0.289 ± 0.057 b | 1.106 ± 0.062 a |
| Rb | - | - | 0.956 ± 0.050 a | 0.198 ± 0.010 b |
| Sr | - | - | 0.041 ± 0.002 b | 0.049 ± 0.002 a |
| Ti | - | - | 0.048 ± 0.002 a | 0.031 ± 0.001 b |
| Total (%) | 0.9 | 1.4 | 1.7 | 2.1 |
| Source of Variation | ||||||||
|---|---|---|---|---|---|---|---|---|
| GR Samples | GRash Samples | |||||||
| Element | Model df = 25 | G df = 12 | E df = 1 | G × E df = 12 | Model df = 25 | G df = 12 | E df = 1 | G × E df = 12 |
| K | 18.79 *** | 2.42 * | 414.17 *** | 2.22 * | 30.50 *** | 2.64 ** | 689.68 *** | 3.43 ** |
| P | 4.48 *** | 1.27 ns | 60.17 *** | 3.04 ** | 5.14 *** | 4.51 *** | 0.09 ns | 6.19 *** |
| Ca | 13.04 *** | 12.69 *** | 153.72 *** | 1.67 ns | 19.12 *** | 9.46 *** | 333.76 *** | 2.56 * |
| S | 2.38 ** | 1.61 ns | 23.86 *** | 1.36 ns | - | - | - | - |
| Cl | 6.45 *** | 6.44 *** | 64.60 *** | 1.62 ns | - | - | - | - |
| Fe | 2.72 ** | 3.06 ** | 2.39 ns | 2.41 * | 16.45 *** | 4.84 *** | 327.25 *** | 2.16 * |
| Mn | 4.18 *** | 6.71 *** | 1.36 ns | 1.88 ns | 11.30 *** | 8.41 *** | 159.45 *** | 1.84 ns |
| Zn | 9.15 *** | 1.97 * | 178.17 *** | 2.25 * | 5.62 *** | 5.35 *** | 24.53 *** | 4.31 *** |
| Cu | 11.82 *** | 1.50 ns | 256.47 *** | 1.76 ns | 6.36 *** | 6.43 *** | 49.67 *** | 2.68 ** |
| Si | - | - | - | - | 6.74 *** | 2.38 * | 120.89 *** | 1.44 ns |
| Rb | - | - | - | - | 12.44 *** | 1.61 ns | 270.89 *** | 1.74 ns |
| Sr | - | - | - | - | 6.74 *** | 10.26 *** | 19.26 *** | 2.18 * |
| Ti | - | - | - | - | 6.07 *** | 4.87 *** | 75.10 *** | 1.53 ns |
| Genotype | K (g/kg) | P (g/kg) | Fe (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|
| AA | ||||
| Antalis | 8.24 ± 0.14 ab | 1.69 ± 0.05 a–d | 46.5 ± 2.5 a–c | 22.5 ± 2.1 c–f |
| Arcoduro | 8.21 ± 0.29 ab | 1.78 ± 0.08 a–d | 45.2 ± 2.5 a–c | 22.5 ± 1.5 c–f |
| Aventadur | 8.74 ± 0.29 ab | 1.60 ± 0.04 cd | 47.9 ± 3.3 a–c | 20.6 ± 1.5 d–f |
| Bridão | 7.84 ± 0.43 a–c | 1.84 ± 0.13 a–d | 38.3 ± 3.4 a–c | 22.2 ± 1.5 c–f |
| Celta | 7.65 ± 0.64 a–c | 1.41 ± 0.08 d | 34.9 ± 0.5 bc | 19.2 ± 1.5 ef |
| DNorman | 8.84 ± 0.70 ab | 1.87 ± 0.11 a–d | 46.0 ± 2.5 a–c | 20.6 ± 2.1 d–f |
| DRicardo | 7.06 ± 0.14 b–g | 1.40 ± 0.08 d | 39.5 ± 0.8 a–c | 17.3 ± 1.2 f |
| Fado | 9.43 ± 1.33 a | 1.88 ± 0.31 a–d | 51.8 ± 4.4 a | 24.0 ± 3.6 b–f |
| Gingão | 7.33± 0.37 a–e | 1.73 ± 0.14 a–d | 48.4 ± 2.8 a–c | 22.2 ± 0.2 c–f |
| Kenobi | 7.80 ± 0.32 a–c | 1.81 ± 0.13 a–d | 38.9 ± 2.2 a–c | 18.4 ± 1.5 ef |
| Massimo | 8.19 ± 0.30 ab | 1.79 ± 0.13 a–d | 41.8 ± 1.7 a–c | 22.2 ± 0.4 c–f |
| Trimulato | 7.64 ± 0.06 a–d | 1.64 ± 0.11 b–d | 50.3 ± 4.4 ab | 26.0 ± 3.2 a–f |
| Vadio | 7.31 ± 0.25 a–f | 1.60 ± 0.08 cd | 42.2 ± 2.5 a–c | 19.5 ± 1.2 ef |
| BA | ||||
| Antalis | 5.20 ± 0.15 f–h | 2.21 ± 0.09 a–c | 45.0 ± 0.7 a–c | 31.8 ± 0.7 ab |
| Arcoduro | 4.82 ± 0.01 h | 2.06 ± 0.01 a–d | 47.3 ± 1.5 a–c | 30.9 ± 0.4 a–c |
| Aventadur | 5.52 ± 0.23 d–h | 2.02 ± 0.13 a–d | 38.7 ± 2.7 a–c | 31.0 ± 2.0 a–c |
| Bridão | 4.85 ± 0.28 h | 1.78 ± 0.15 a–d | 32.8 ± 1.8 c | 27.1 ± 1.7 a–e |
| Celta | 5.88 ± 0.11 c–h | 2.29 ± 0.09 ab | 43.7 ± 1.6 a–c | 33.5 ± 1.4 a |
| DNorman | 5.17 ± 0.14 gh | 2.24 ± 0.02 a–c | 41.7 ± 1.7 a–c | 33.2 ± 0.8 a |
| DRicardo | 5.03 ± 0.09 gh | 2.23 ± 0.04 a–c | 45.9 ± 2.2 a–c | 29.7 ± 1.1 a–d |
| Fado | 4.64 ± 0.25 h | 1.83 ± 0.13 a–d | 40.1 ± 1.7 a–c | 27.1 ± 1.6 a–e |
| Gingão | 4.81 ± 0.17 h | 2.06 ± 0.15 a–d | 40.2 ± 1.9 a–c | 29.5 ± 1.2 a–d |
| Kenobi | 5.26 ± 0.12 e–h | 2.34 ± 0.10 a | 47.4 ± 2.5 a–c | 29.9 ± 0.8 a–c |
| Massimo | 4.17 ± 0.27 h | 1.75 ± 0.17 a–d | 34.4 ± 3.4 bc | 25.5 ± 2.6 a–f |
| Trimulato | 4.30 ± 0.21 h | 1.98 ± 0.11 a–d | 49.5 ± 3.0 a–c | 33.0 ± 1.4 ab |
| Vadio | 4.60 ± 0.10 h | 2.05 ± 0.12 a–d | 40.6 ± 1.6 a–c | 29.0 ± 1.6 a–d |
| Genotype | Ca (g/kg) | S (g/kg) | Cl (g/kg) | Mn (mg/kg) | Cu (mg/kg) |
|---|---|---|---|---|---|
| AA | |||||
| Antalis | 0.502 ± 0.032 BC | 0.538 ± 0.063 A | 0.135 ± 0.021 AB | 36.5 ± 4.7 AB | 3.23 ± 0.97 A |
| Arcoduro | 0.524 ± 0.028 A–C | 0.514 ± 0.036 A | 0.104 ± 0.007 AB | 36.4 ± 2.6 AB | 3.87 ± 0.44 A |
| Aventadur | 0.589 ± 0.005 AB | 0.564 ± 0.039 A | 0.211 ± 0.049 AB | 32.4 ± 2.3 AB | 3.60 ± 0.51 A |
| Bridão | 0.493 ± 0.027 BC | 0.526 ± 0.037 A | 0.137 ± 0.042 AB | 37.0 ± 0.7 AB | 3.60 ± 1.32 A |
| Celta | 0.604 ± 0.046 AB | 0.448 ± 0.036 A | 0.231 ± 0.048 A | 31.4 ± 0.7 B | 1.97 ± 0.47 A |
| DNorman | 0.572 ± 0.035 AB | 0.589 ± 0.032 A | 0.186 ± 0.029 AB | 39.3 ± 4.7 AB | 5.23 ± 0.28 A |
| DRicardo | 0.366 ± 0.016 C | 0.459 ± 0.037 A | 0.036 ± 0.024 B | 30.5 ± 1.7 B | 2.43 ± 1.39 A |
| Fado | 0.577± 0.081 AB | 0.575 ± 0.102 A | 0.151 ± 0.037 AB | 34.6 ± 3.8 AB | 4.17 ± 0.69 A |
| Gingão | 0.700± 0.044 A | 0.532 ± 0.031 A | 0.264 ± 0.035 A | 45.2 ± 3.0 AB | 4.70 ± 0.35 A |
| Kenobi | 0.471 ± 0.016 BC | 0.527 ± 0.015 A | 0.185 ± 0.024 AB | 30.8 ± 2.3 B | 2.90 ± 1.10 A |
| Massimo | 0.514± 0.006 BC | 0.557 ± 0.053 A | 0.208 ± 0.040 AB | 32.1 ± 3.4 AB | 2.80 ± 0.60 A |
| Trimulato | 0.504 ± 0.018 BC | 0.607 ± 0.031 A | 0.168 ± 0.050 AB | 48.1 ± 4.4 A | 3.93 ± 0.80 A |
| Vadio | 0.507± 0.017 BC | 0.486 ± 0.020 A | 0.084 ± 0.028 AB | 32.4 ± 2.7 AB | 2.97 ± 1.23 A |
| BA | |||||
| Antalis | 0.394 ± 0.013 d–f | 0.594 ± 0.021 bc | 0.229 ± 0.005 cd | 37.5 ± 0.6 a–e | 8.47 ± 0.80 ab |
| Arcoduro | 0.388 ± 0.005 d–f | 0.610 ± 0.011 a–c | 0.181 ± 0.007 e | 43.4 ± 0.3 ab | 8.03 ± 0.26 ab |
| Aventadur | 0.466 ± 0.006 bc | 0.554 ± 0.009 c | 0.284 ± 0.009 b | 33.0 ± 1.5 c–e | 7.03 ± 0.24 b |
| Bridão | 0.357 ± 0.014 ef | 0.593 ± 0.015 bc | 0.265 ± 0.010 bc | 31.2 ± 2.8 de | 7.87 ± 0.33 ab |
| Celta | 0.490 ±0.002 ab | 0.585 ± 0.020 bc | 0.253 ± 0.003 b–d | 38.7 ± 1.1 a–e | 8.00 ± 0.55 ab |
| DNorman | 0.413 ± 0.003 c–e | 0.562 ± 0.013 c | 0.258 ± 0.019 b–d | 37.0 ± 1.1 b–e | 8.07 ± 0.23 ab |
| DRicardo | 0.344 ± 0.009 f | 0.573 ± 0.018 bc | 0.220 ± 0.006 de | 36.5 ± 0.7 b–e | 8.10 ± 0.70 ab |
| Fado | 0.384 ± 0.006 d–f | 0.571 ± 0.004 bc | 0.237 ± 0.007 cd | 30.6 ± 1.3 de | 7.53 ± 0.48 b |
| Gingão | 0.524 ± 0.017 a | 0.604 ± 0.025 a–c | 0.336 ± 0.005 a | 41.7 ± 1.3 a–c | 8.27 ± 0.09 ab |
| Kenobi | 0.393 ± 0.008 d–f | 0.676 ± 0.005 a | 0.218 ± 0.002 de | 39.3 ± 1.8 a–d | 10.27 ± 0.49 a |
| Massimo | 0.353 ± 0.015 f | 0.601 ± 0.003 a–c | 0.225 ± 0.003 cd | 29.6 ± 3.1 e | 7.47 ± 0.88 b |
| Trimulato | 0.359 ± 0.027 ef | 0.647 ± 0.005 ab | 0.240 ± 0.003 cd | 46.3 ± 2.3 a | 7.23 ± 0.17 b |
| Vadio | 0.436 ±0.016 b–d | 0.594 ± 0.028 bc | 0.218 ± 0.011 de | 37.0 ± 2.5 b–e | 6.57 ± 0.43 b |
| Genotype | K (g/kg) | P (g/kg) | Ca (g/kg) | Fe (g/kg) | Zn (g/kg) | Cu (g/kg) | Sr (g/kg) |
|---|---|---|---|---|---|---|---|
| AA | |||||||
| Antalis | 196.6 ± 17.5 ab | 52.0 ± 4.7 a–c | 13.0 ± 1.1 b–f | 1.320 ± 0.121 a–d | 0.729 ± 0.079 a–d | 0.152 ± 0.029 a–f | 0.036 ± 0.003 c–f |
| Arcoduro | 191.6 ± 1.1 ab | 52.5 ± 0.2 a–c | 13.6 ± 0.5 b–e | 1.334 ± 0.002 a–d | 0.783 ± 0.002 ab | 0.176 ± 0.003 a–d | 0.040 ± 0.005 c–f |
| Aventadur | 215.8 ± 6.2 a | 50.0 ± 2.0 a–d | 17.0 ± 0.7 ab | 1.343 ± 0.042 a–d | 0.737 ± 0.007 a–d | 0.133 ± 0.002 b–f | 0.051 ± 0.002 a–d |
| Bridão | 215.5 ± 9.0 a | 60.4 ± 1.6 ab | 14.8 ± 0.3 b–d | 1.290 ± 0.057 a–d | 0.816 ± 0.043 ab | 0.162 ± 0.013 a–e | 0.038 ± 0.005 c–f |
| Celta | 182.8 ± 9.7 ab | 45.6 ± 2.6 cd | 16.0 ± 1.3 a–c | 1.064 ± 0.061 c–g | 0.662 ± 0.052 b–e | 0.126 ± 0.018 d–f | 0.052 ± 0.008 a–d |
| DNorman | 200.2 ± 4.1 ab | 55.0 ± 1.3 a–c | 14.2 ± 0.5 b–e | 1.296 ± 0.009 a–d | 0.673 ± 0.022 b–e | 0.135 ± 0.003 b–f | 0.048 ± 0.005 a–e |
| DRicardo | 201.5 ± 9.9 ab | 53.6± 3.7 a–c | 11.4 ± 0.9 c–h | 1.440 ± 0.084 a–c | 0.721 ± 0.046 a–d | 0.159 ± 0.010 a–e | 0.033 ± 0.008 c–f |
| Fado | 181.4 ± 12.7 ab | 47.4 ± 4.1 b–d | 11.5 ± 1.2 c–h | 1.157 ± 0.093 b–e | 0.602 ± 0.031 b–e | 0.121 ± 0.015 d–f | 0.025 ± 0.002 ef |
| Gingão | 185.5 ± 3.6 ab | 55.7 ± 1.7 a–c | 19.7 ± 1.3 a | 1.483 ± 0.007 ab | 0.800 ± 0.018 ab | 0.184 ± 0.011 a–c | 0.066 ± 0.006 ab |
| Kenobi | 211.3 ± 0.6 ab | 57.2 ± 1.3 a–c | 14.0 ± 0.1 b–e | 1.325 ± 0.012 a–d | 0.738 ± 0.025 a–d | 0.202 ± 0.009 a | 0.042 ± 0.003 b–f |
| Massimo | 180.2 ± 9.6 ab | 47.4 ± 3.1 b–d | 12.5 ± 0.9 b–g | 1.085 ± 0.055 c–g | 0.660 ± 0.036 b–e | 0.127 ± 0.012 c–f | 0.035 ± 0.002 c–f |
| Trimulato | 214.6 ± 26.8 ab | 63.4 ± 7.0 a | 16.0 ± 2.9 a–c | 1.658 ± 0.248 a | 0.927 ± 0.133 a | 0.187 ± 0.022 ab | 0.040 ± 0.011 c–f |
| Vadio | 169.6 ± 8.5 bc | 48.0 ± 4.7 b–d | 11.9 ± 0.7 c–h | 1.108 ± 0.050 b–f | 0.600 ± 0.043 b–e | 0.121 ± 0.009 d–f | 0.021 ± 0.003 f |
| BA | |||||||
| Antalis | 114.8 ± 0.6 de | 57.0 ± 0.9 a–c | 8.0 ± 0.2 g–j | 0.801 ± 0.003 e–g | 0.681 ± 0.008 b–e | 0.129 ± 0.001 c–f | 0.051 ± 0.002 a–e |
| Arcoduro | 80.8 ± 0.4 e | 36.6 ± 0.1 d | 5.4 ± 0.1 j | 0.744 ± 0.010 fg | 0.481 ± 0.005 e | 0.101 ± 0.002 f | 0.026 ± 0.002 d–f |
| Aventadur | 120.3 ± 6.5 de | 48.8 ± 3.4 b–d | 9.6 ± 0.6 e–j | 0.763 ± 0.034 e–g | 0.667 ± 0.042 b–e | 0.112 ± 0.006 ef | 0.054 ± 0.008 a–c |
| Bridão | 125.6 ± 1.3 c–e | 56.4 ± 1.0 a–c | 8.4 ± 0.1 f–j | 0.721 ± 0.007 fg | 0.675 ± 0.011 b–e | 0.125 ± 0.002 d–f | 0.045 ± 0.002 b–f |
| Celta | 128.7 ± 1.7 cd | 56.8 ± 0.8 a–c | 10.5 ± 0.5 d–i | 0.765 ± 0.018 e–g | 0.719 ± 0.019 a–d | 0.117 ± 0.003 ef | 0.068 ± 0.002 ab |
| DNorman | 107.1 ± 2.8 de | 50.9 ± 0.9 a–c | 8.1 ± 0.2 g–j | 0.707 ± 0.021 g | 0.634 ± 0.021 b–e | 0.123 ± 0.008 d–f | 0.051 ± 0.002 a–e |
| DRicardo | 96.3 ± 0.1 de | 46.0 ± 0.0 cd | 5.9 ± 0.1 ij | 0.732 ± 0.002 fg | 0.553 ± 0.006 c–e | 0.107 ± 0.001 ef | 0.040 ± 0.001 c–f |
| Fado | 115.9 ± 4.9 de | 58.1 ± 2.0 a–c | 8.5 ± 0.1 f–j | 0.830 ± 0.011 e–g | 0.667 ± 0.003 b–e | 0.118 ± 0.002 ef | 0.045 ± 0.002 b–f |
| Gingão | 116.7 ± 1.8 de | 57.8 ± 1.4 a–c | 11.2 ± 0.1 d–h | 0.839 ± 0.014 e–g | 0.735 ± 0.012 a–d | 0.147 ± 0.002 a–f | 0.073 ± 0.008 a |
| Kenobi | 94.2 ± 1.6 de | 47.0 ± 1.2 b–d | 6.3 ± 0.1 ij | 0.719 ± 0.006 fg | 0.520 ± 0.007 de | 0.143 ± 0.002 b–f | 0.039 ± 0.002 c–f |
| Massimo | 117.1 ± 1.6 de | 58.0 ± 0.9 a–c | 8.5 ± 0.2 f–j | 0.799 ± 0.018 e–g | 0.683 ± 0.013 b–e | 0.130 ± 0.001 c–f | 0.053 ± 0.004 a–c |
| Trimulato | 103.2 ±4.0 de | 54.4 ± 1.5 a–c | 7.4 ± 0.3 h–j | 1.040 ± 0.077 d–g | 0.760 ± 0.035 a–c | 0.144 ± 0.009 b–f | 0.049 ± 0.005 a–e |
| Vadio | 109.9 ± 2.3 de | 56.5 ± 0.9 a–c | 8.6 ± 0.2 f–j | 0.798 ± 0.012 e–g | 0.669 ± 0.02 b–e | 0.116 ± 0.003 ef | 0.044 ± 0.003 b–f |
| K | P | Ca | S | Cl | Fe | Mn | Zn | Cu | |
|---|---|---|---|---|---|---|---|---|---|
| Ash content | −0.25 * | 0.43 *** | −0.05 ns | 0.29 * | 0.30 ** | −0.04 ns | 0.12 ns | 0.34 ** | 0.40 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, J.; Rodrigo, S.; Pinheiro, N.; Costa, R.; Costa, A.; Dôres, J.; Patanita, M.; Maçãs, B.; Leitão, R.; Guerra, M.; et al. Durum Wheat Kernel: Influence of the Genotype and Environment on the Mineral Profile of Grains and Ashes. Plants 2025, 14, 3414. https://doi.org/10.3390/plants14223414
Moreira J, Rodrigo S, Pinheiro N, Costa R, Costa A, Dôres J, Patanita M, Maçãs B, Leitão R, Guerra M, et al. Durum Wheat Kernel: Influence of the Genotype and Environment on the Mineral Profile of Grains and Ashes. Plants. 2025; 14(22):3414. https://doi.org/10.3390/plants14223414
Chicago/Turabian StyleMoreira, José, Sara Rodrigo, Nuno Pinheiro, Rita Costa, Armindo Costa, José Dôres, Manuel Patanita, Benvindo Maçãs, Roberta Leitão, Mauro Guerra, and et al. 2025. "Durum Wheat Kernel: Influence of the Genotype and Environment on the Mineral Profile of Grains and Ashes" Plants 14, no. 22: 3414. https://doi.org/10.3390/plants14223414
APA StyleMoreira, J., Rodrigo, S., Pinheiro, N., Costa, R., Costa, A., Dôres, J., Patanita, M., Maçãs, B., Leitão, R., Guerra, M., & Bagulho, A. S. (2025). Durum Wheat Kernel: Influence of the Genotype and Environment on the Mineral Profile of Grains and Ashes. Plants, 14(22), 3414. https://doi.org/10.3390/plants14223414







